Tissue management system
a tissue management and tissue technology, applied in the field of tissue management system, can solve the problems of not allowing such staff members to carry out a critical step for handling, storing, transporting, reconstituting or using such tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
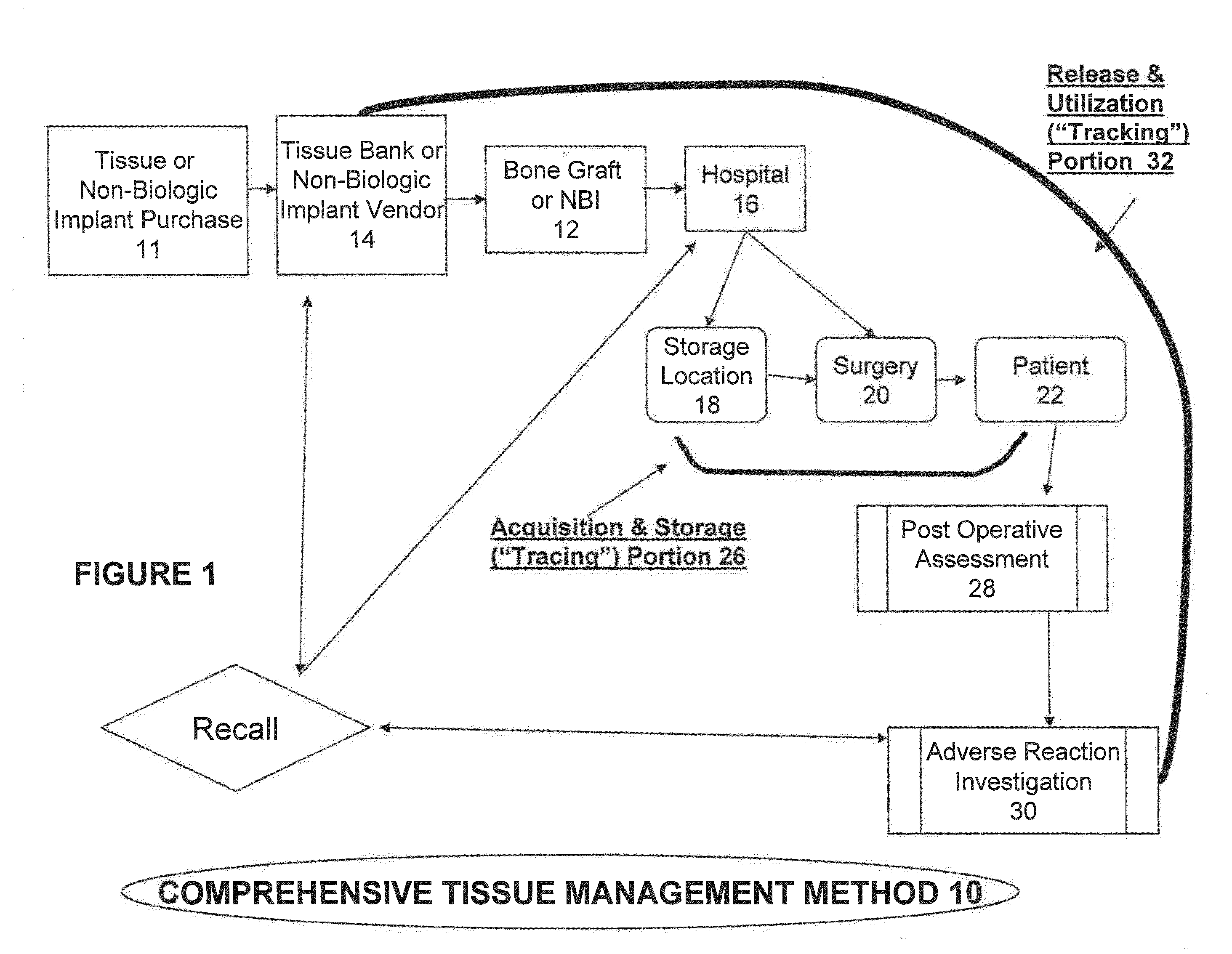
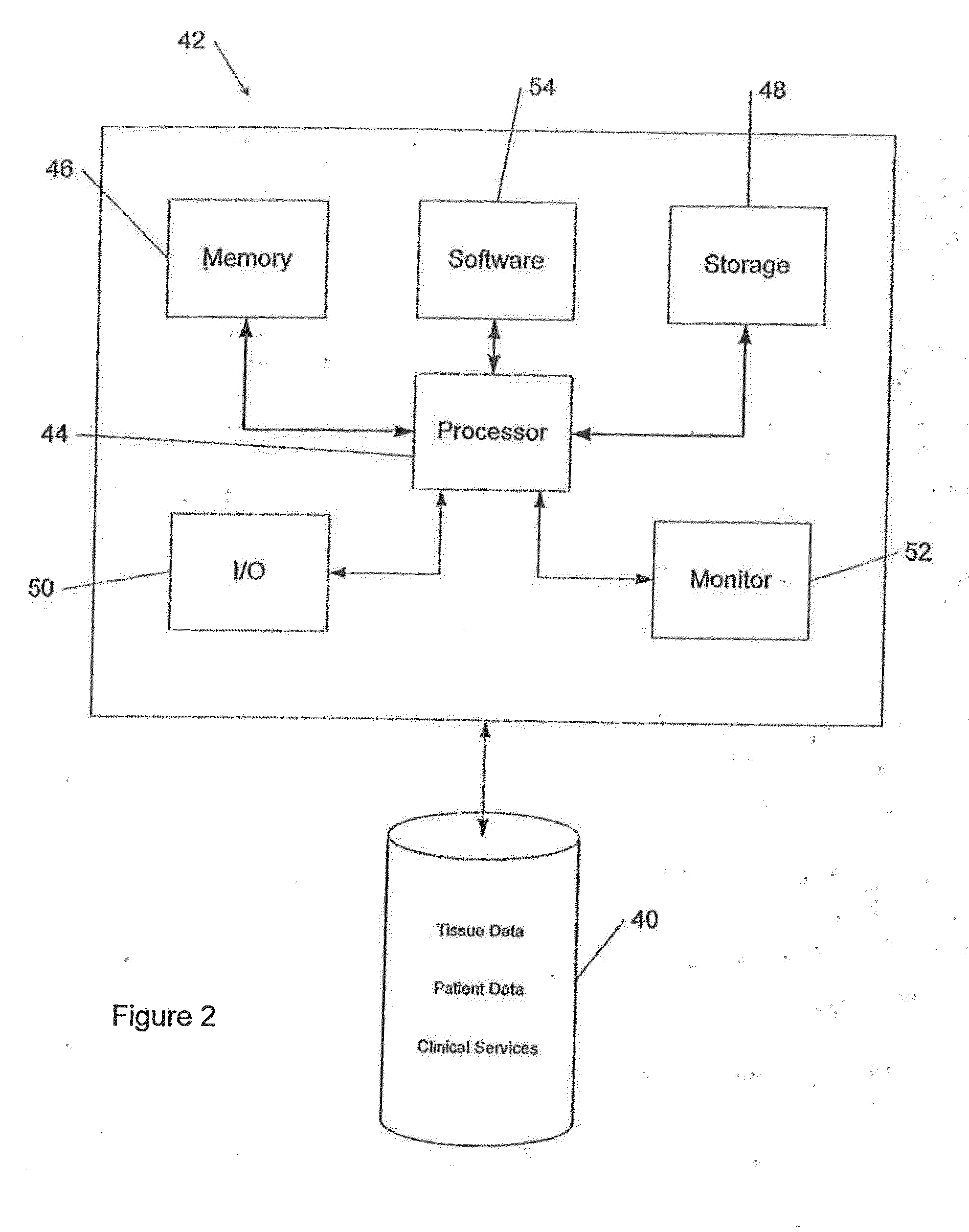
[0058]A comprehensive system for safe management of transplantable material is provided by the invention. In its preferred embodiment, the system is computer-based. Such invention enables the proper qualification of suppliers; logging and inspection of incoming transplantable material; product maintenance; integrated tracking and verification of the handling, storage, and use of transplantable materials in a safe and regulatory-compliant manner from the point of receipt to the point of issuance / final disposition throughout the medical establishment. The system also provides for prompt investigation of any adverse reaction suffered by a patient who receives the transplantable material through a surgical procedure. Additionally, it ensures a complete documented history of the transplantable material within the medical establishment so that the transplantable material can be traced back to its supplier in the event that a product recall of transplantable material is warranted either by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com