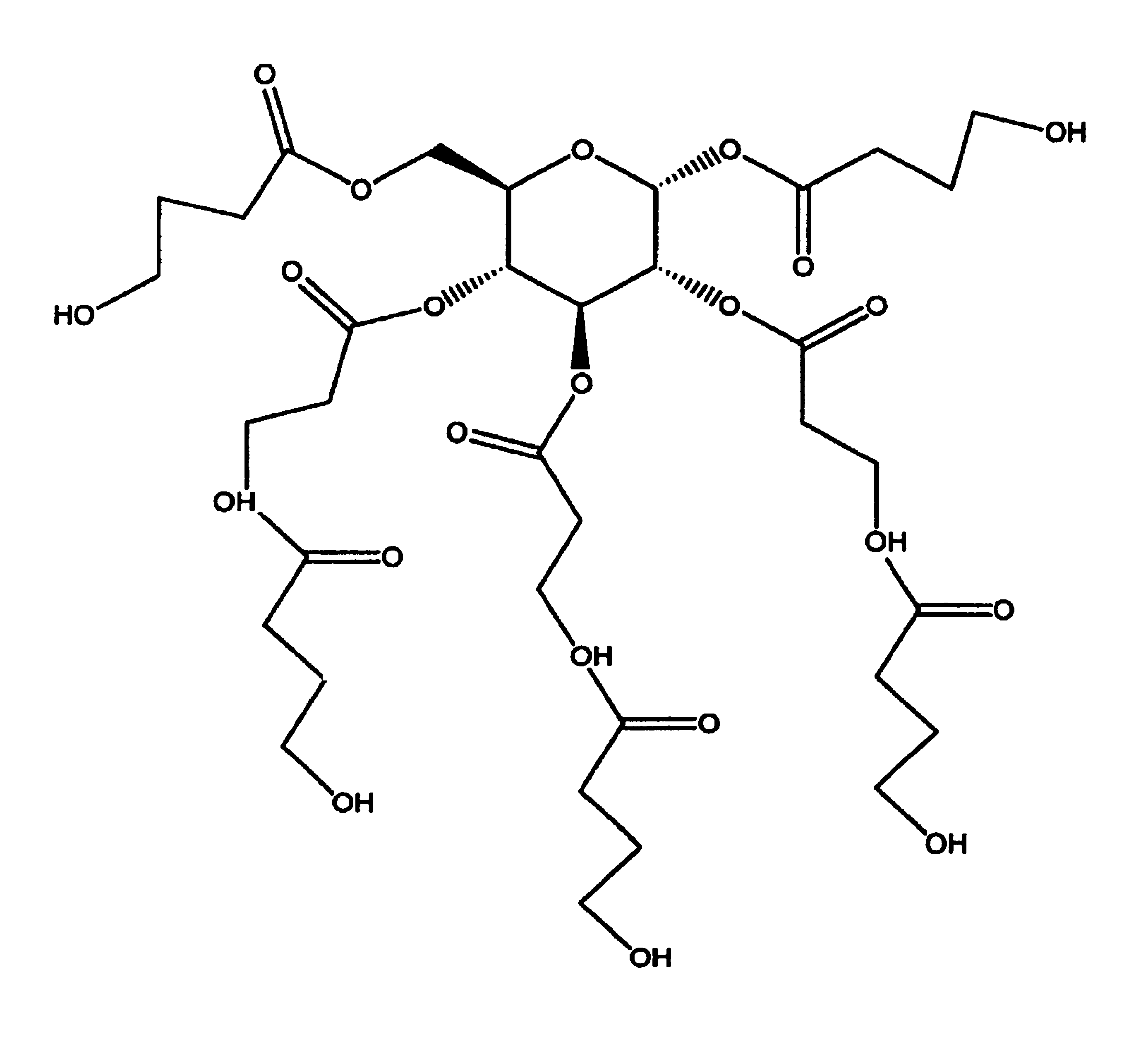
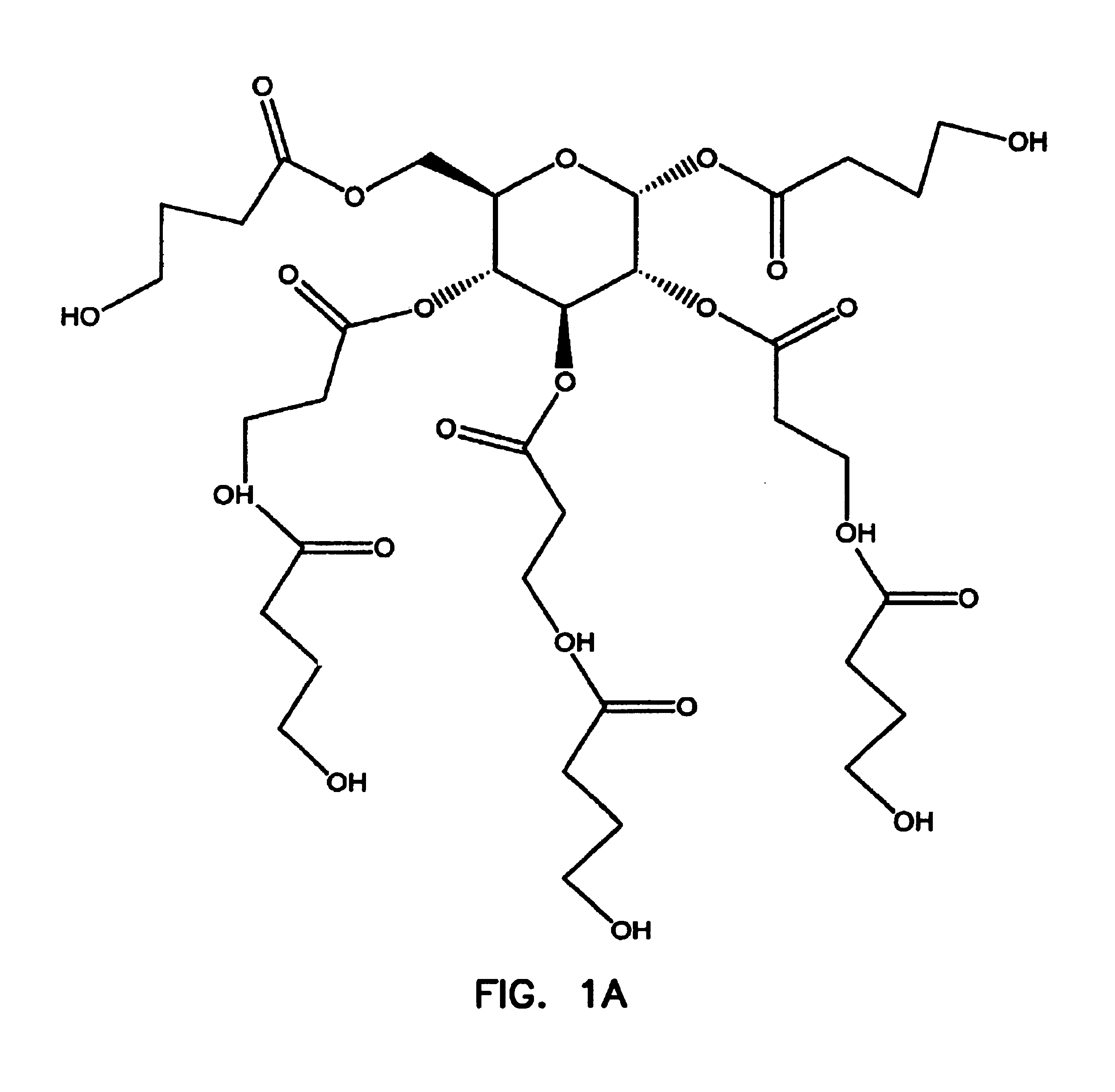
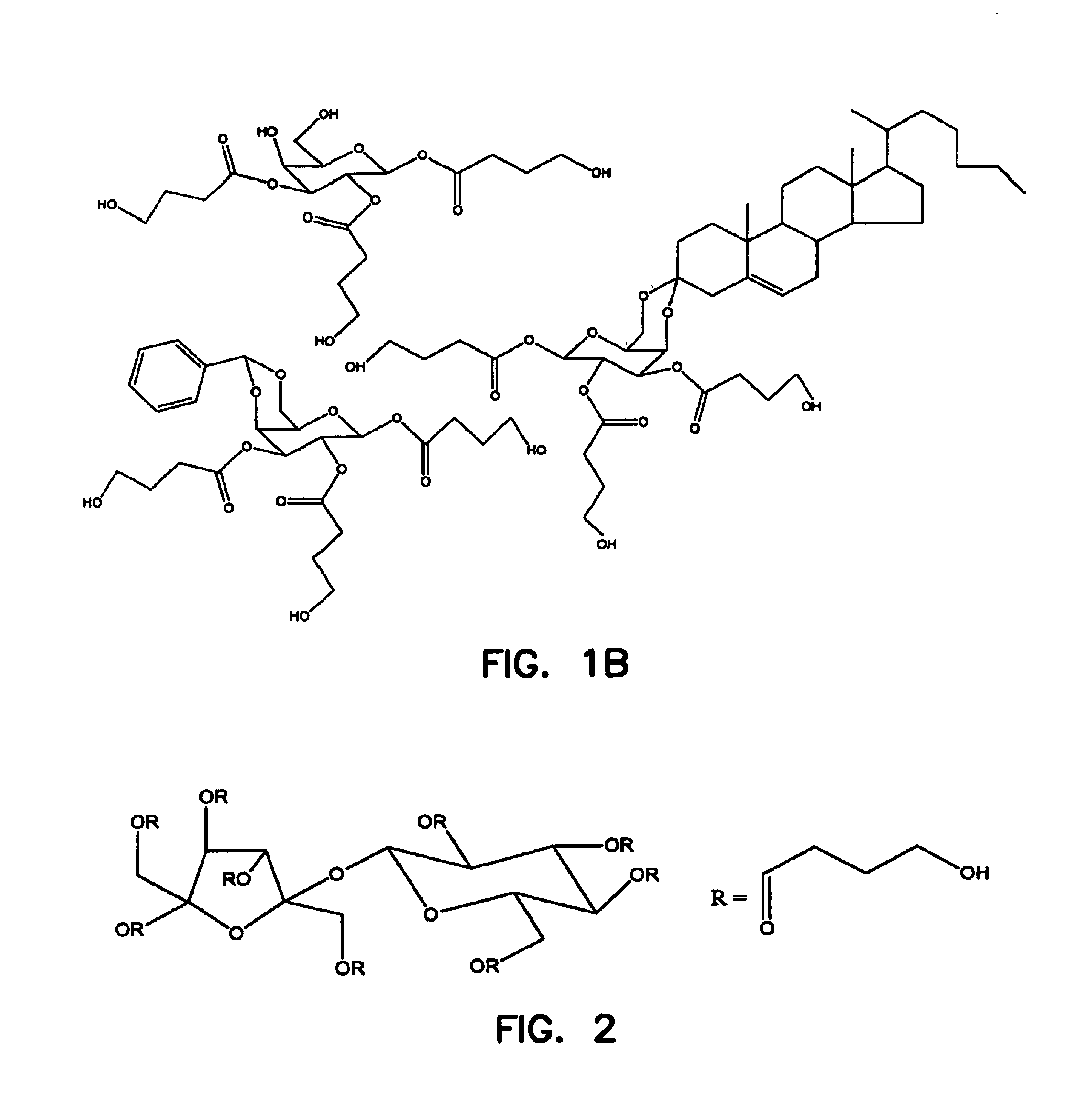
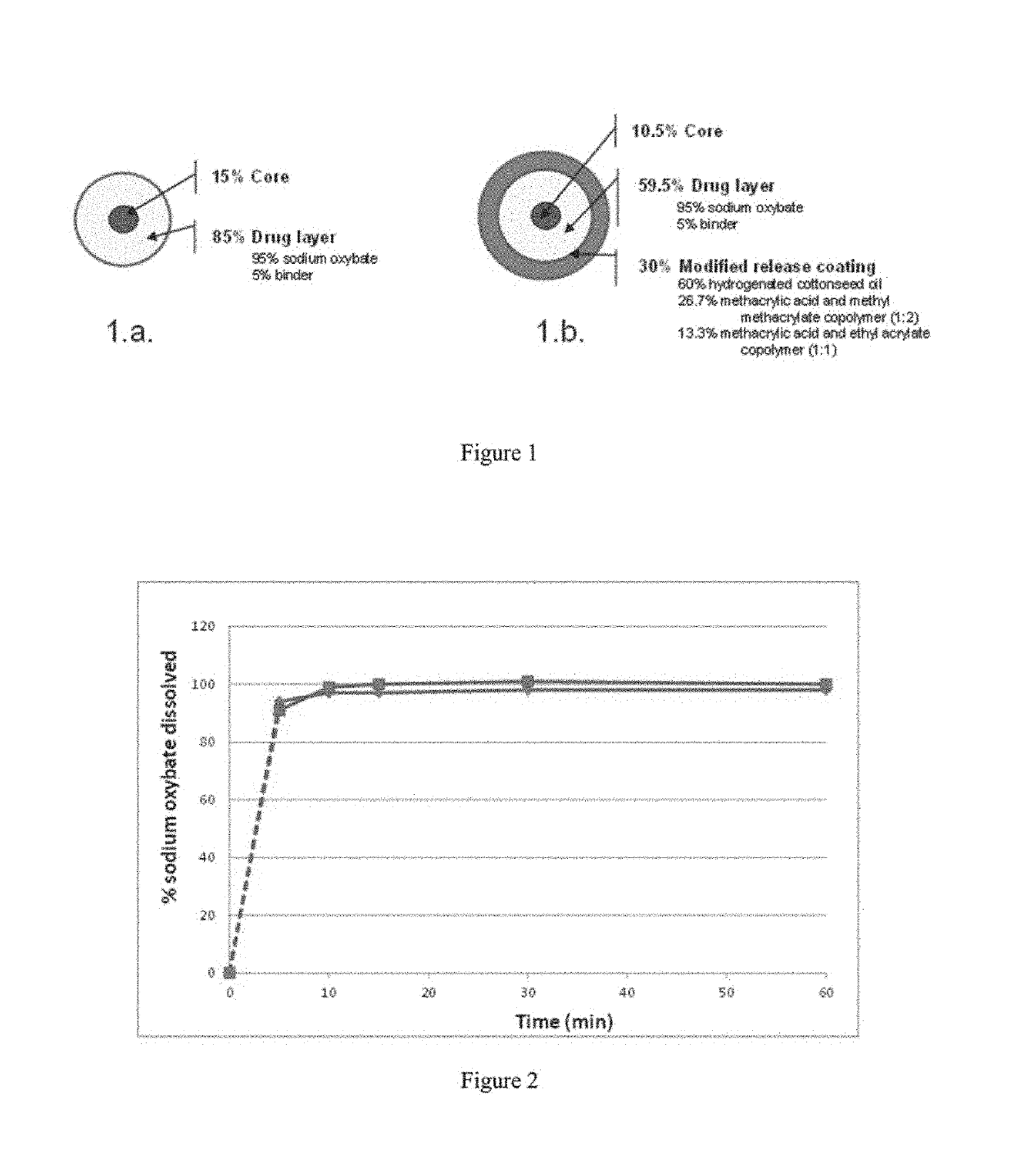
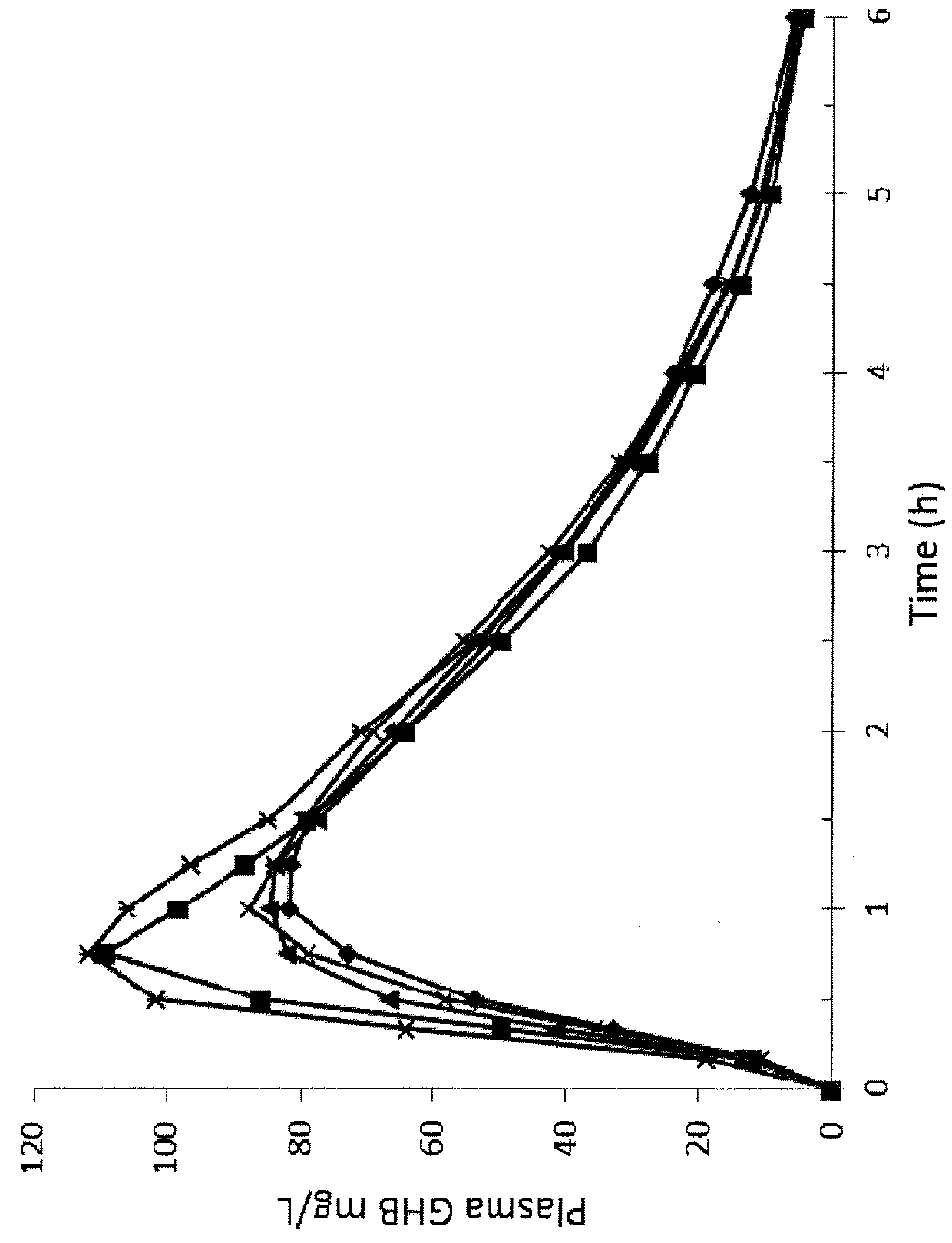
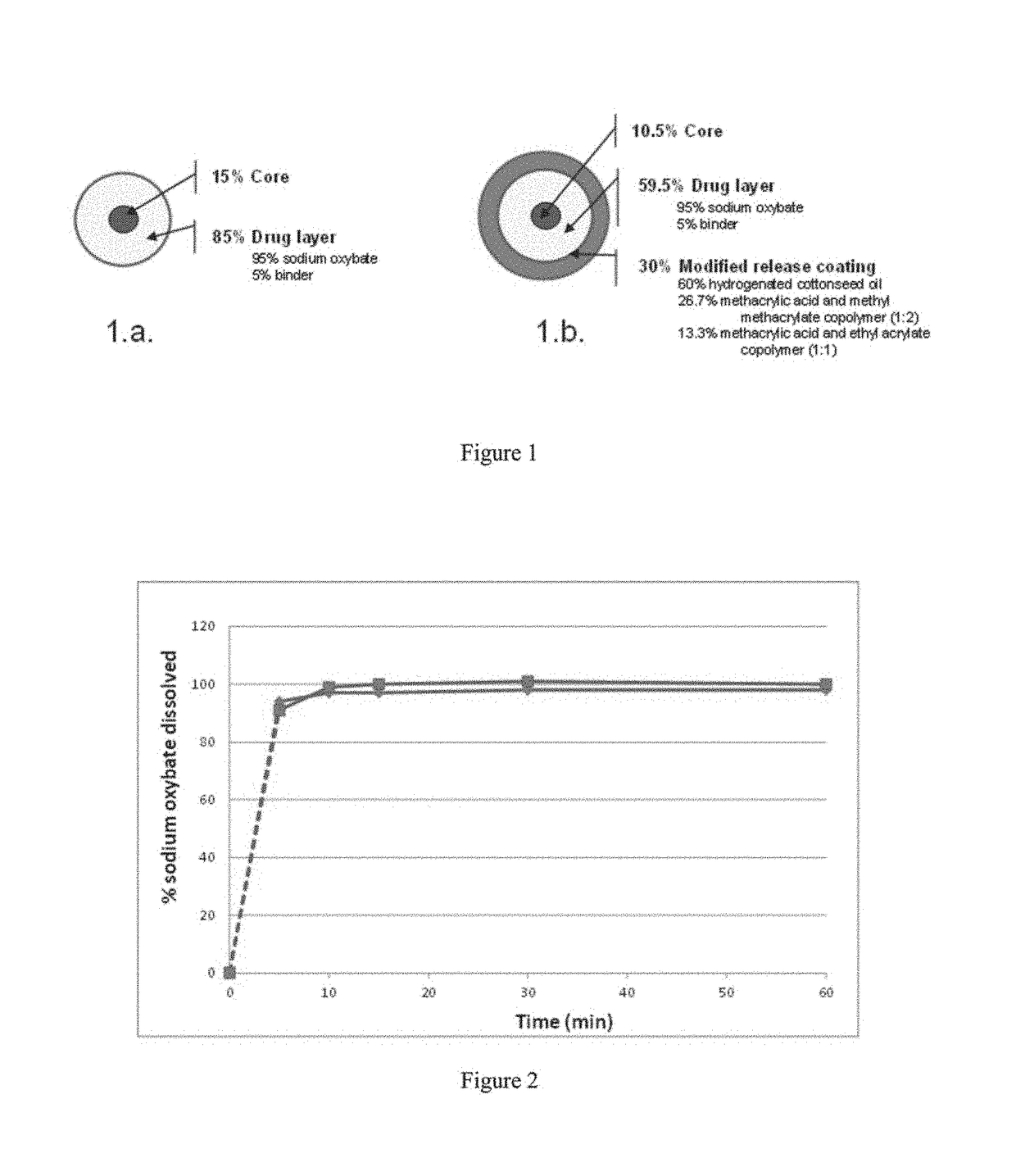
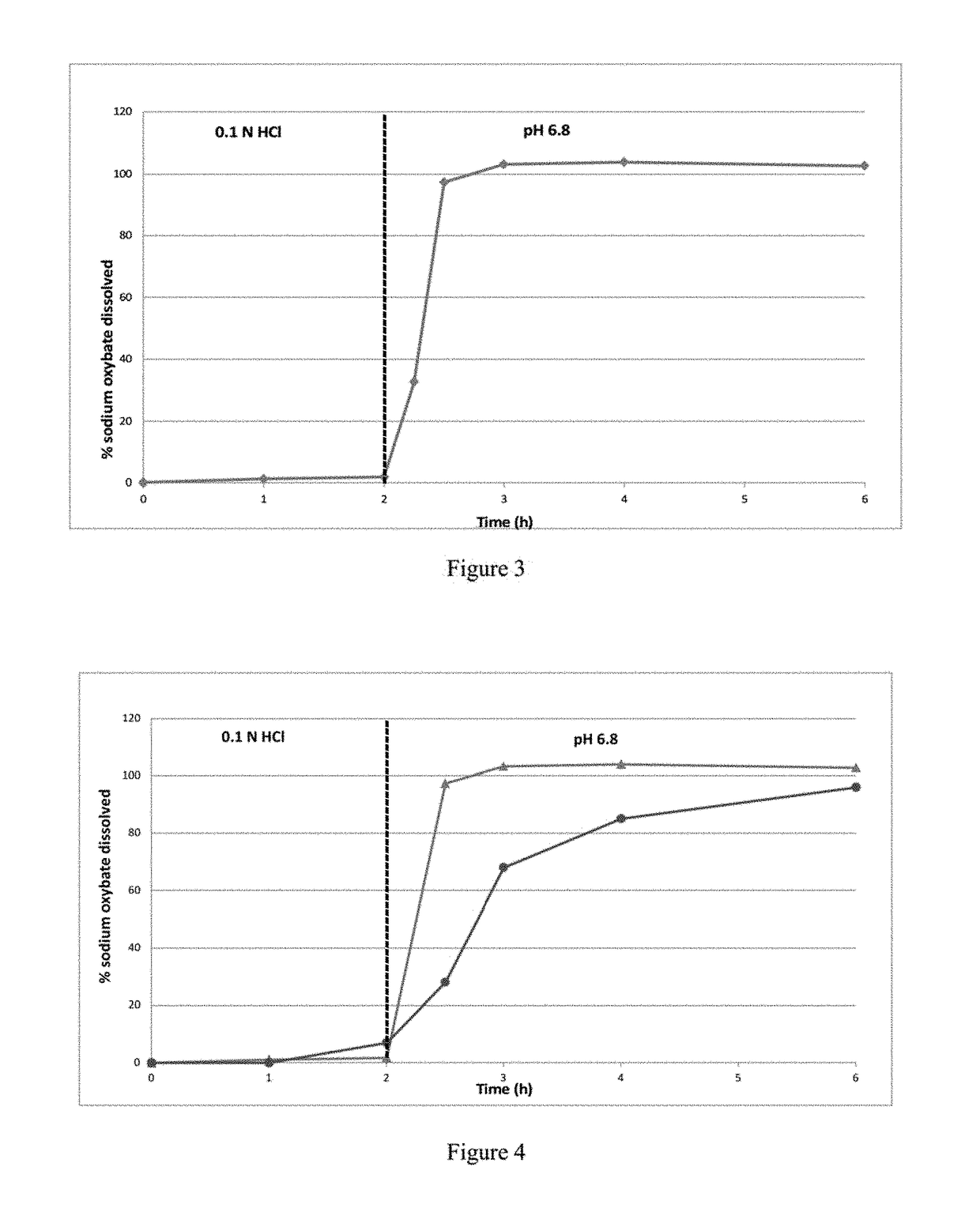
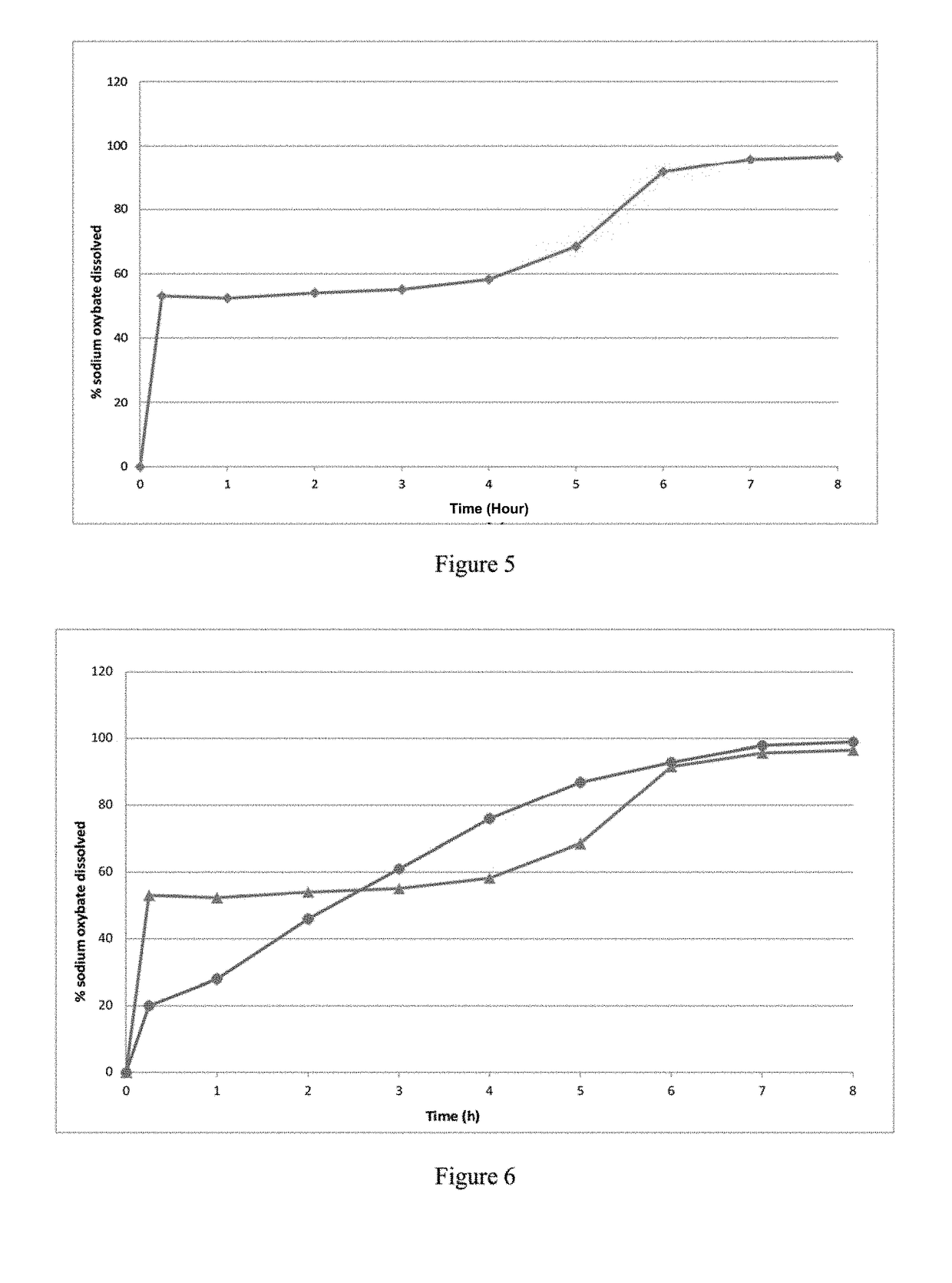
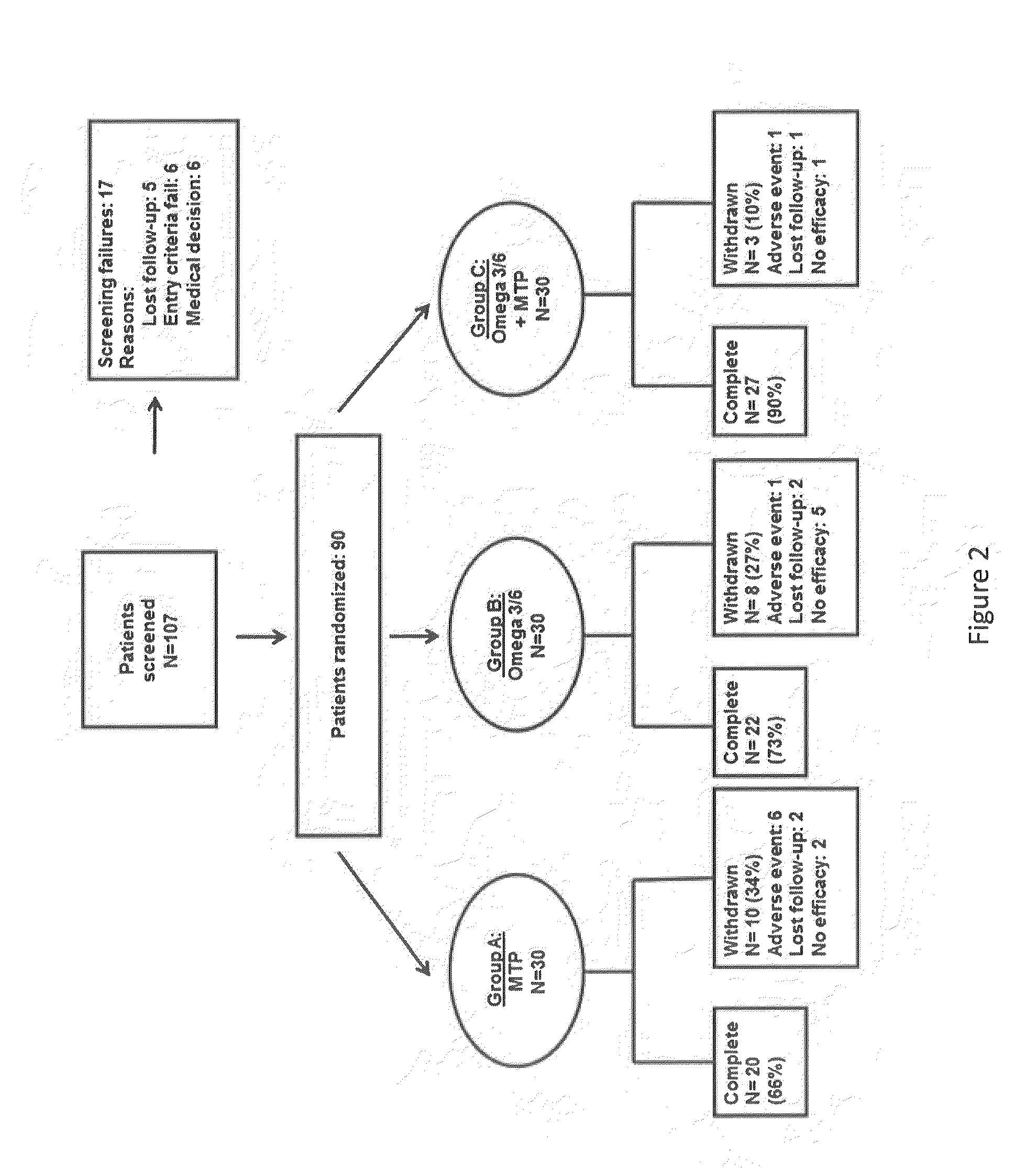
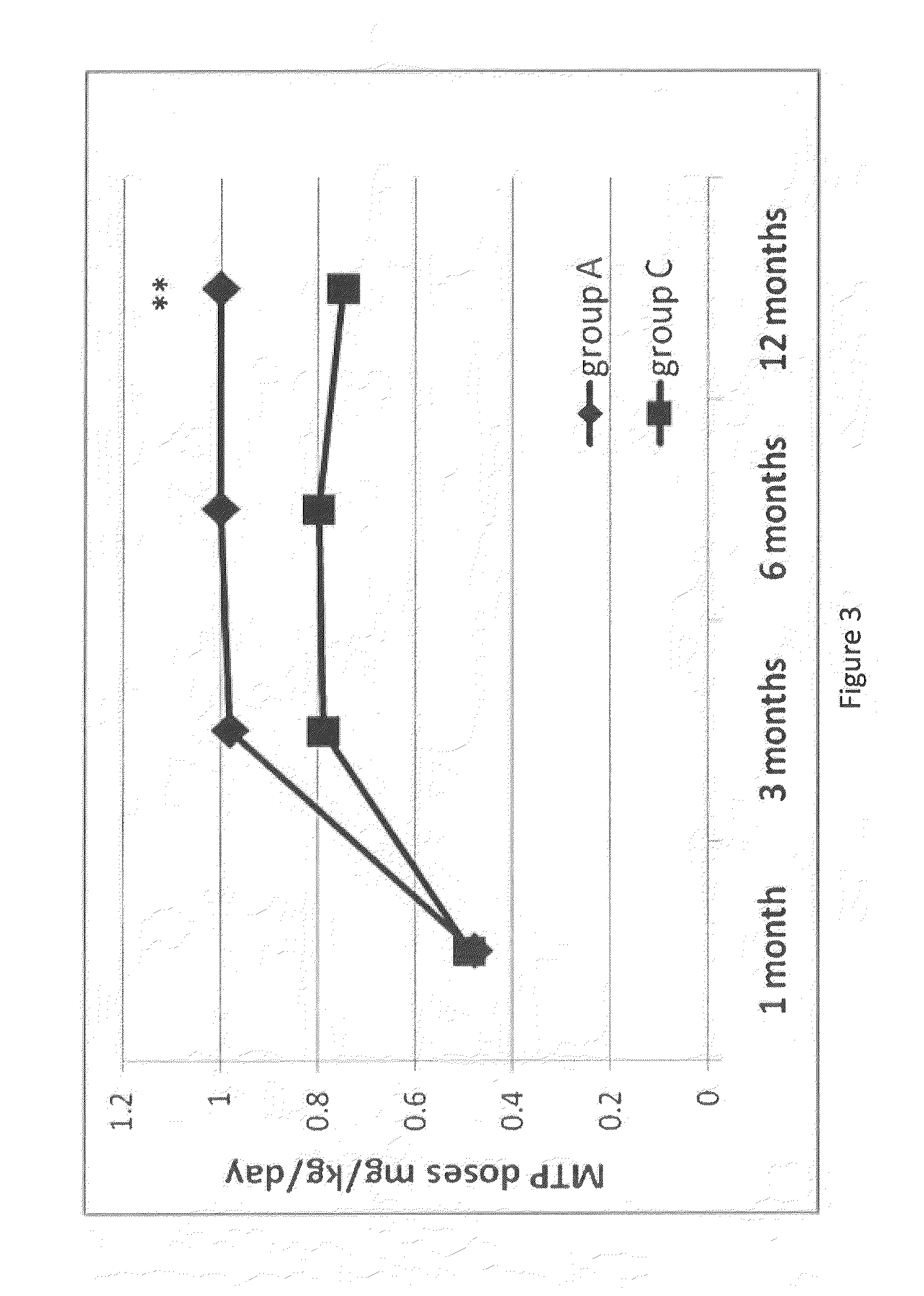
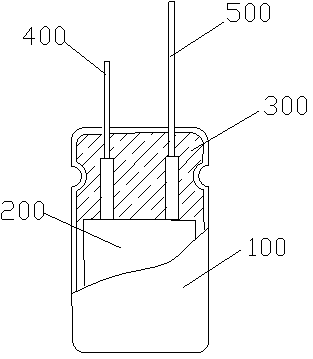
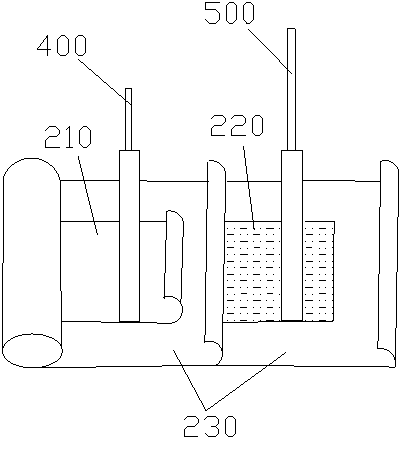
Patents
Literature
34 results about "Gamma hydroxybutyrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
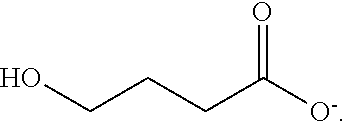
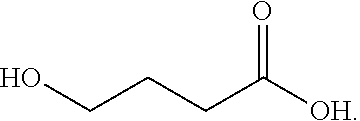
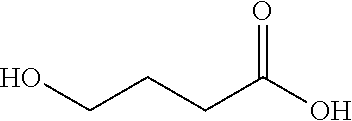
Hydroxy derivative of butyric acid substituted at the gamma, or 4 position; intermediate in the metabolism of gamma-aminobutyric acid (GABA).
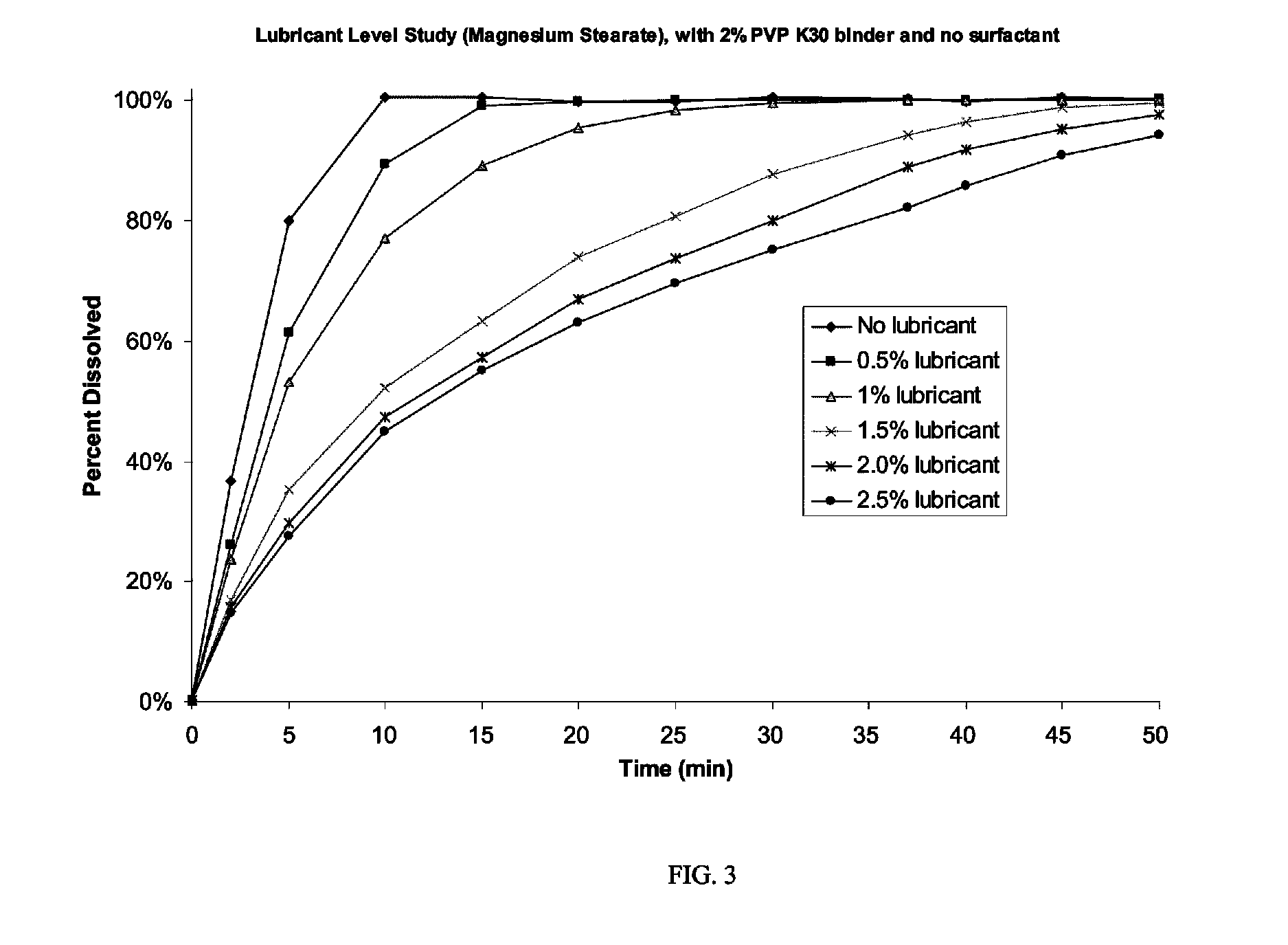
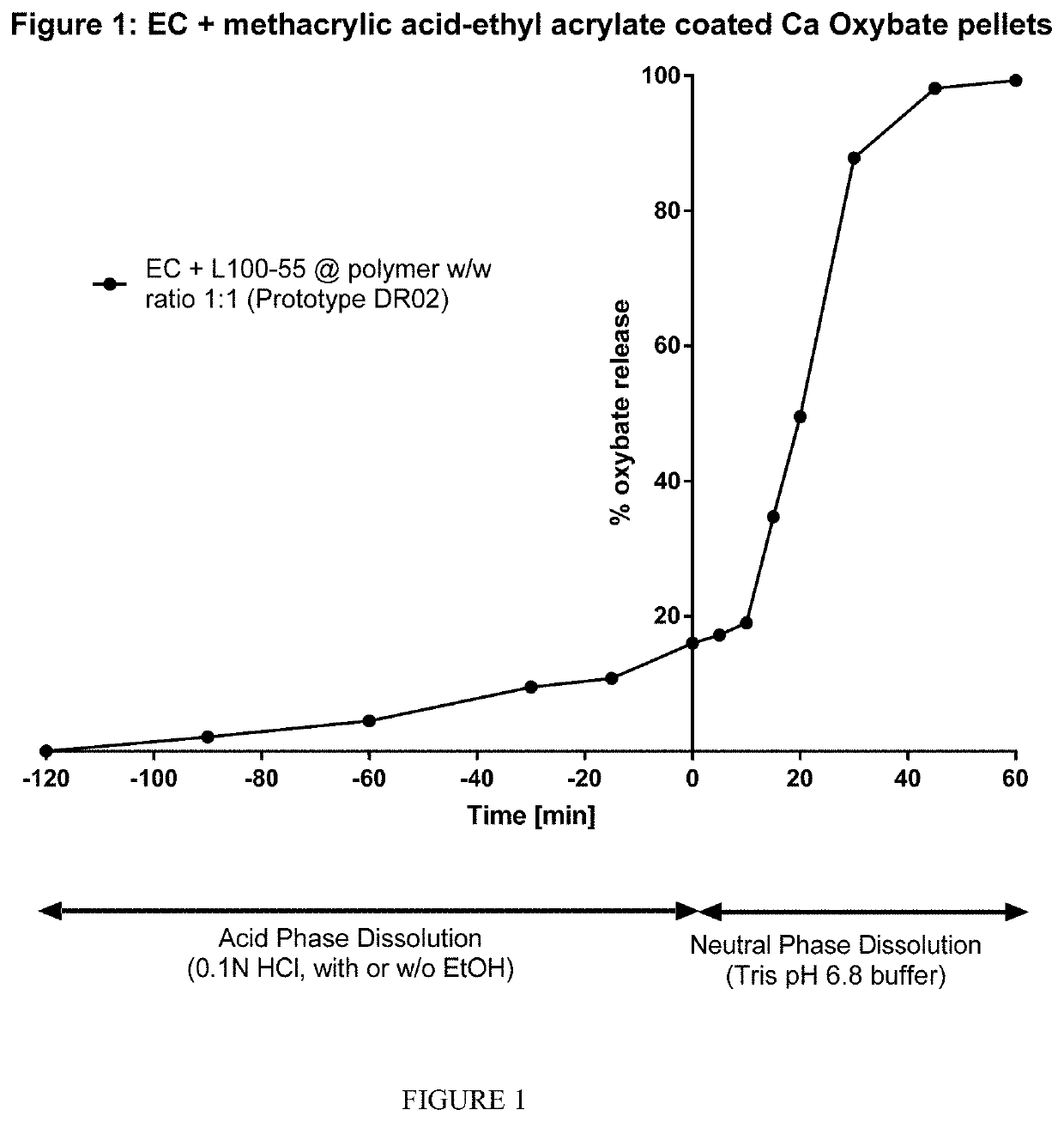
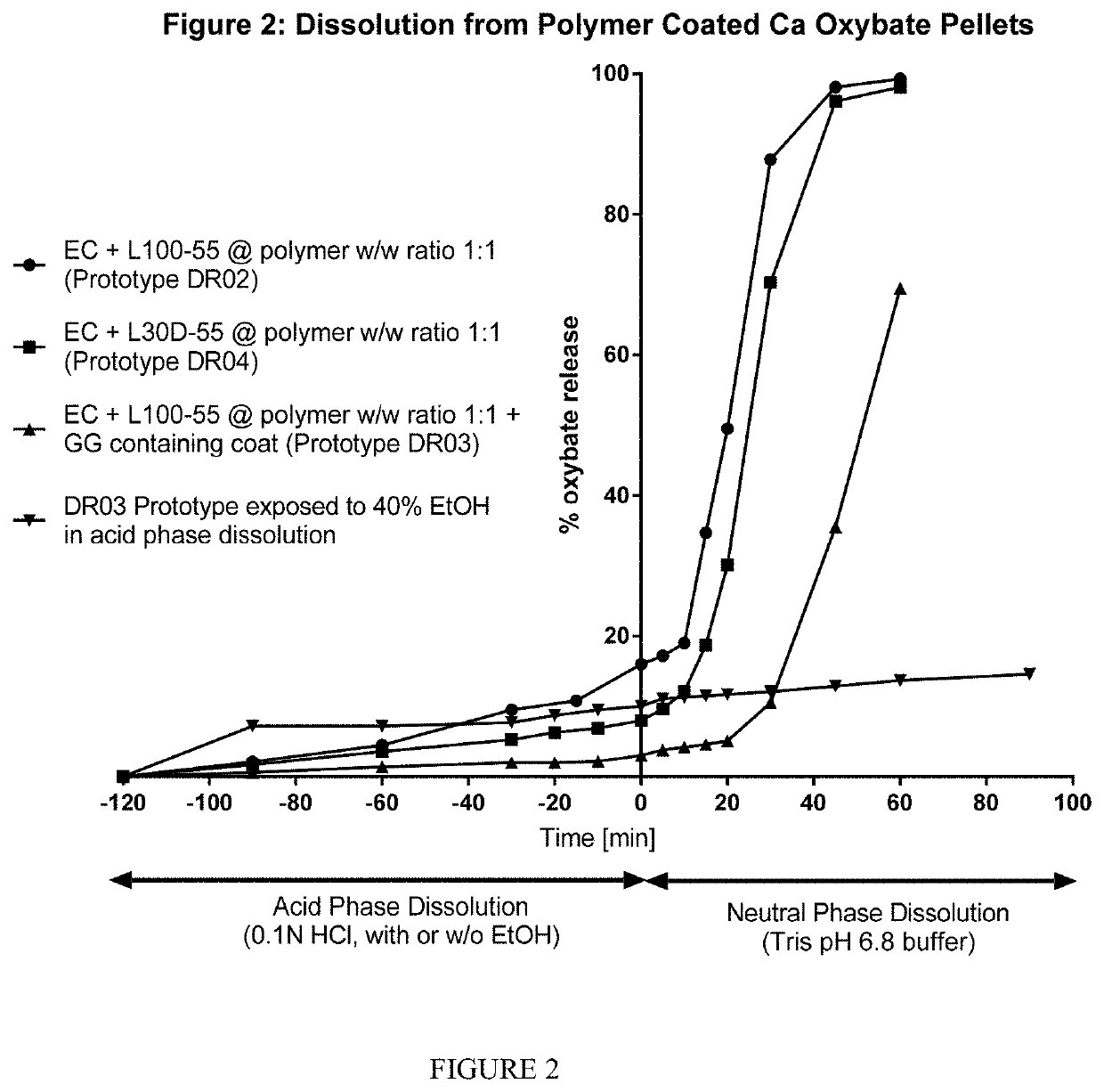
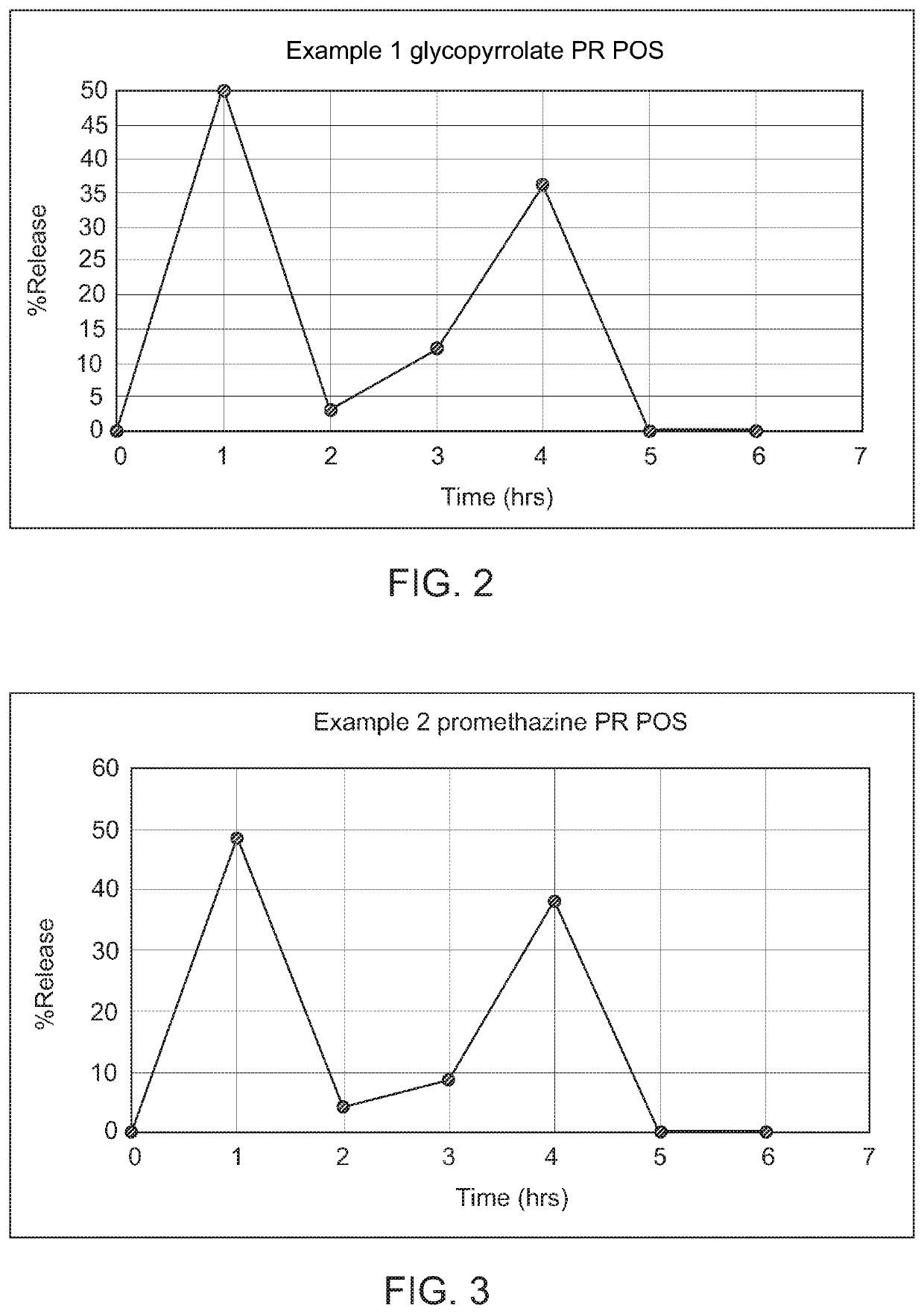
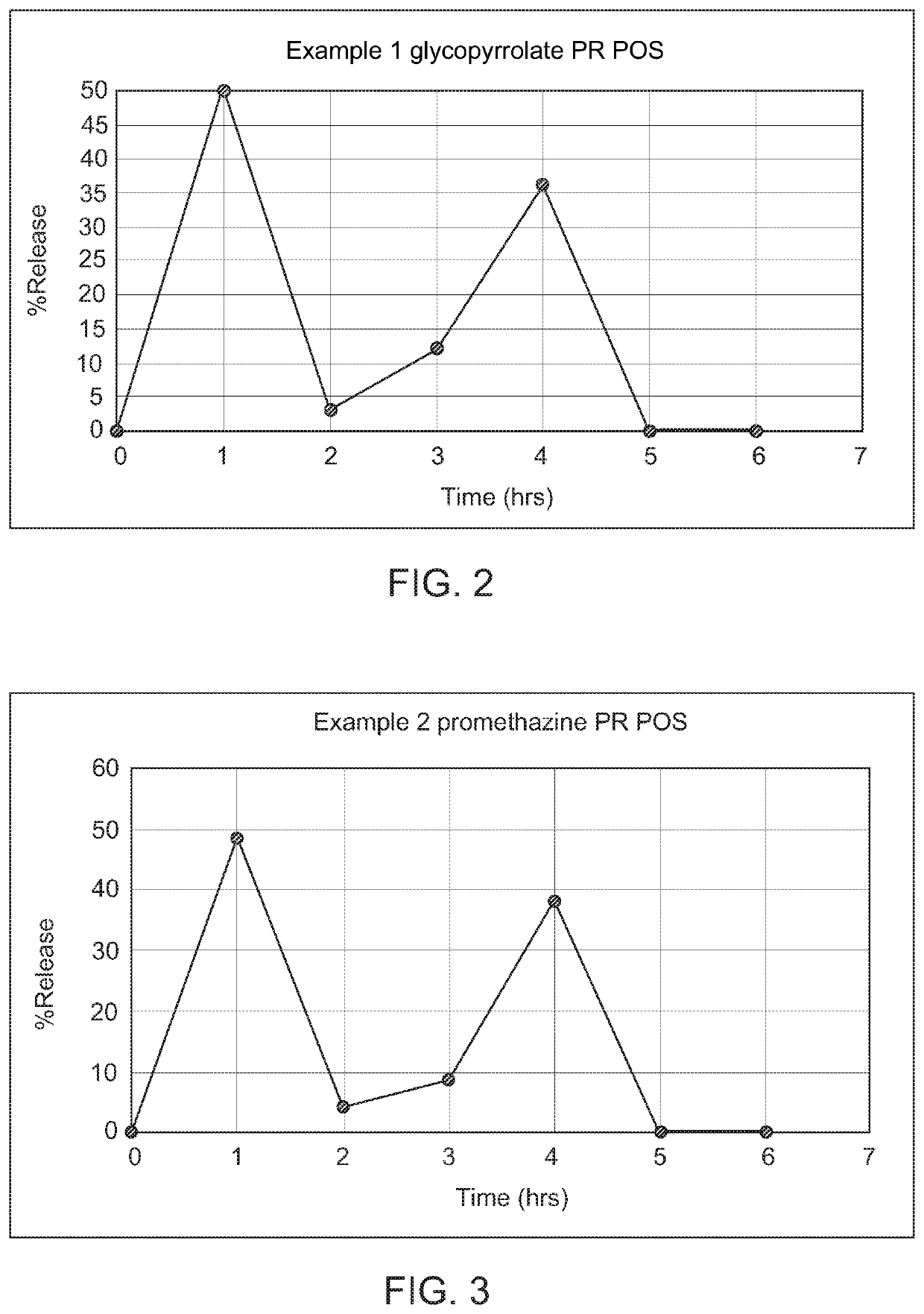
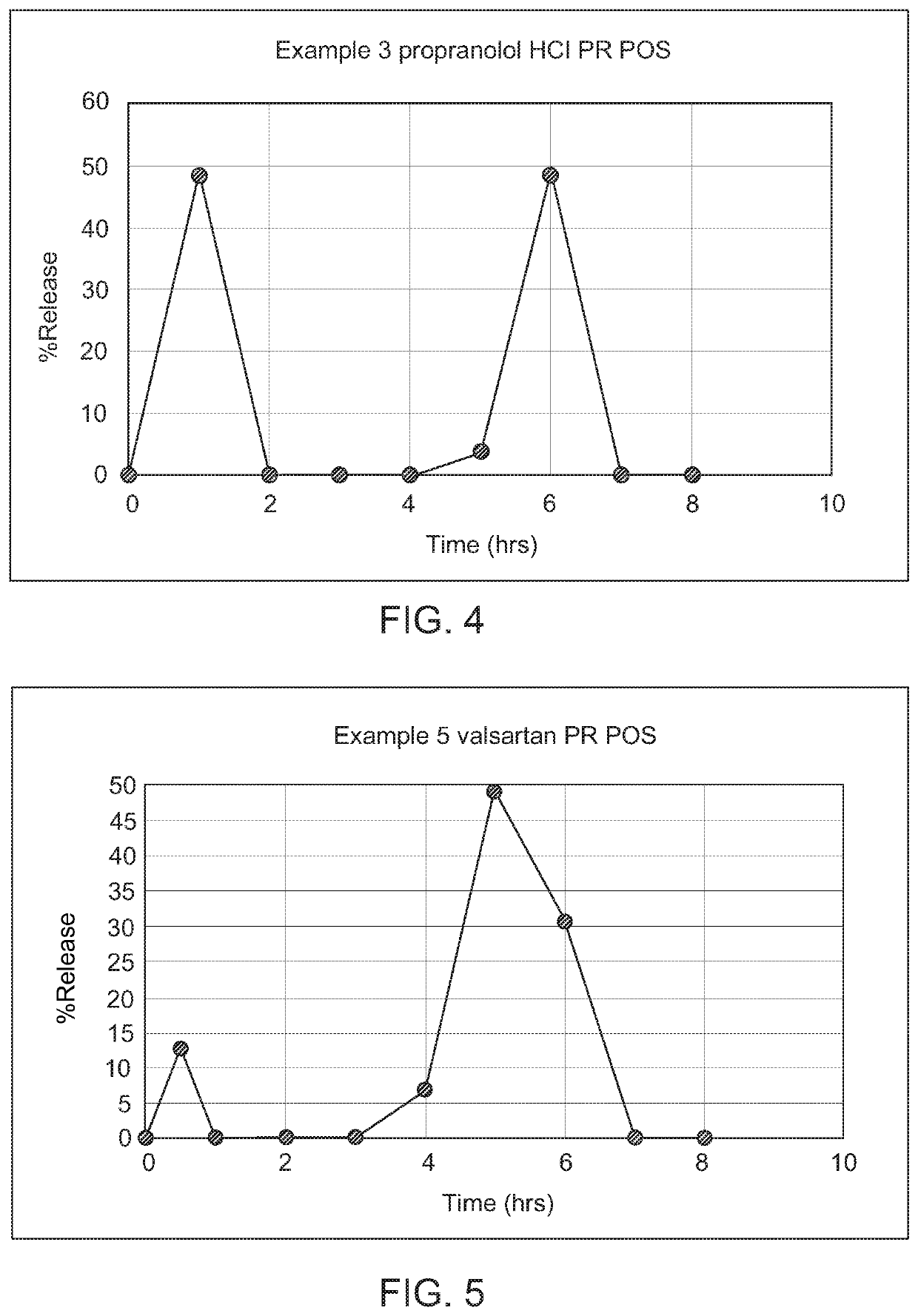
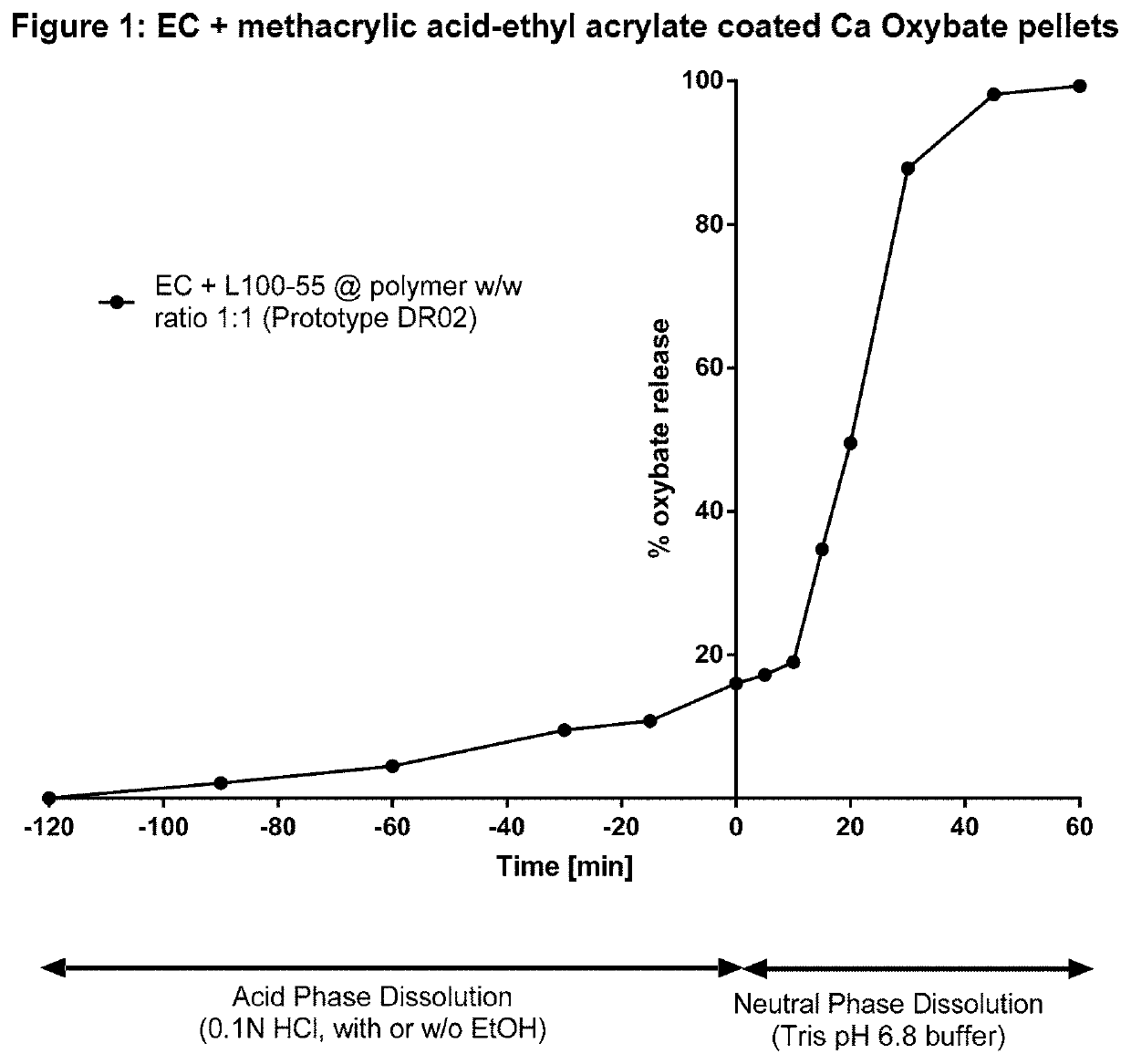
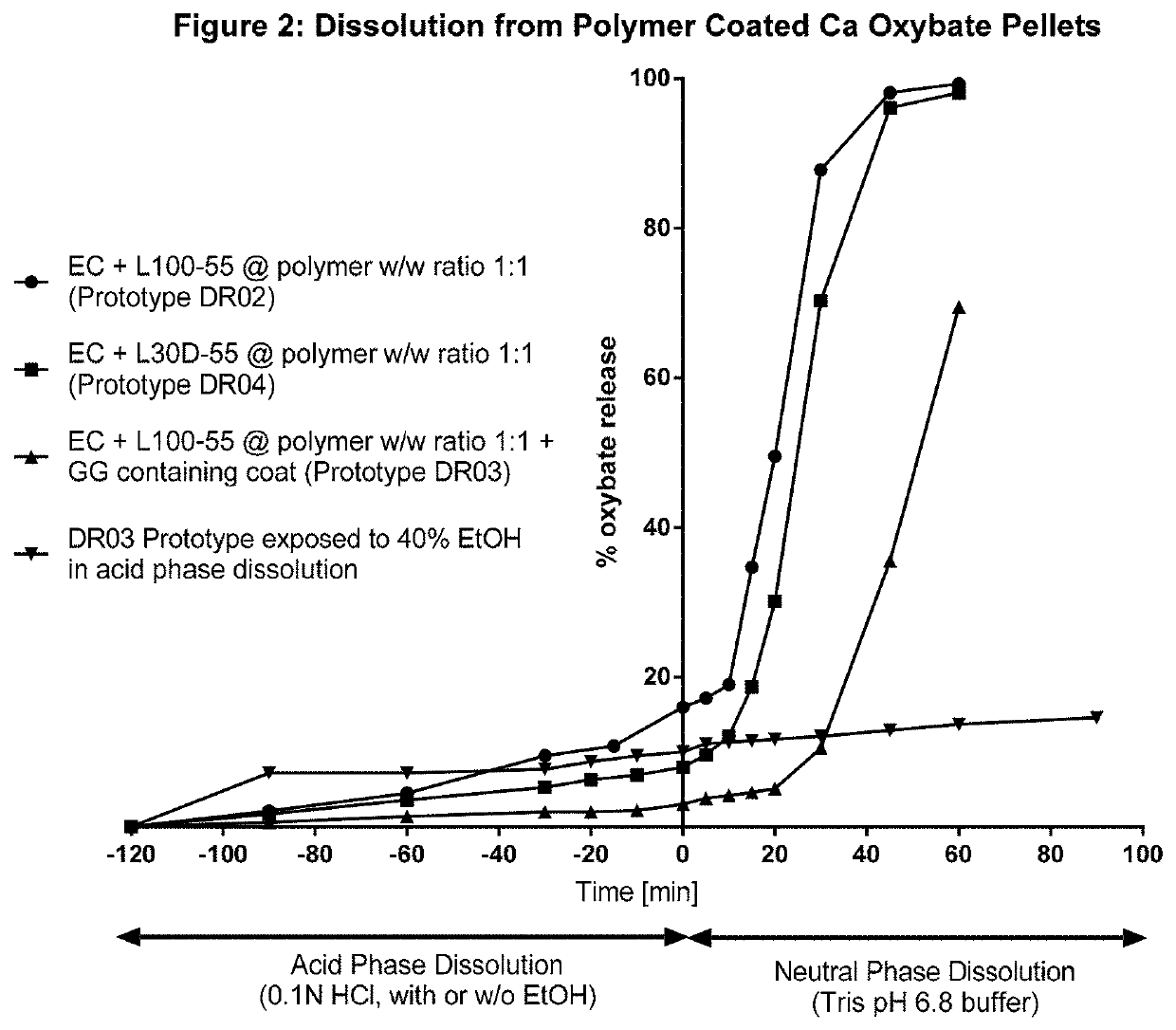
Controlled release compositions of gamma-hydroxybutyrate
ActiveUS20060210630A1Improve gastro-stabilityImprove efficiencyBiocideNervous disorderControlled releaseImmediate release
The present invention is directed to oral pulse-release pharmaceutical dosage form containing an immediate release component of gamma-hydroxybutyric acid, and one or more delayed / controlled release components of gamma-hydroxybutyric acid.
Owner:SUPERNUS PHARM INC
Microbiologically sound and stable solutions of gamma-hydroxybutyrate salt for the treatment of narcolepsy
InactiveUS7851506B2Lower Level RequirementsImprove the level ofBiocideNervous disorderAlcoholAqueous medium
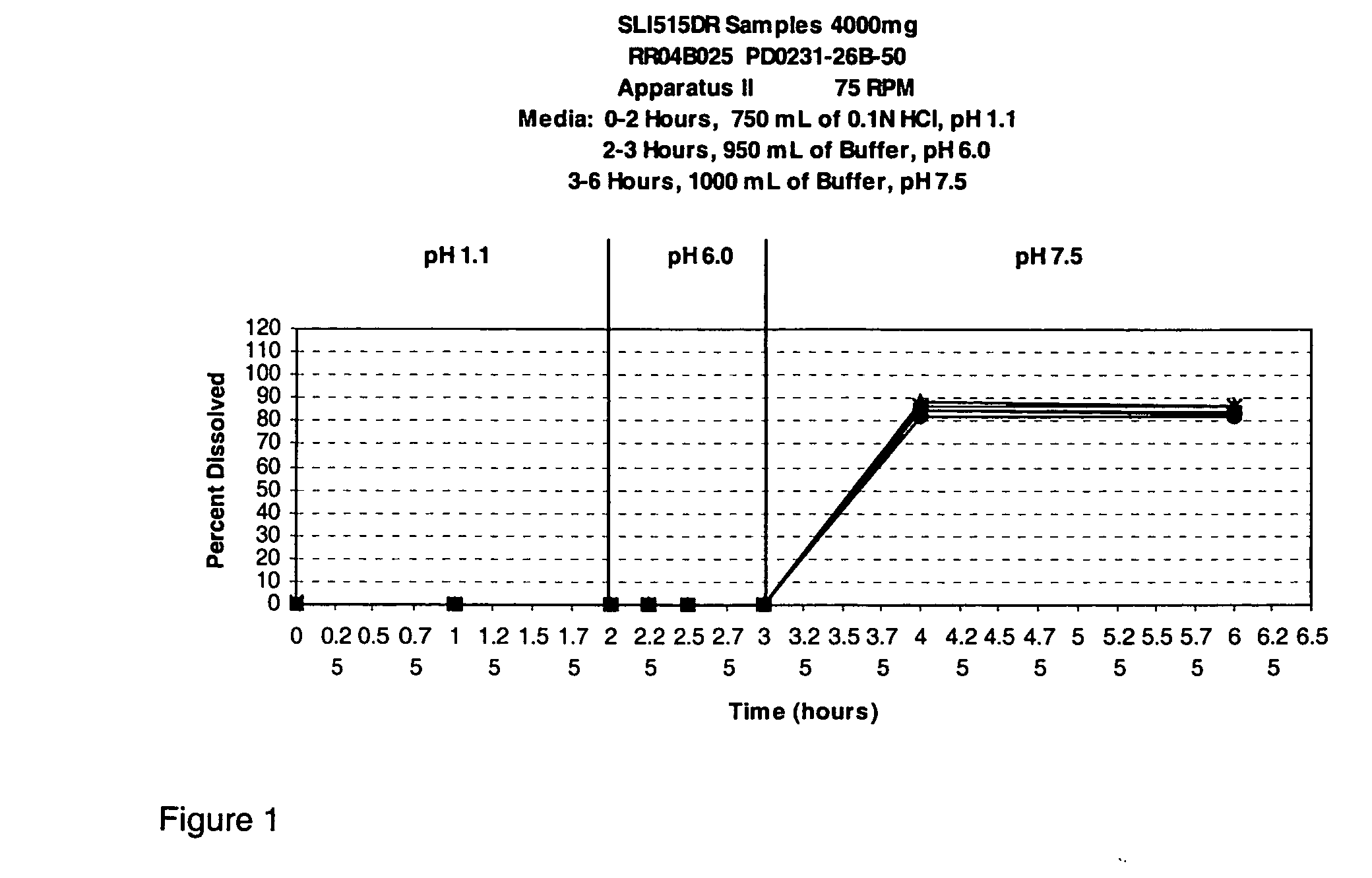
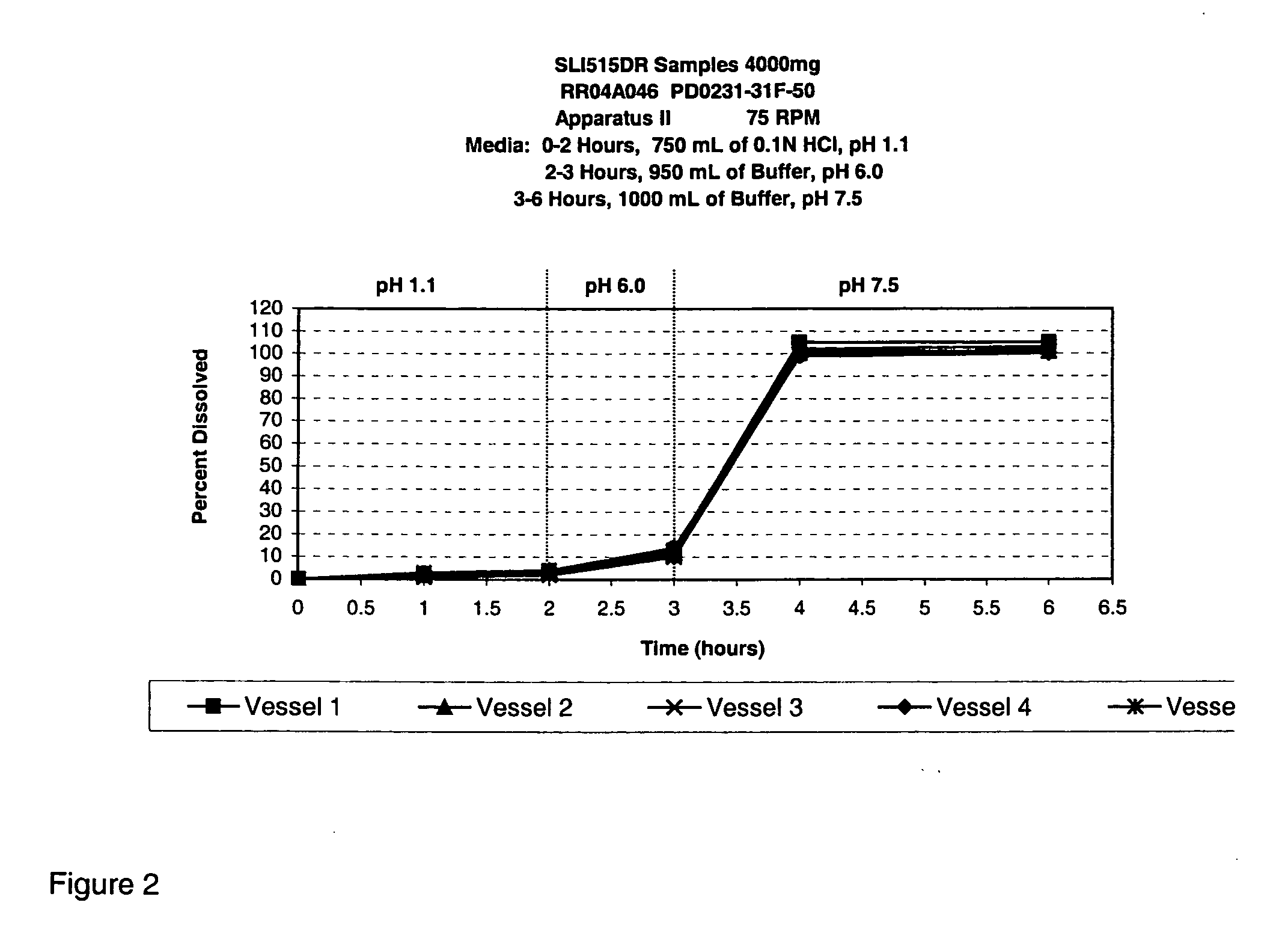
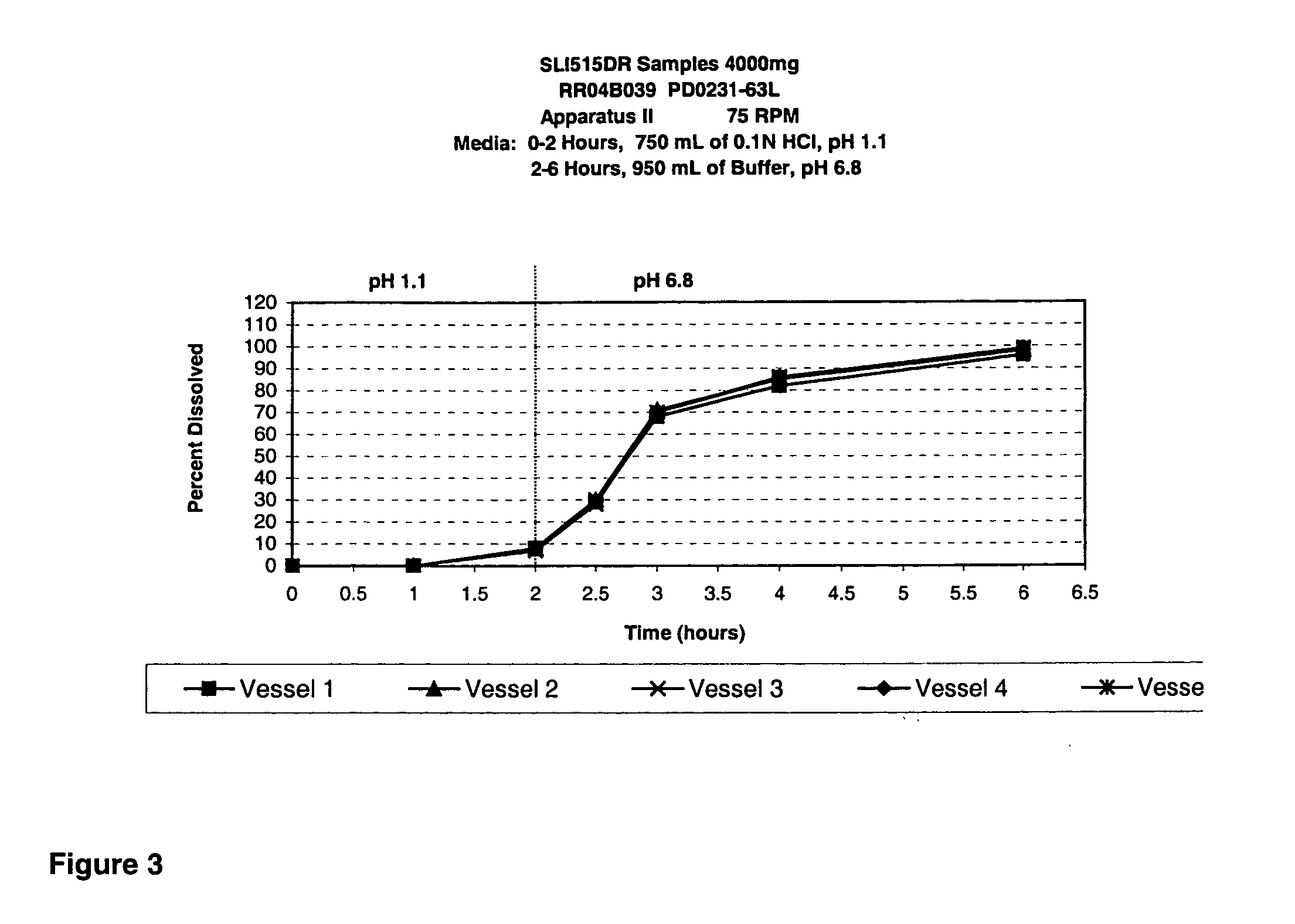
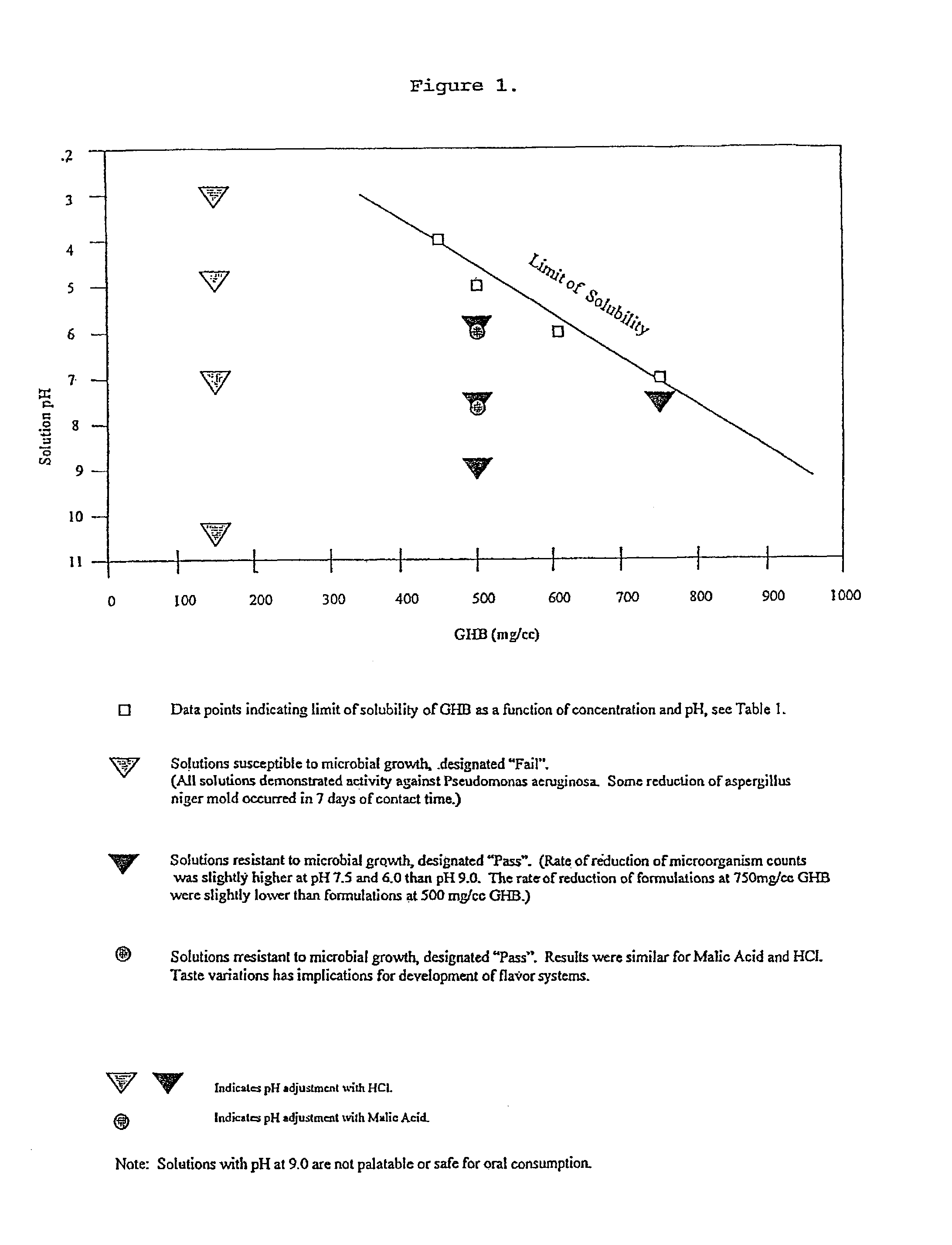
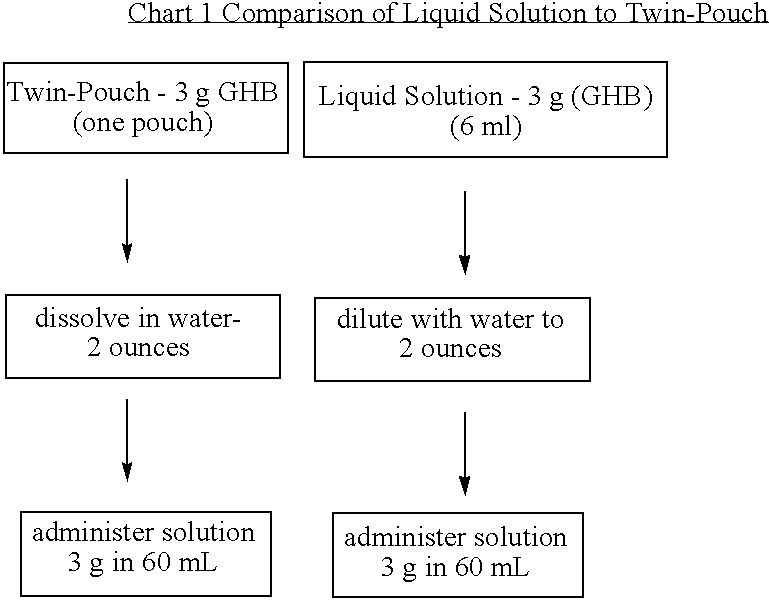
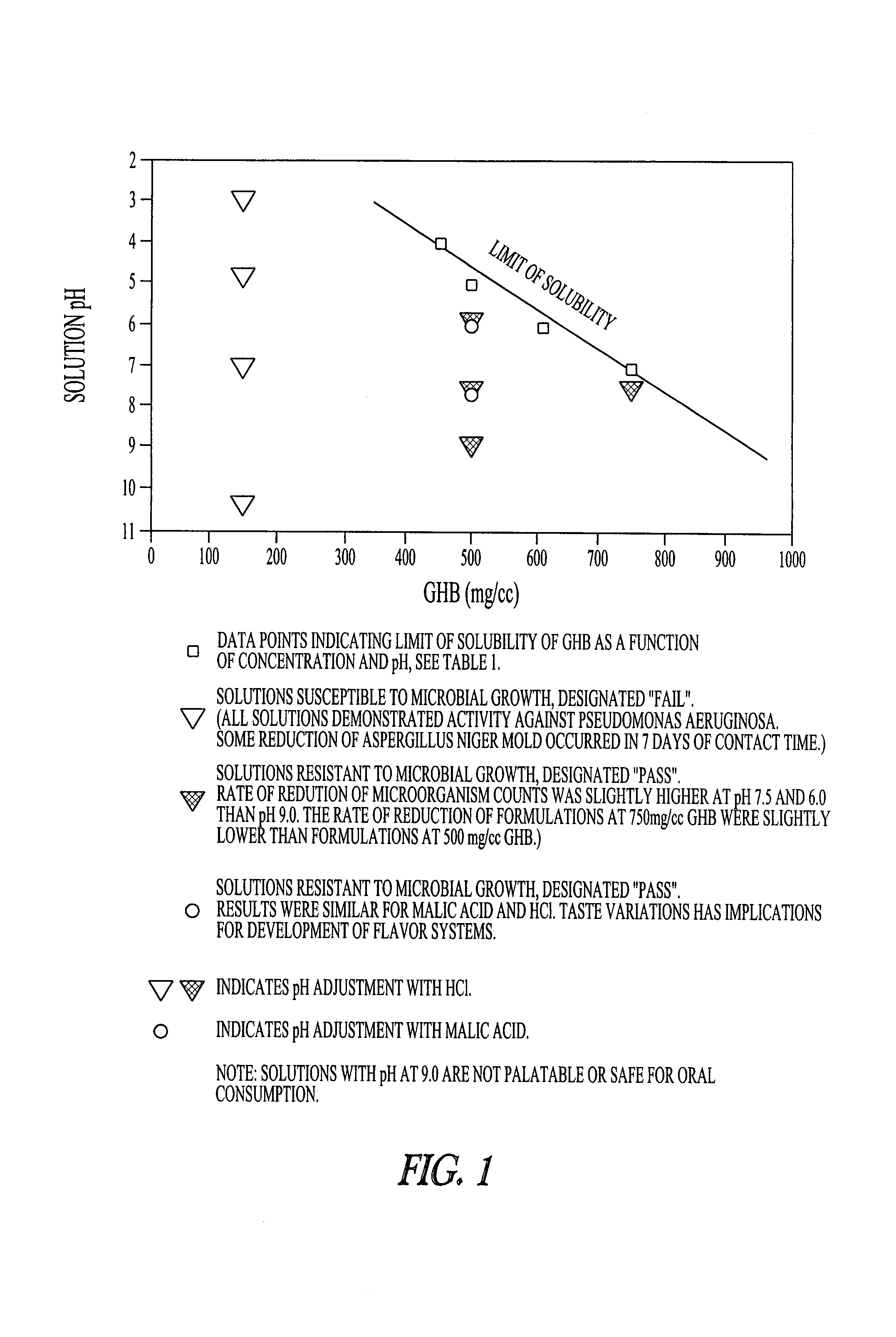
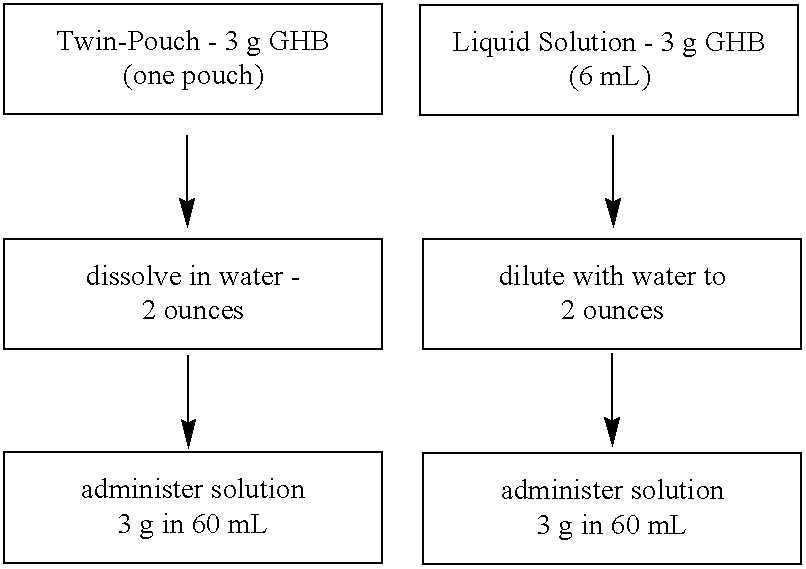
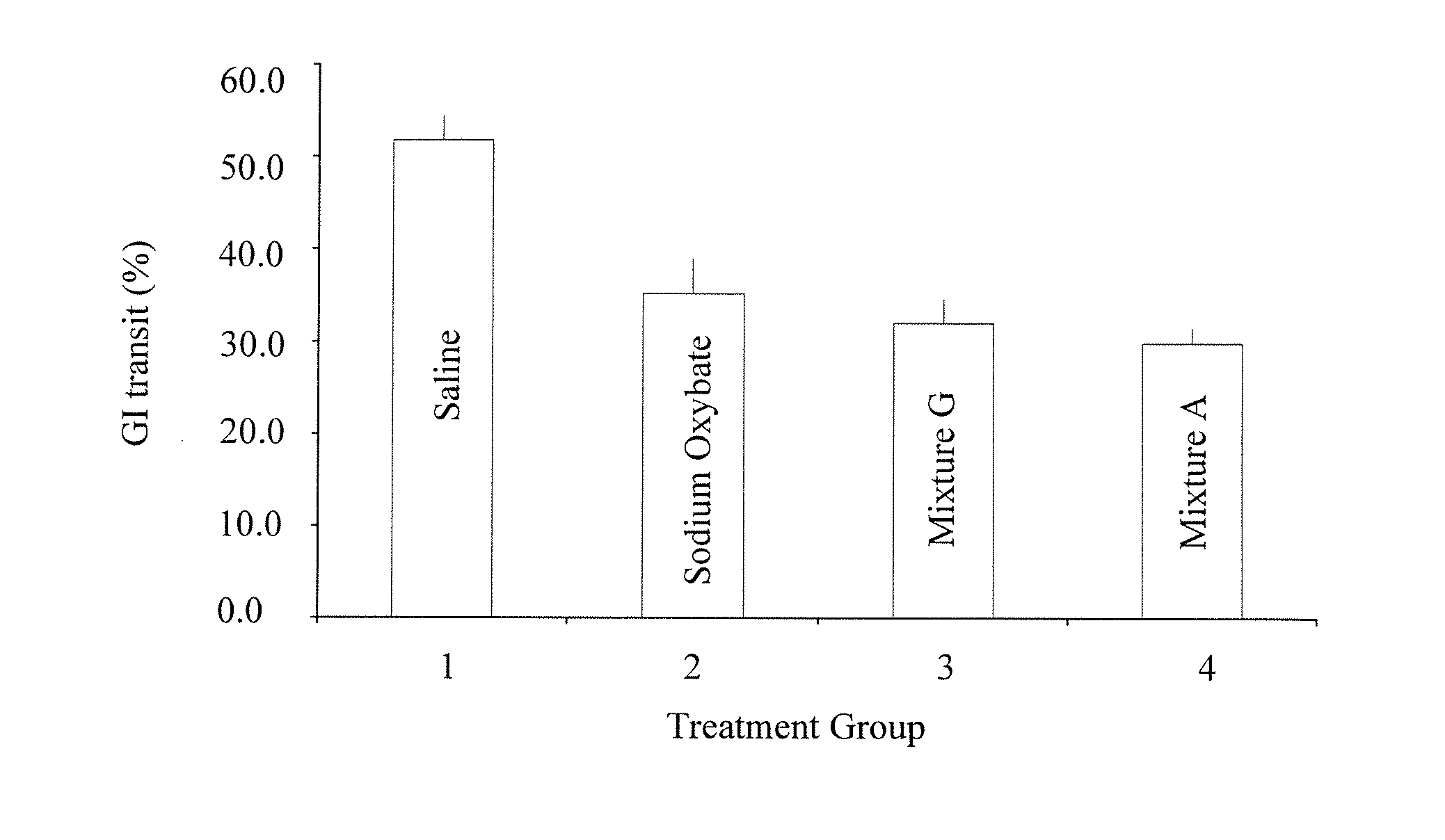
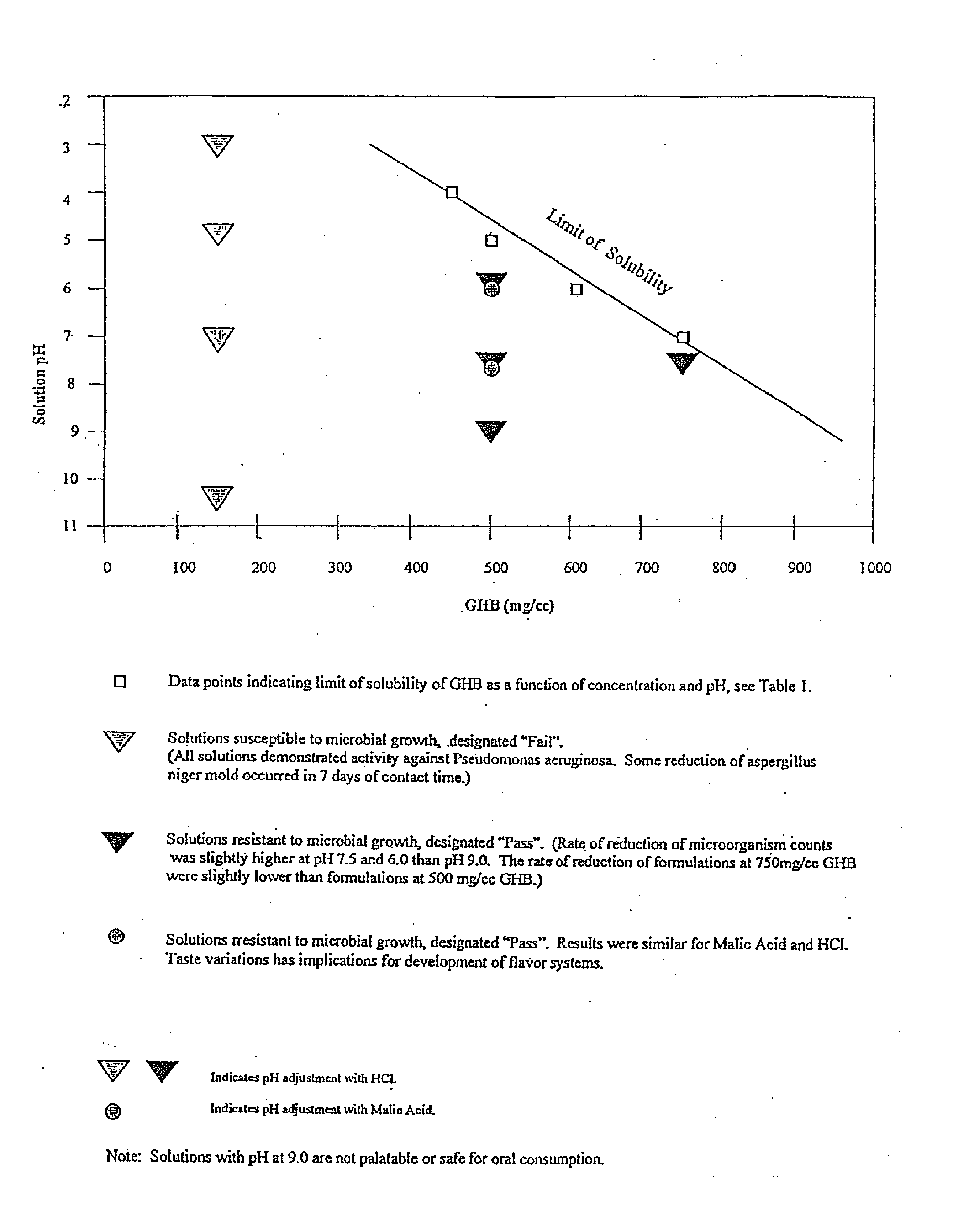
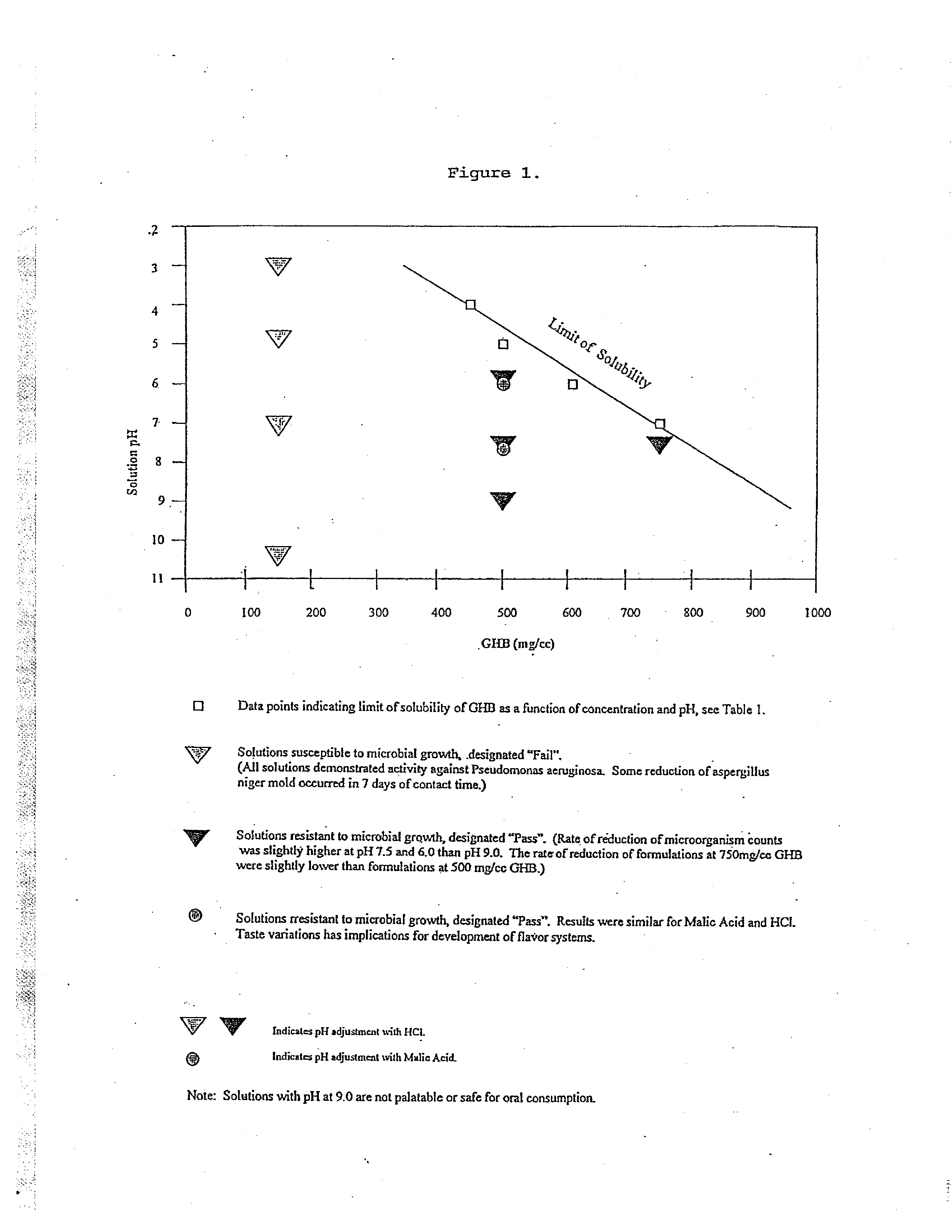
Disclosed are formulations of gamma-hydroxybutyrate in an aqueous medium that are resistant to microbial growth. Also disclosed are formulations of gamma-hydroxybutyrate that are also resistant to the conversion into GBL. Disclosed are methods to treat sleep disorders, including narcolepsy, with these stable formulations of GHB. The present invention also provides methods to treat alcohol and opiate withdrawal, reduced levels of growth hormone, increased intracranial pressure, and physical pain in a patient.
Owner:JAZZ PHARMA INC
Microbiologically sound and stable solutions of gamma-hydroxybutyrate salt for the treatment of narcolepsy
InactiveUS7262219B2Lower Level RequirementsImprove the level ofBiocideNervous disorderAlcoholAqueous medium
Disclosed are formulations of gamma-hydroxybutyrate in an aqueous medium that are resistant to microbial growth. Also disclosed are formulations of gamma-hydroxybutyrate that are also resistant to the conversion into GBL. Disclosed are methods to treat sleep disorders, including narcolepsy, with these stable formulations of GHB. The present invention also provides methods to treat alcohol and opiate withdrawal, reduced levels of growth hormone, increased intracranial pressure, and physical pain in a patient.
Owner:JAZZ PHARMA
Gamma-hydroxybutyrate compositions and their use for the treatment of disorders
Provided herein are pharmaceutical compositions and formulations comprising mixed salts of gamma-hydroxybutyrate (GHB). Also provided herein are methods of making the pharmaceutical compositions and formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Microbiologically sound and stable solutions of gamma-hydroxybutyrate salt for the treatment of narcolepsy
InactiveUS20070270491A1Lower Level RequirementsImprove the level ofBiocideNervous disorderOpioid withdrawalNarcolepsy
Disclosed are formulations of gamma-hydroxybutyrate in an aqueous medium that are resistant to microbial growth. Also disclosed are formulations of gamma-hydroxybutyrate that are also resistant to the conversion into GBL. Disclosed are methods to treat sleep disorders, including narcolepsy, with these stable formulations of GHB. The present invention also provides methods to treat alcohol and opiate withdrawal, reduced levels of growth hormone, increased intracranial pressure, and physical pain in a patient.
Owner:JAZZ PHARMA INC
Immediate release formulations and dosage forms of gamma-hydroxybutyrate
The present invention provides a solid immediate release dosage form adapted for oral administration of GHB. The solid immediate release dosage form includes an immediate release formulation comprising a relatively high weight-percentage of GHB with a bioavailability similar to that of a liquid GHB dosage form.
Owner:JAZZ PHARMA INC
Controlled release compositions of gamma-hydroxybutyrate
ActiveUS8193211B2Reduce in quantityReduce the possibilityBiocideNervous disorderControl releaseImmediate release
The present invention is directed to oral pulse-release pharmaceutical dosage form containing an immediate release component of gamma-hydroxybutyric acid, and one or more delayed / controlled release components of gamma-hydroxybutyric acid.
Owner:SUPERNUS PHARM INC
Gamma-hydroxybutyrate compositions containing carbohydrate, lipid or amino acid carriers
Owner:JAZZ PHARMA INC
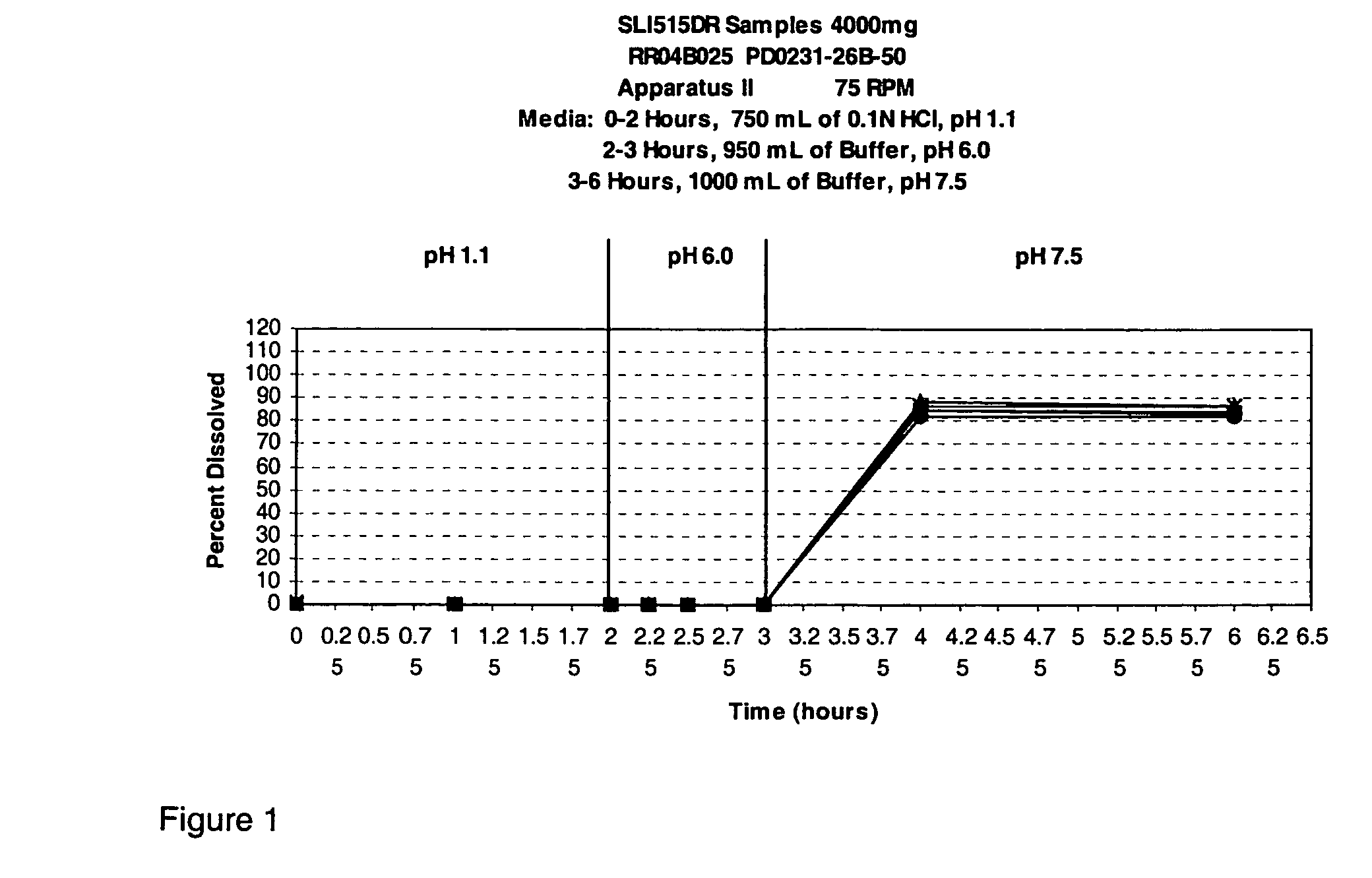
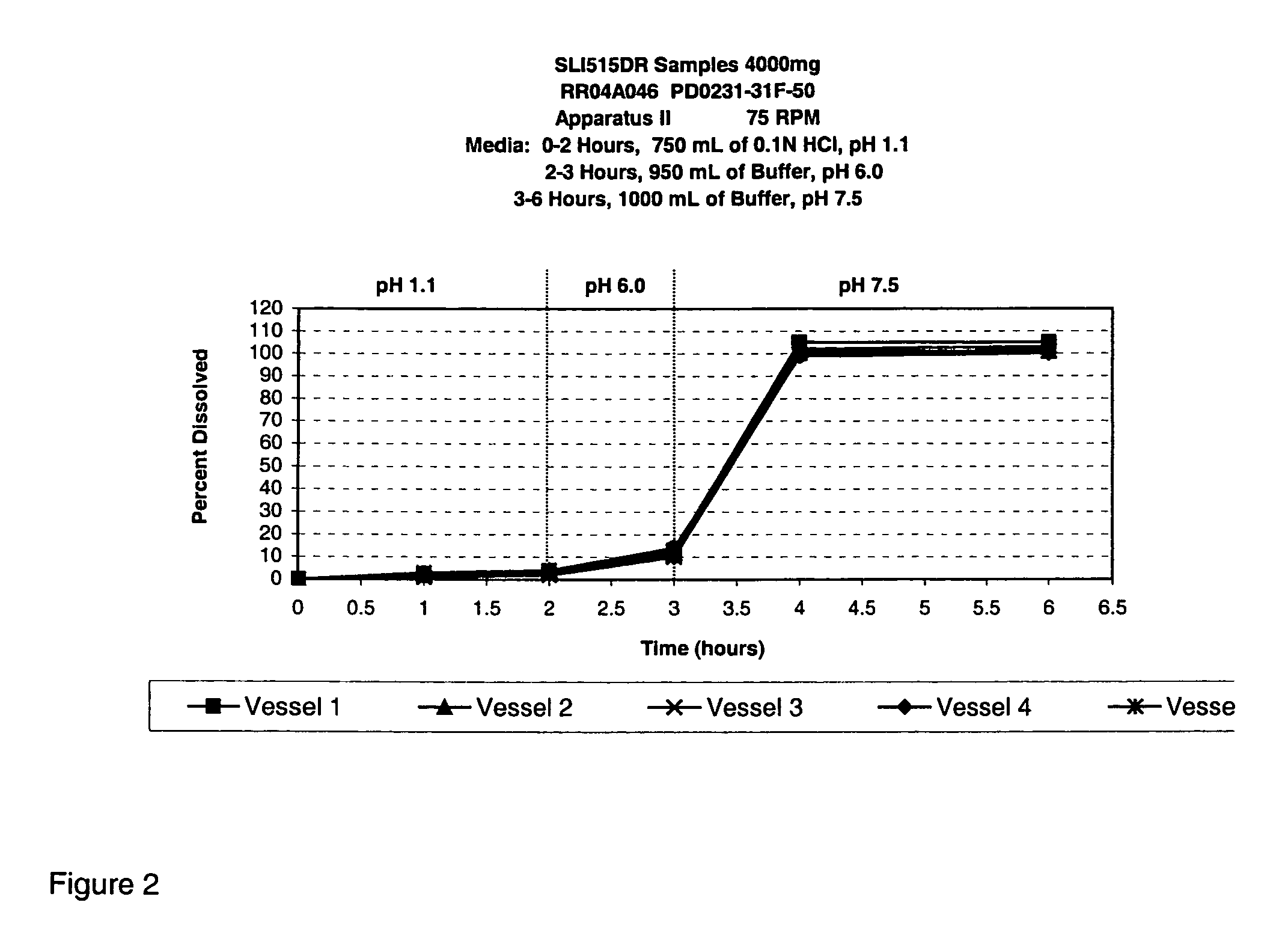
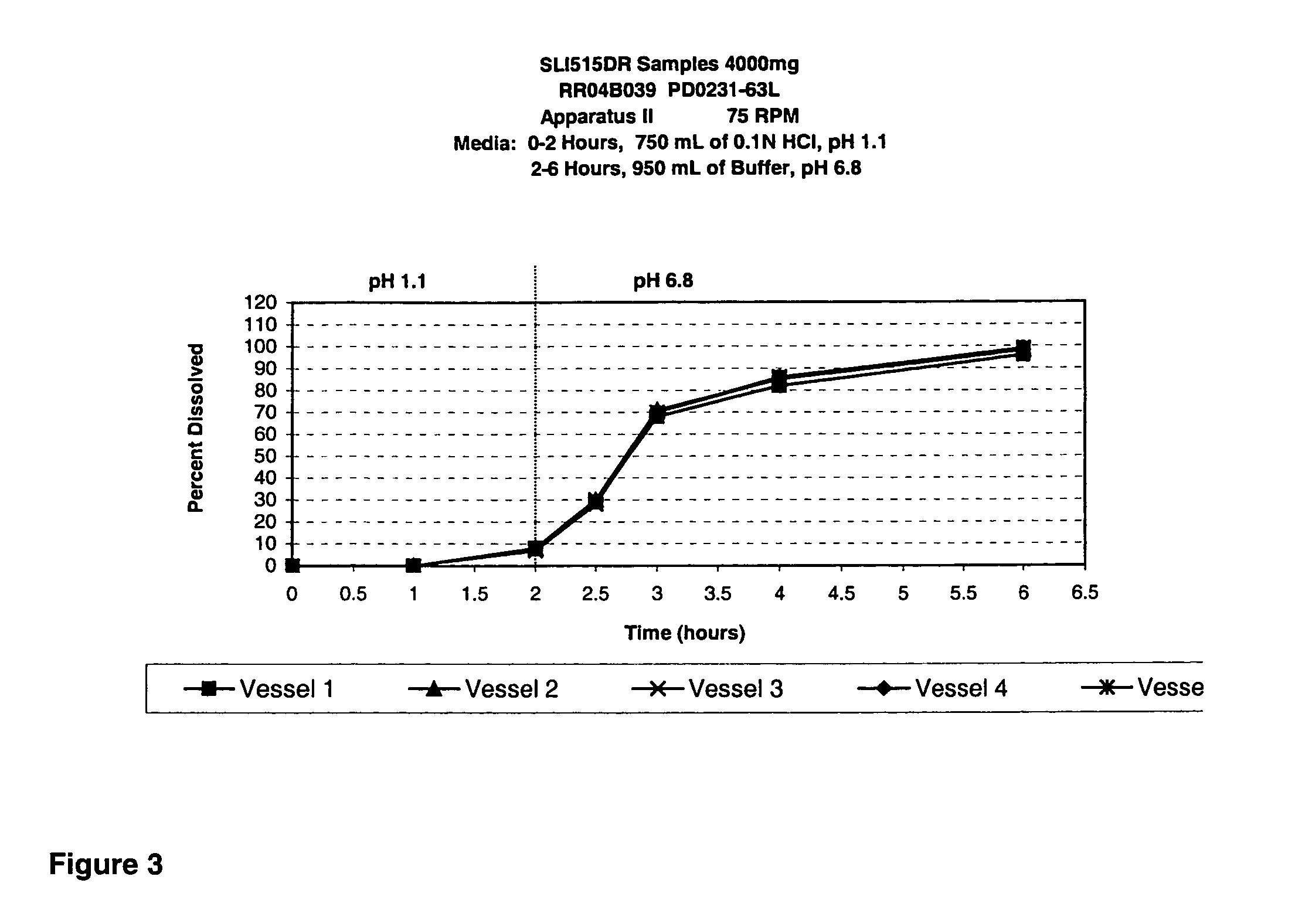
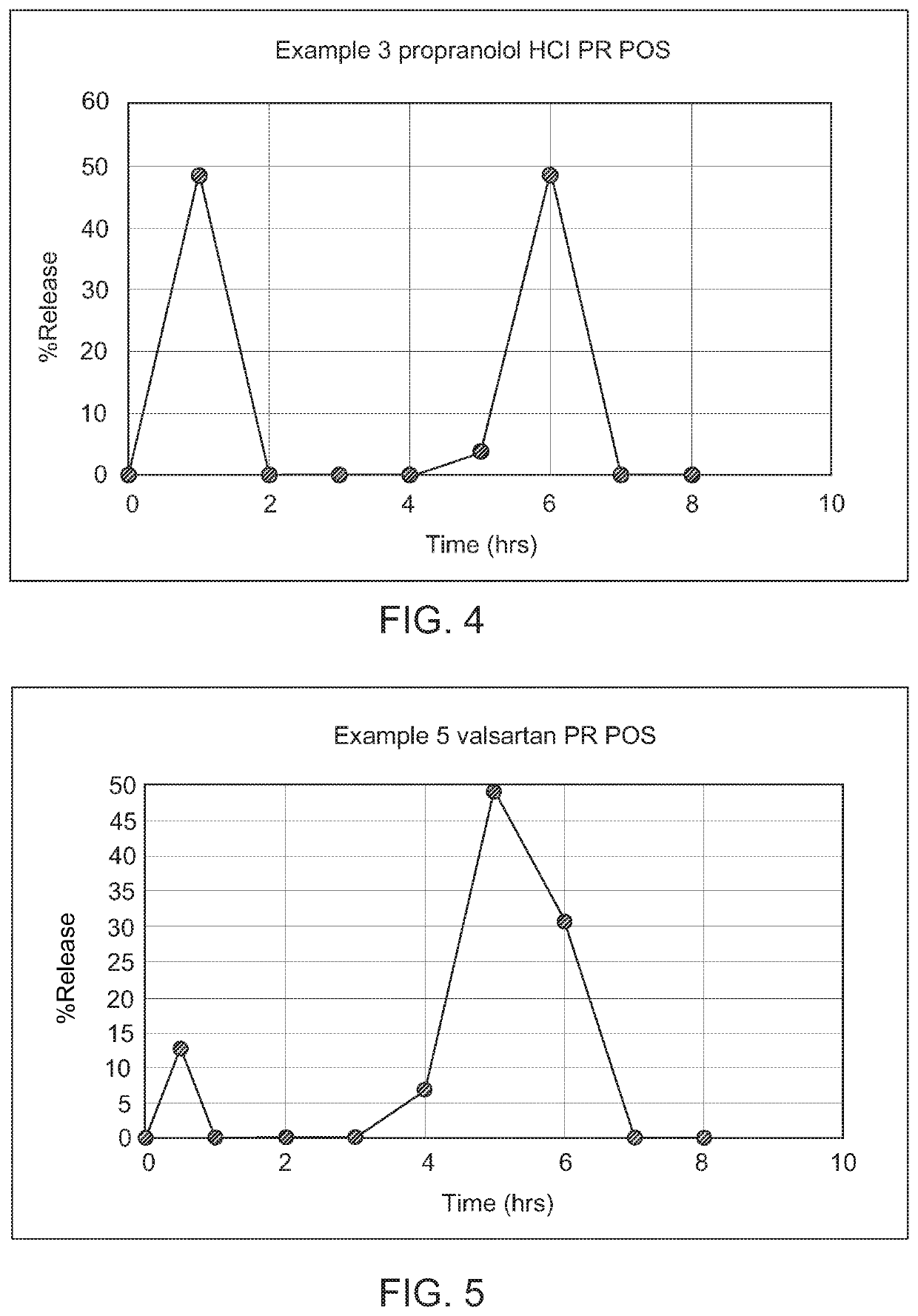
Modified release gamma-hydroxybutyrate formulations having improved pharmacokinetics
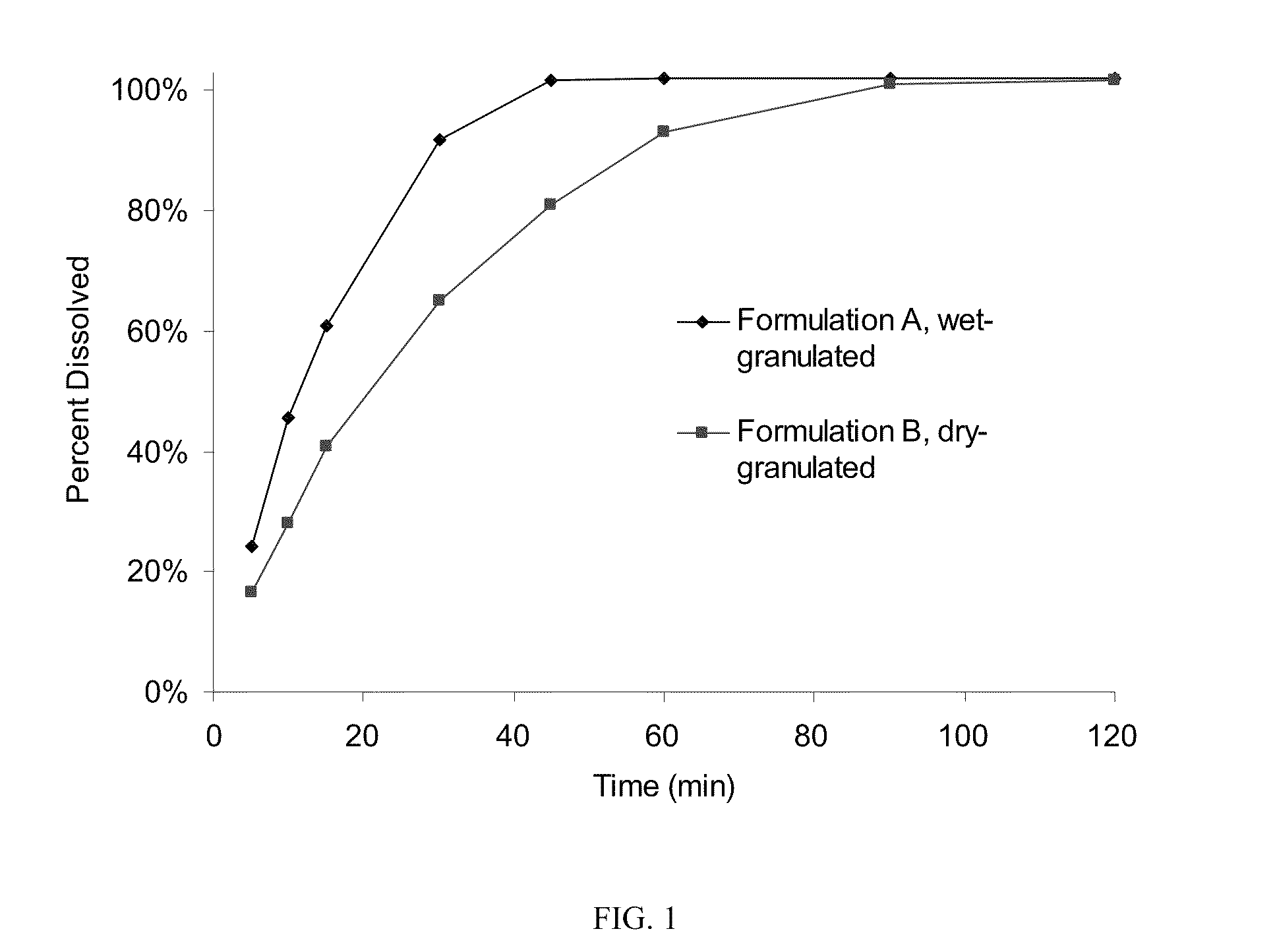
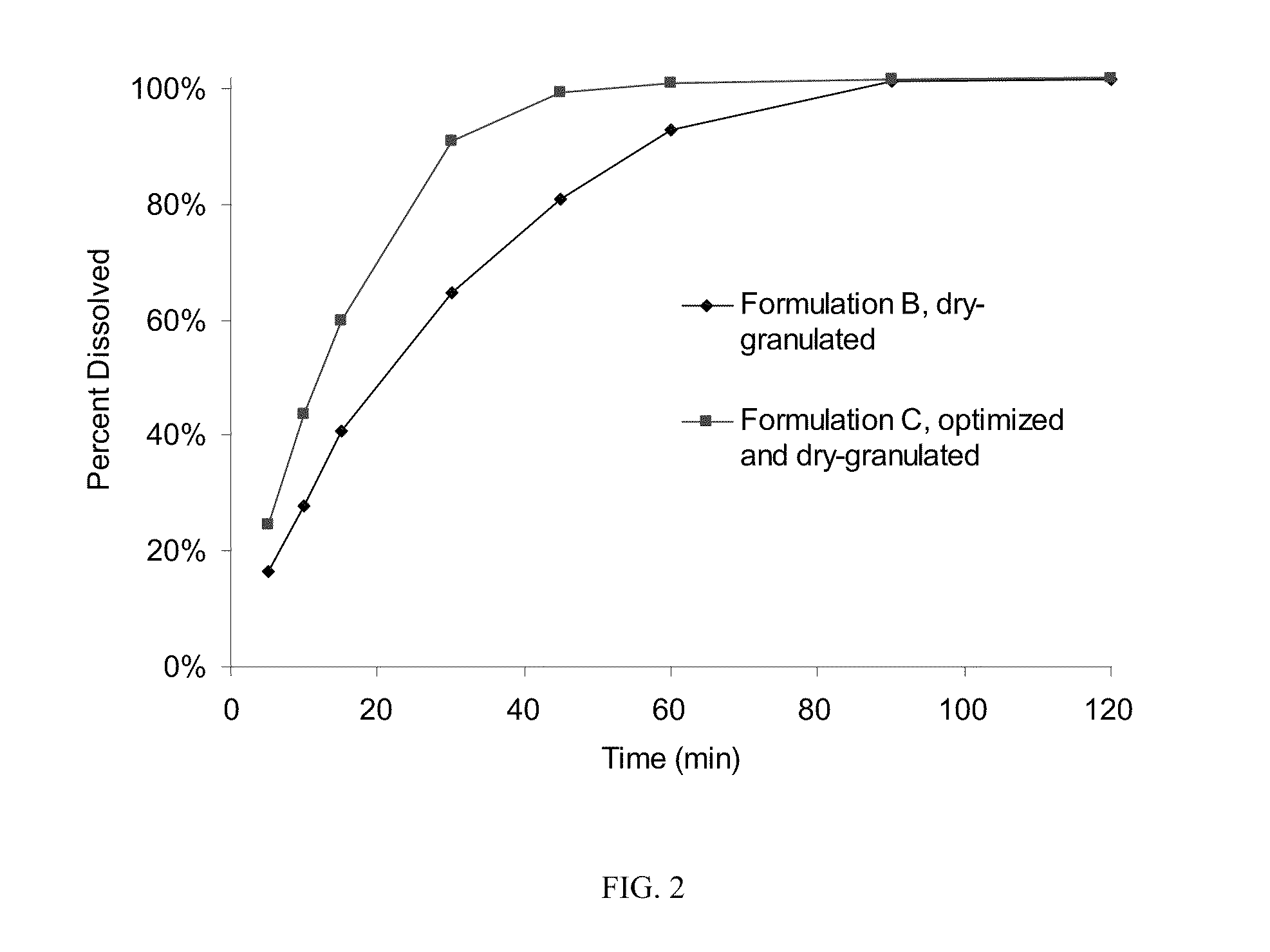
Modified release formulations of gamma-hydroxybutyrate having improved dissolution and pharmacokinetic properties are provided, and therapeutic uses thereof.
Owner:FLAMEL IRELAND
Gamma-hydroxybutyrate compositions and their use for the treatment of disorders
InactiveUS20180263936A1Lower Level RequirementsNervous disorderAnhydride/acid/halide active ingredientsSleep timeSleep arousal
Provided herein are pharmaceutical compositions and formulations comprising mixed salts of gamma-hydroxybutyrate (GHB). Also provided herein are methods of making the pharmaceutical compositions and formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Modified release gamma- hydroxybutyrate formulations having improved pharmacokinetics
ActiveUS20180021284A1Improve therapeutic effectivenessImprove safety profilePowder deliveryNervous disorderDissolutionHydroxybutyrates
Modified release formulations of gamma-hydroxybutyrate having improved dissolution and pharmacokinetic properties are provided, and therapeutic uses thereof.
Owner:FLAMEL IRELAND
Gamma-hydroxybutyrate compositions and their use for the treatment of disorders
Provided herein are pharmaceutical compositions and formulations comprising mixed salts of gamma-hydroxybutyrate (GHB). Also provided herein are methods of making the pharmaceutical compositions and formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Production of salts of 4-hydroxybutyrate using biobased raw materials
Gamma-butyrolactone (“GBL”) and Gamma-hydroxybutyrate (“GHB”) having a unique carbon footprint as defined by the percent modern carbon (pmc) are described herein. The percent modern carbon can be controlled by varying the amounts of biobased, renewable starting materials and petroleum-based starting materials to prepare GBL or GHB having a defined pmc or by preparing mixtures of GBL or GHB prepared from biobased renewable starting materials and GBL or GHB prepared from petroleum-based starting materials.
Owner:CJ CHEILJEDANG CORP
Alcohol-resistant drug formulations
ActiveUS20200330393A1Eliminate side effectsNervous disorderGranular deliverySleep arousalParacetamolum
The invention relates to modified release oral formulations of therapeutic agents, including gamma hydroxybutyrate (GHB), paracetamol, codeine or oxycodone, which are resistant to alcohol induced dose dumping. Provided are formulations that have improved resistance to rapid release of the active ingredient in the presence of increasing amounts of alcohol. Also provided are formulations that can reduce or prevent the release of the active ingredient following exposure to alcohol-containing media. The invention also relates to methods of making the formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Modified release gamma-hydroxybutyrate formulations having improved pharmacokinetics
Modified release formulations of gamma-hydroxybutyrate having improved dissolution and pharmacokinetic properties are provided, and therapeutic uses thereof.
Owner:FLAMEL IRELAND
Therapeutic uses of polymers and oligomers comprising gamma-hydroxybutyrate
Oligomers and polymer compositions are provided which comprise GHB and produce GHB after administration in vivo. Devices for the storage and delivery of these polymers and oligomers are also provided. These oligomers and polymer compositions are useful in a variety of applications. The compositions can be used therapeutically, for example, in the treatment of patients with narcolepsy, chronic schizophrenia, catatonic schizophrenia, atypical psychoses, chronic brain syndrome, neurosis, alcoholism, drug addiction and withdrawal, Parkinson's disease and other neuropharmacological illnesses, hypertension, ischemia, circulatory collapse, radiation exposure, cancer, and myocardial infarction. Other uses for the compositions include anesthesia induction, sedation, growth hormone production, heightened sexual desire, anorectic effects, euphoria, smooth muscle relaxation, muscle mass production, and sleep, including rapid eye movement sleep. In a still further embodiment, the oligomers and polymers may be used to produce absence seizures.
Owner:TEPHA INC
Use of gamma substituted gamma-butyrolactones to increase levels of their corresponding substituted gamma-hydroxybutyrate derivatives in humans
This invention relates to a method of increasing C-4 substituted gamma-hydroxybutyrate levels in humans by administration of their respective gamma substituted gamma-butyrolactones, for the purposes of increasing human growth hormone levels, aiding sleep, and enhancing well-being.
Owner:STONE CALEB
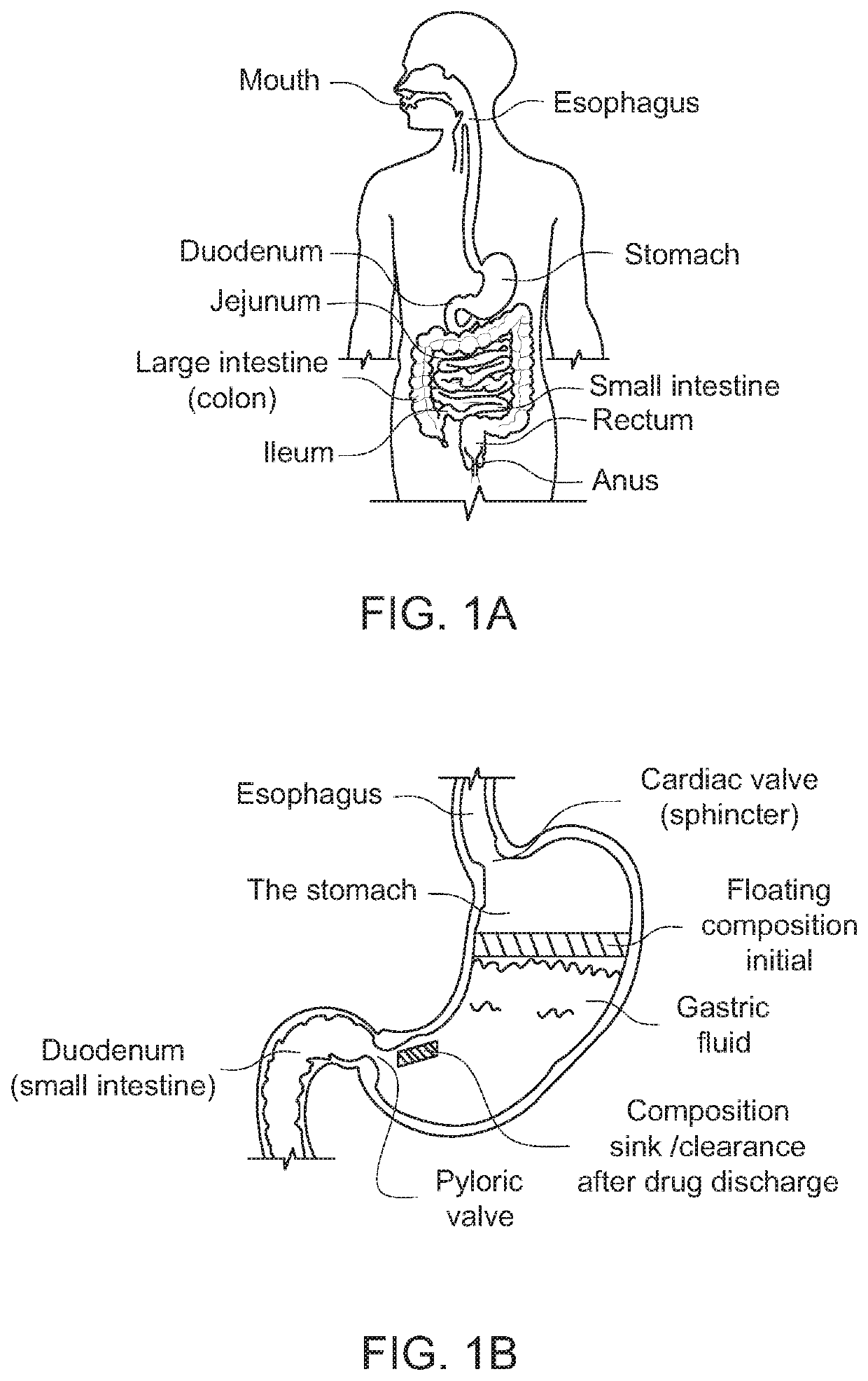
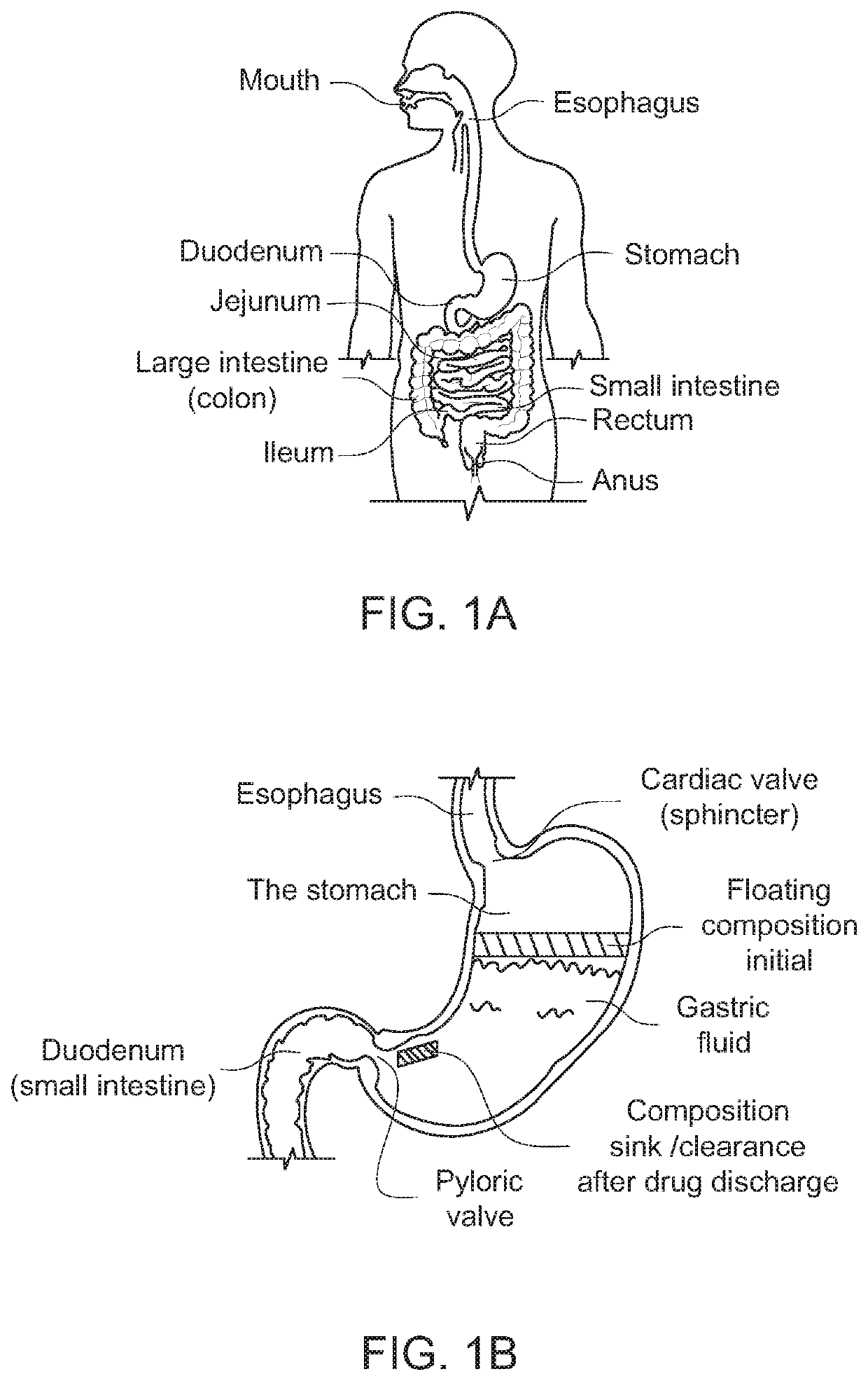
Modified release drug powder composition comprising gastro-retentive raft forming systems having trigger pulse drug release
ActiveUS20210015745A1Long retentionDispersion deliverySolution deliveryHydroxybutyric acidImmediate release
An orally administrable drug powder composition which forms a gastro-retentive RAFT having at least two trigger pulses is provided. The composition contains, at a minimum, (a) at least one drug in an immediate release pulse release form; (b) at least one drug in a delayed trigger release form; (c) at least one non-toxic gas generating agent and (d) a RAFT system, wherein following oral ingestion, the composition provides a self-assembling gastro-retentive RAFT having entrapped therein, the at least one drug of (a) and (b) and the gas generated in situ by the non-toxic gas generating agent, thereby providing a floating gastro-retentive RAFT having a dual pulse system wherein at least the second pulse is a trigger pulse and which retains the at least one drug in the stomach for at least about 3 hours, provided that the composition does not include a gamma hydroxybutyrate and its salts, hydrates, tautomers, or solvates, or complexes thereof.
Owner:TRIS PHARMA
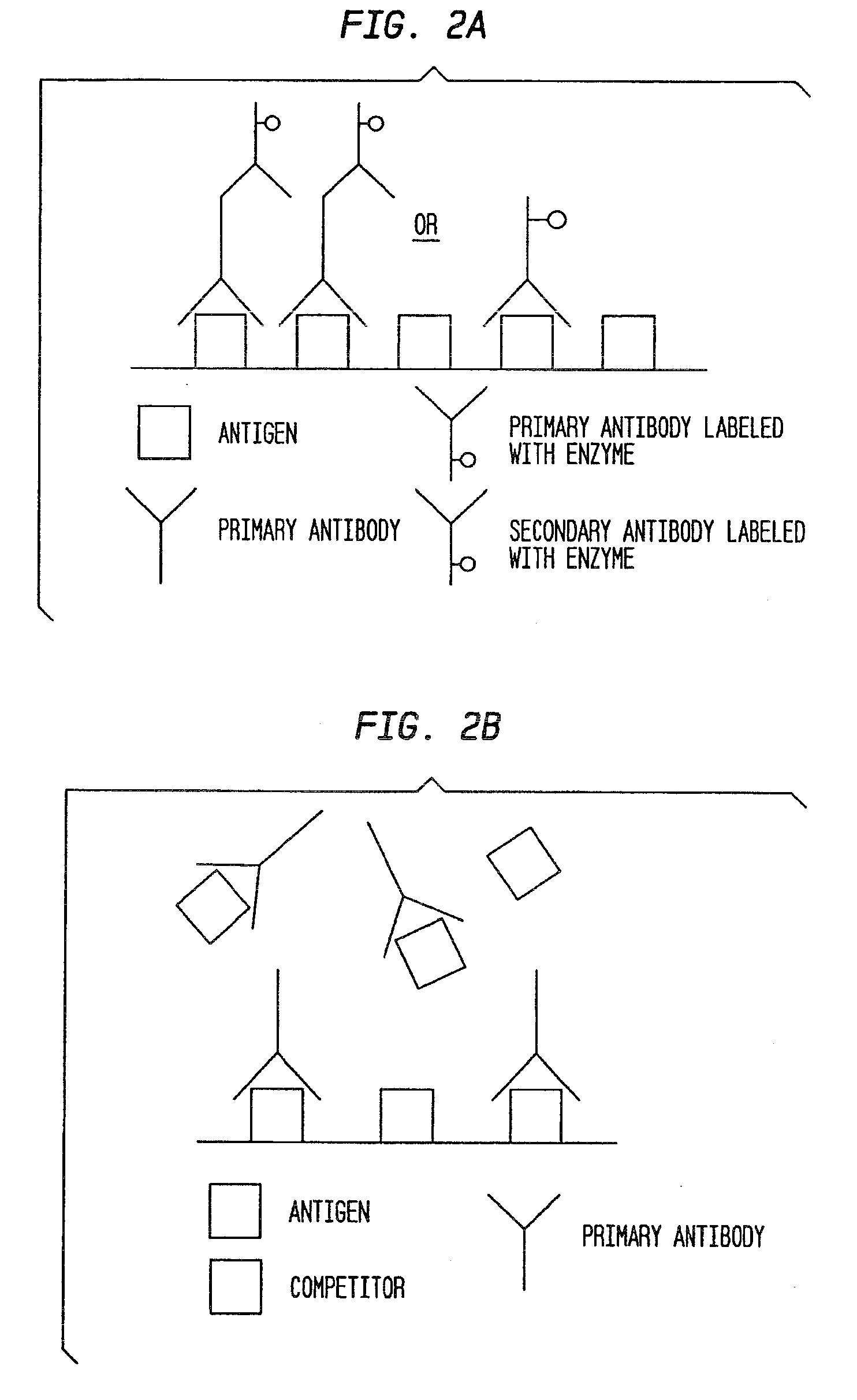
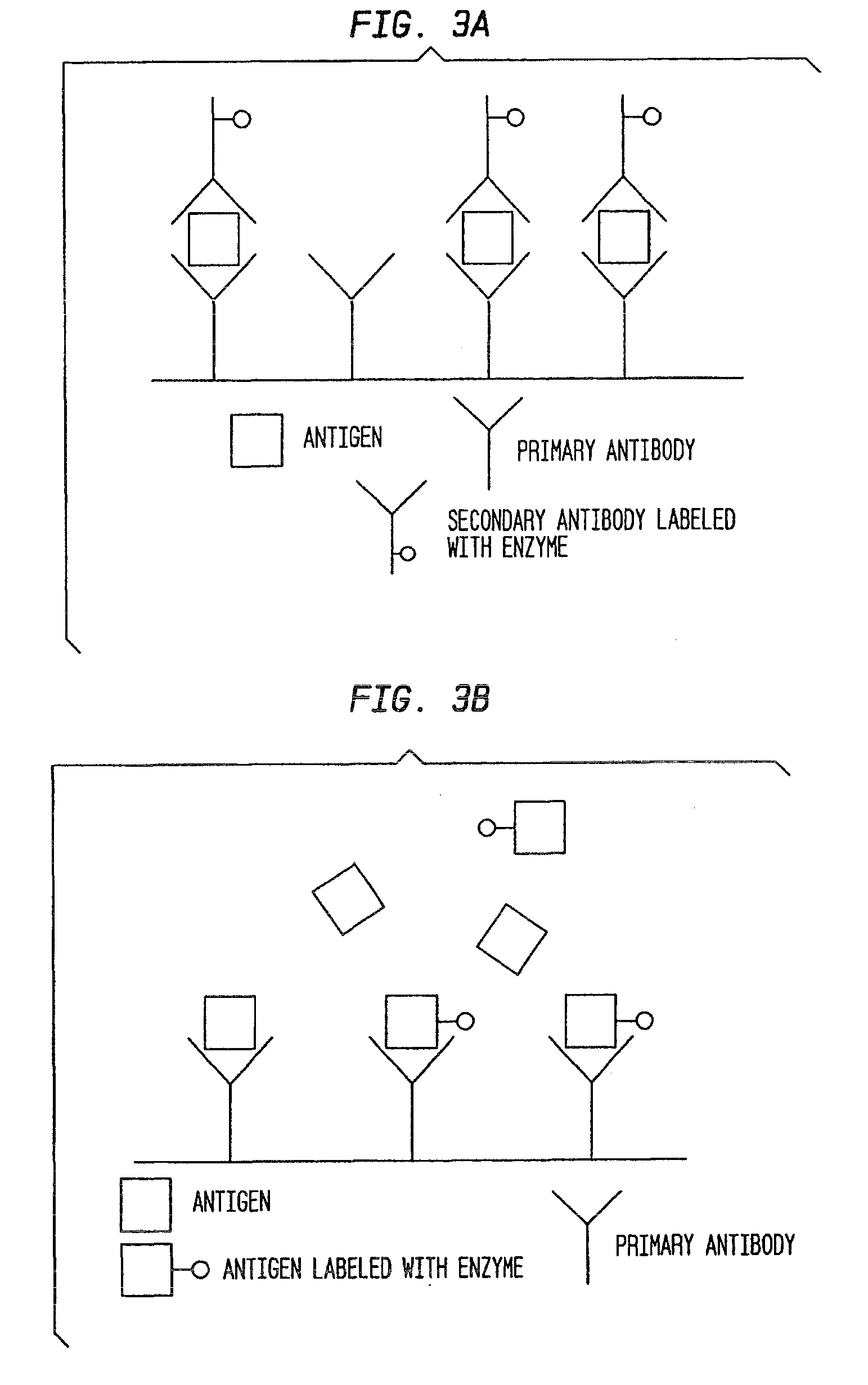
Antibody-based gamma-hydroxybutyrate (GHB) detection method and device
Gamma-hydroxybutyrate (GHB) can be used as a recreational party drug, aphrodisiac, and attenuator of other drugs and make a person a vulnerable target of robbery or rape. The present invention provides methods and kits for detection of GHB in a sample using an antibody-based assay. Antibodies that specifically bind to GHB or the conjugates of GHB and its derivatives to larger molecules and methods for detecting GHB or its derivatives in bodily fluids and non-alcoholic and alcoholic drinks by employing such antibodies in ELISA or RIA assays are provided by the present invention.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Treatment of addictive disorders
The present invention is directed to the treatment of addiction, and particularly to addictions with a chemical dependency component, with gamma-hydroxybutyrate (GHB) and at least one gamma-aminobutyric acid (B) receptor agonist (GABAB receptor agonist), for example, baclofen. Pharmaceutical compositions therefor are also provided.
Owner:AMEISEN OLIVIER
Preparation method of 4-hydroxybutyric acid test paper resisting interference of ascorbic acid and pigments
InactiveCN104111253AEliminate distractionsReliable resultsMaterial analysis by observing effect on chemical indicatorASCORBIC ACID/ZINCAscorbic acid
The invention relates to a preparation method of a 4-hydroxybutyric acid test paper resisting the interference of ascorbic acid and pigments. The method includes: infiltrating a filter paper in a first phase immersion liquid, then conducting hot air drying at 30-50DEG C to obtain an enzyme reagent layer; infiltrating a filter paper in a second phase immersion liquid, then conducting hot air drying at 30-50 DEG C to obtain a color developing agent layer; infiltrating a sponge stick in an anti-ascorbic acid infiltration liquid, performing hot air drying at 40-60DEG C to obtain an anti-ascorbic acid sponge stick; and pressing test paper pieces obtained by cutting the color developing agent layer and the enzyme reagent layer under an upper cover color developing hole and at one end of the anti-ascorbic acid sponge stick in order, thus obtaining the test paper. The method provided by the invention provides a solution to the problems that: existing test papers can only be preserved under refrigeration and is unstable under normal temperature; GHB (gamma-hydroxybutyrate) dehydrogenase and ascorbic acid can undergo cross reaction to make ascorbic acid existing samples undergo false positive reaction, thereby obtaining wrong results; and many beverages have colors in itself, thus interfering detection results and like.
Owner:武汉高丰生物科技有限公司 +1
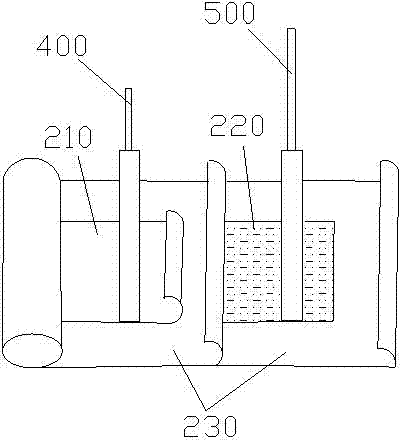
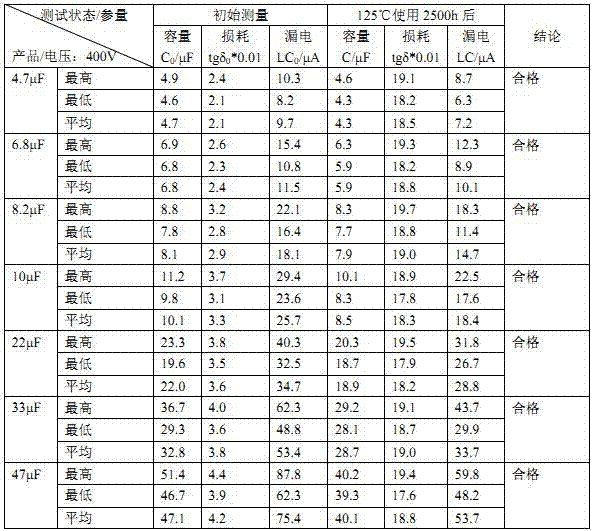
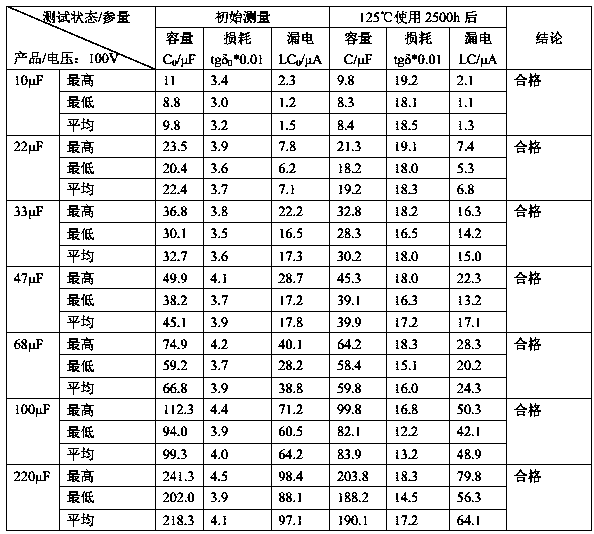
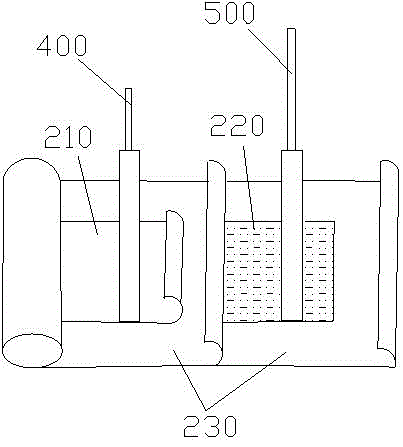
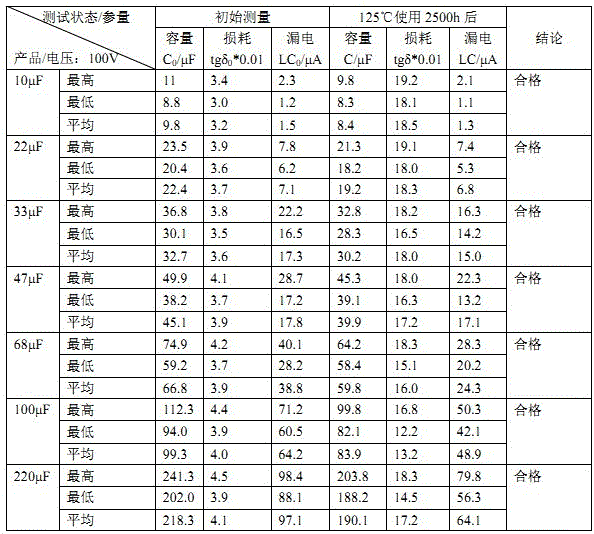
400 V electrolytic capacitor and production technology thereof
ActiveCN103680980AIncrease in sizeGuaranteed to workLiquid electrolytic capacitorsCapacitor terminalsElectrolysisO-Nitroanisole
The invention discloses a 400 V electrolytic capacitor and a production technology thereof. The 400 V electrolytic capacitor comprises a shell, a core, a sealing plug, a negative wire and a positive wire, wherein the core comprises a cathode foil and an anode foil; the core further comprises insulating electrolytic paper; electrolyte is added to the insulating electrolytic paper; the electrolyte is composed of ethylene glycol accounting for 40%-50%, diethylene glycol accounting for 10%-20%, triethylene glycol accounting for 10%-20%, glycerol accounting for 10%-20%, gamma-hydroxybutyrate lactone accounting for 10%-20%, sebacic acid accounting for 1%-10%, ammonium sebacate accounting for 1%-10%, o-nitroanisole accounting for 0.1%-0.9%, p-nitrobenzyl alcohol accounting for 0.1%-0.9%, benzene accounting for 0.1%-1%, polyvinyl alcohol accounting for 0.1%-1% and citric acid accounting for 0%-0.5%. The electrolytic capacitor has good temperature tolerance and long service life, and the load life reaches to 10000-15000 hours at 105 DEG C.
Owner:FOSHAN LIMING ELECTRONICS GAOMING
Gamma-hydroxybutyrate compositions containing carbohydrate, lipid or amino acid carriers
ActiveUS20050250848A1Effective treatmentAlter cellular metabolismBiocideNervous disorderLipid formationEffective treatment
Owner:JAZZ PHARMA
Gamma-hydroxybutyrate compositions and their use for the treatment of disorders
Provided herein are pharmaceutical compositions and formulations comprising mixed salts of gamma-hydroxybutyrate (GHB). Also provided herein are methods of making the pharmaceutical compositions and formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Modified release drug powder composition comprising gastro-retentive RAFT forming systems having trigger pulse drug release
ActiveUS11337919B2Long retentionDispersion deliverySolution deliveryHydroxybutyric acidImmediate release
An orally administrable drug powder composition which forms a gastro-retentive RAFT having at least two trigger pulses is provided. The composition contains, at a minimum, (a) at least one drug in an immediate release pulse release form; (b) at least one drug in a delayed trigger release form; (c) at least one non-toxic gas generating agent and (d) a RAFT system, wherein following oral ingestion, the composition provides a self-assembling gastro-retentive RAFT having entrapped therein, the at least one drug of (a) and (b) and the gas generated in situ by the non-toxic gas generating agent, thereby providing a floating gastro-retentive RAFT having a dual pulse system wherein at least the second pulse is a trigger pulse and which retains the at least one drug in the stomach for at least about 3 hours, provided that the composition does not include a gamma hydroxybutyrate and its salts, hydrates, tautomers, or solvates, or complexes thereof.
Owner:TRIS PHARMA
Novel omega-3 and omega-6 fatty acid compositions and uses thereof
InactiveUS20130295179A1Preventing functionsReducing secondary adverse eventsHeavy metal active ingredientsBiocideSertralineStimulant
Owner:TERREAUX CHRISTIAN +3
100V electrolytic capacitor and producing process thereof
ActiveCN103700507AGuaranteed to workImprove temperature resistanceLiquid electrolytic capacitorsCapacitor electrodesElectrolysisElectrolytic capacitor
The invention discloses a 100V electrolytic capacitor and a producing process thereof, wherein the capacitor comprises a shell, a core, a seal plug, a cathode lead and an anode lead; the core comprises a cathode foil and an anode foil and also comprises insulation electrolytic paper; components of electrolyte added to the insulation electrolytic paper comprise 40-70% of ethylene glycol, 10-40% of glycerin, 10-40% of gamma-hydroxybutyrate lactone, 1-10% of ammonium benzoate, 1-10% of azelaic acid EG (ethylene glycol) solution, 2-8% of ammonium hydrogen maleate, 0.1-0.9% of ortho-nitroanisole, 0-0.5% of citric acid, 0.1-0.5% of phosphotungstic acid and 0-0.1% of molybdic acid. The electrolytic capacitor is good in temperature resistance and long in service life, and achieves the load life of 10,000-15,000 hours under the condition of 105 DEG C.
Owner:FOSHAN LIMING ELECTRONICS GAOMING
Methods and pharmaceutical compositions for treating cancer
The invention relates to methods and pharmaceutical compositions for treating cancer. In particular, the present invention relates to a compound selected from the group consisting of gamma-hydroxybutyrate (GHB), GHB derivatives, and GHB structurally-related compounds thereof, or a pharmaceutically acceptable salt thereof for use in the treatment of cancer in a subject in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
Alcohol-resistant drug formulations
PendingUS20220160640A1Eliminate side effectsNervous disorderGranular deliverySleep arousalParacetamolum
The invention relates to modified release oral formulations of therapeutic agents, including gamma hydroxybutyrate (GHB), paracetamol, codeine or oxycodone, which are resistant to alcohol induced dose dumping. Provided are formulations that have improved resistance to rapid release of the active ingredient in the presence of increasing amounts of alcohol. Also provided are formulations that can reduce or prevent the release of the active ingredient following exposure to alcohol-containing media. The invention also relates to methods of making the formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
A 100v electrolytic capacitor and its production process
ActiveCN103700507BGuaranteed to workImprove temperature resistanceLiquid electrolytic capacitorsCapacitor electrodesElectrolysisO-Nitroanisole
Owner:FOSHAN LIMING ELECTRONICS GAOMING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com