Sustained-release preparation of compound metformin hydrochloride rosiglitazone and preparation method thereof
A technology for compound metformin hydrochloride and metformin hydrochloride, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, metabolic diseases, etc., and can solve problems such as large dosage, adverse reactions, hypoglycemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
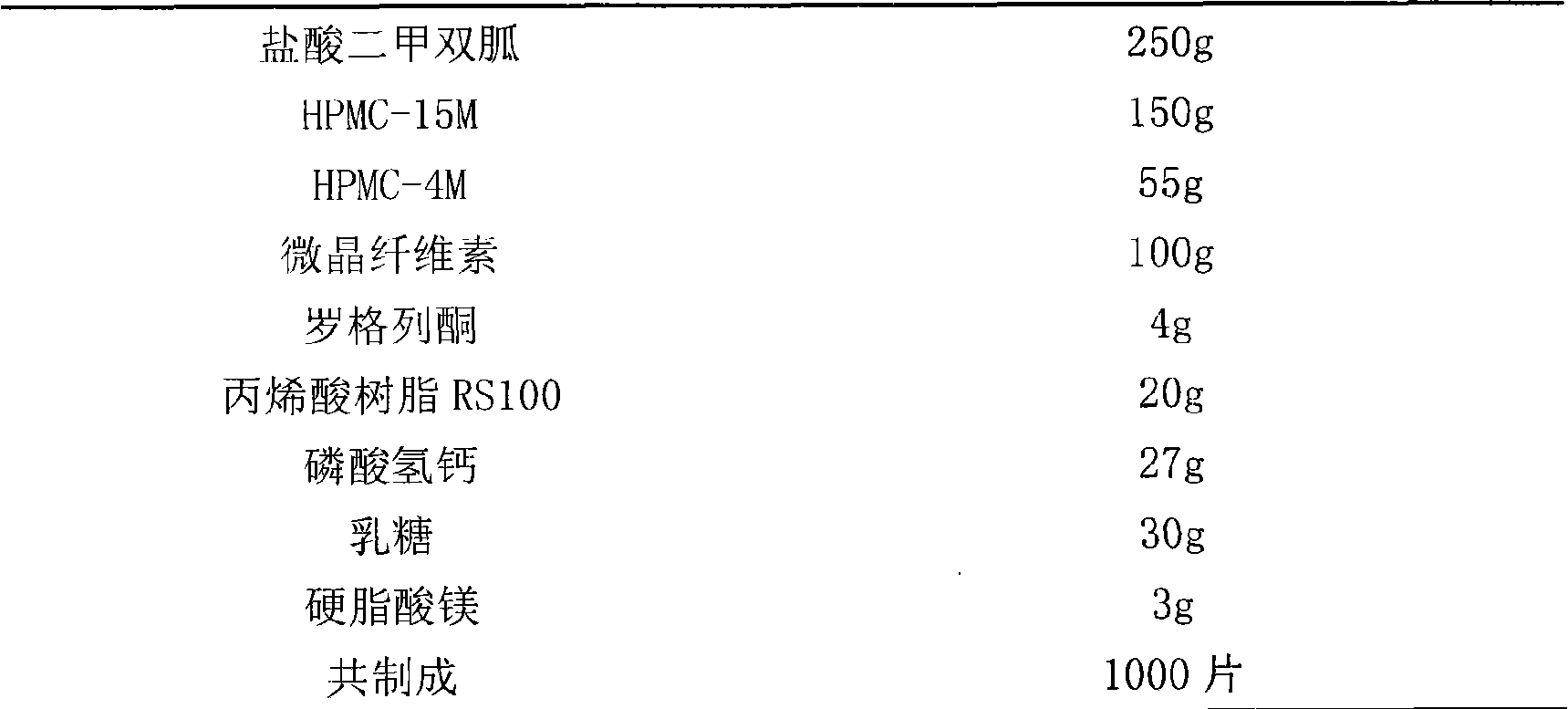
[0015] Embodiment 1: metformin hydrochloride rosiglitazone double-layer sustained-release tablet
[0016] prescription:
[0017]
[0018] Preparation:
[0019] Metformin hydrochloride is passed through a 100-mesh sieve, HPMC-15M, HPMC-4M, and microcrystalline cellulose are passed through a 60-mesh sieve, and after the raw and auxiliary materials are mixed evenly, a soft material is made with 80% ethanol solution, granulated with a 20-mesh sieve, dried at 40°C, and dried for 18 Mesh sieve for granulation, and set aside; in addition, rosiglitazone, lactose, calcium hydrogen phosphate, and acrylic resin RS100 were sieved and mixed three times, and the granules were sized in the same way. The above two granules are weighed in proportion respectively, and after adding magnesium stearate, they are compressed into double-layer sustained-release tablets.
Embodiment 2
[0020] Example 2 Compound metformin hydrochloride rosiglitazone film-coated sustained-release tablet
[0021] prescription:
[0022]
[0023]
[0024] Preparation:
[0025] Pass metformin hydrochloride and rosiglitazone through a 100-mesh sieve, and set aside; take metformin hydrochloride, mix evenly with 300 g of microcrystalline cellulose, make a soft material with 3% povidone K-30 aqueous solution, granulate with a 20-mesh sieve, and dry. 18-mesh sieve for granulation, and set aside; take another rosiglitazone, mix well with the remaining microcrystalline cellulose, calcium hydrogen phosphate, and silicon dioxide, and prepare granules by the same method as above, and set aside; mix the two granules and add hard Magnesium fatty acid, mixed evenly, tableted; configured in 8% EC aqueous dispersion solution, stirred evenly, and set aside; opened the coating granulator, and put the tablet into it. Adjust the air inlet pressure and temperature, adjust the rotating speed o...
Embodiment 3
[0026] Example 3 Compound Metformin Hydrochloride Rosiglitazone Sustained-release Pellet Capsules
[0027] prescription:
[0028]
[0029] Preparation:
[0030] Pass metformin hydrochloride, rosiglitazone, HPMC-K4M, and HPMC-K10M through a 60-mesh sieve, add 60% ethanol solution to make a wet soft material, extrude strips with a length of about 3-5cm in 12-20 mesh aperture, and rotate at the bottom of the turntable Adjust to 600-1200rpm, spheronize for about 5 minutes, fluidize and dry, fill in capsule shells, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com