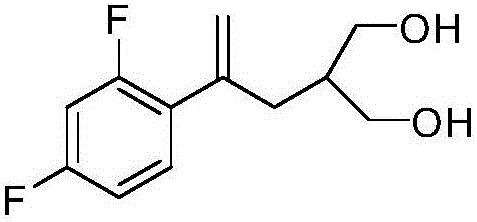
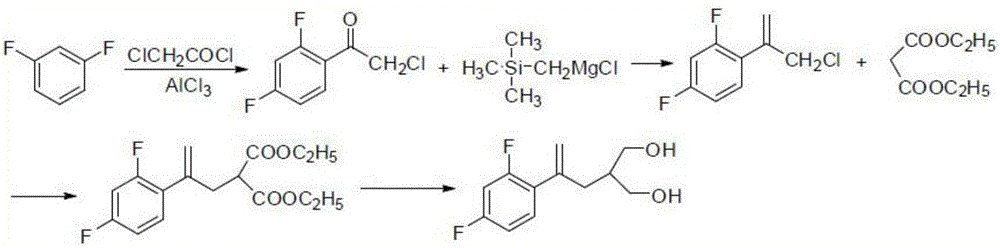
Synthesis method of 2-[2-(2,4-diflurophenyl)-2-propen-1-yl)-1,3-propanediol
A technology of difluorophenyl and synthetic method, which is applied in the field of synthesis of posaconazole intermediates, can solve the problems of affecting the health of experimenters, high production cost of propylene glycol, and difficulty in industrialized production, and achieves low toxicity and easy industrialized production , easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Mix 2-chloromethyl propylene oxide (27.60g, 0.30mol) and 1,3-difluorobenzene (34.23g, 0.30mol) at 0°C and add trichloride in 5 batches while stirring. Aluminum (40.00 g, 0.30 mol) was reacted at room temperature for 7.5 hours after the addition was completed, and then the temperature was raised to 58° C. to continue the reaction for 3 hours. After the reaction was completed, the mixture was carefully added to 300 ml of hydrochloric acid solution with a concentration of 2 mol / l at 0°C, stirred evenly and extracted three times with dichloromethane, 200 ml each time, and the dichloromethane layers were combined, and the dichloromethane layers were sequentially washed with saturated NaHCO 3 Solution, water, and saturated saline were washed once respectively. The washed dichloromethane layer was washed with anhydrous Na 2 SO 4 After drying, filter, and rotary evaporate to remove dichloromethane, 51.65 g (0.25 mol) of oily product 2-(2,4-difluorophenyl)-1-chloro-3-propa...
Embodiment 2
[0046] 1. Mix 2-chloromethyl propylene oxide (22.08g, 0.24mol) and 1,3-difluorobenzene (22.82g, 0.20mol) at 0°C and add trichloride in 4 batches while stirring. Iron (0.24mol), react at room temperature for 8 hours after the addition, then raise the temperature to 60°C and continue the reaction for 3 hours. After the reaction was completed, the mixture was carefully added to 200 ml of hydrochloric acid solution with a concentration of 2 mol / l at 0°C, stirred evenly, extracted three times with dichloromethane, 130 ml each time, combined the dichloromethane layers, and washed with saturated NaHCO 3 Solution, water, and saturated saline were washed once respectively. Anhydrous Na for organic layer 2 SO 4 After drying, filter, and remove methylene chloride by rotary evaporation, 33.06 g (0.16 mol) of oily product 2-(2,4-difluorophenyl)-1-chloro-3-propanol was obtained, with a yield of 80%.
[0047] 2. Add 41.32g (0.20mol) of 2-(2,4-difluorophenyl)-1-chloro-3-propanol and 29.96g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com