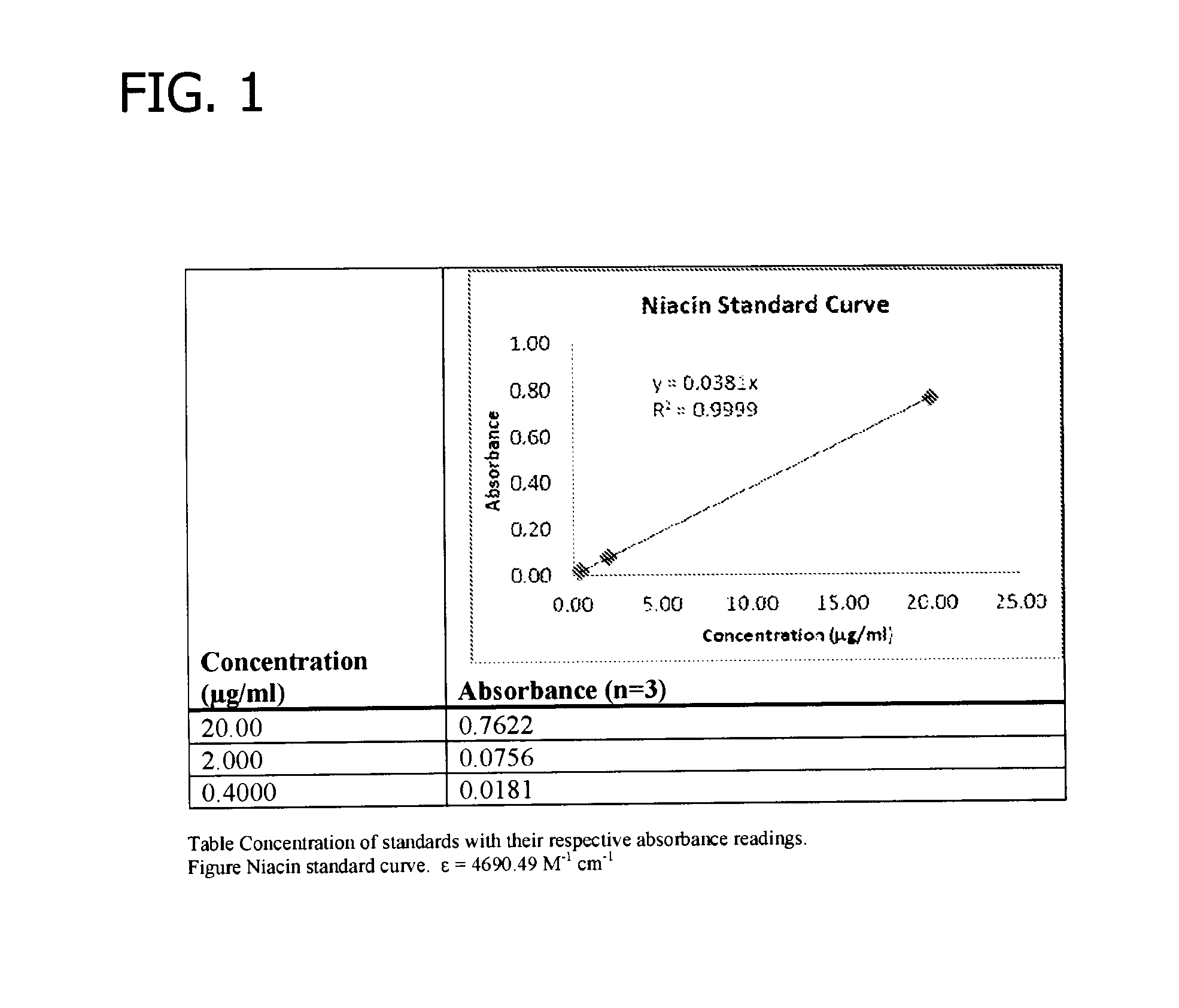
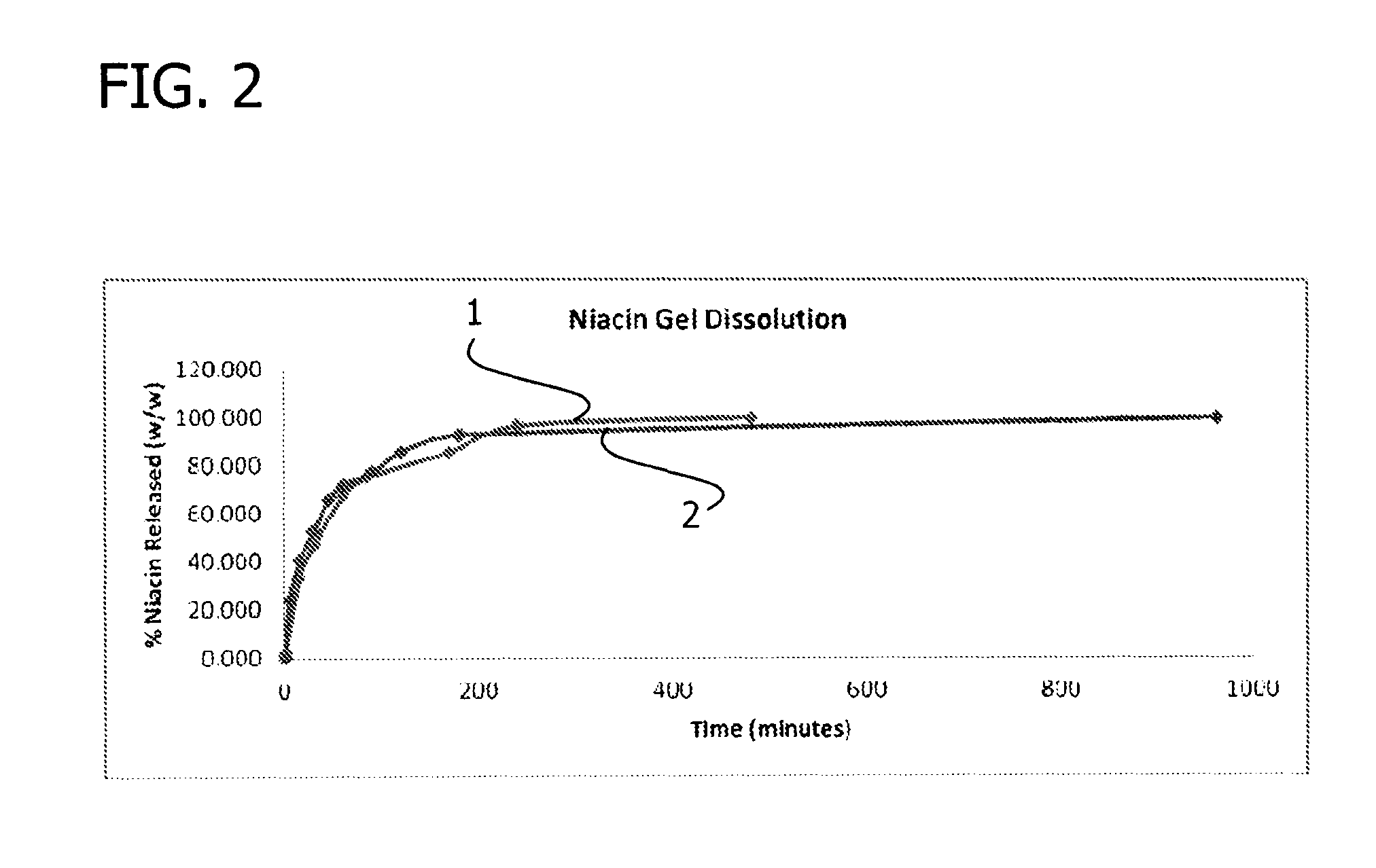
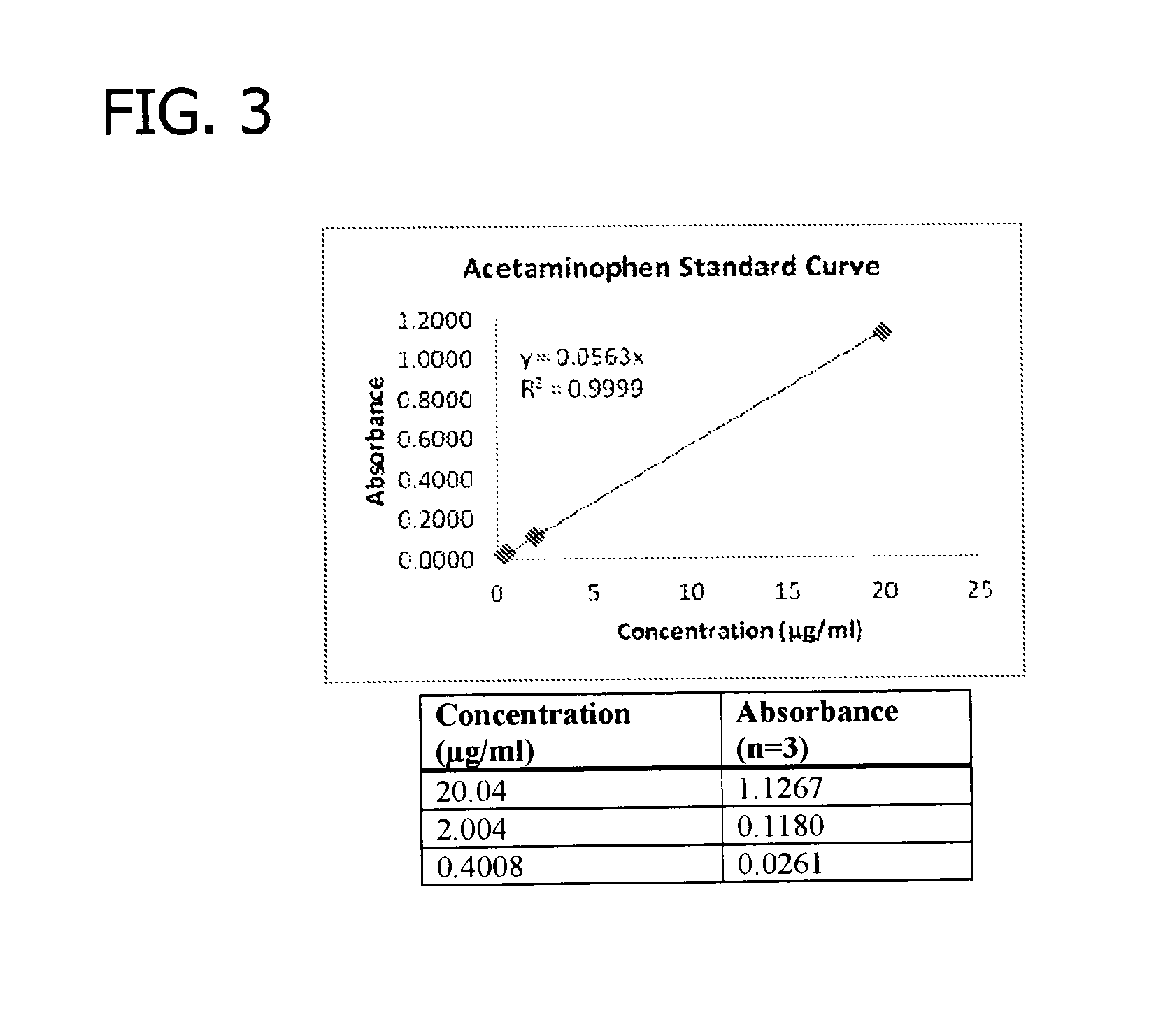
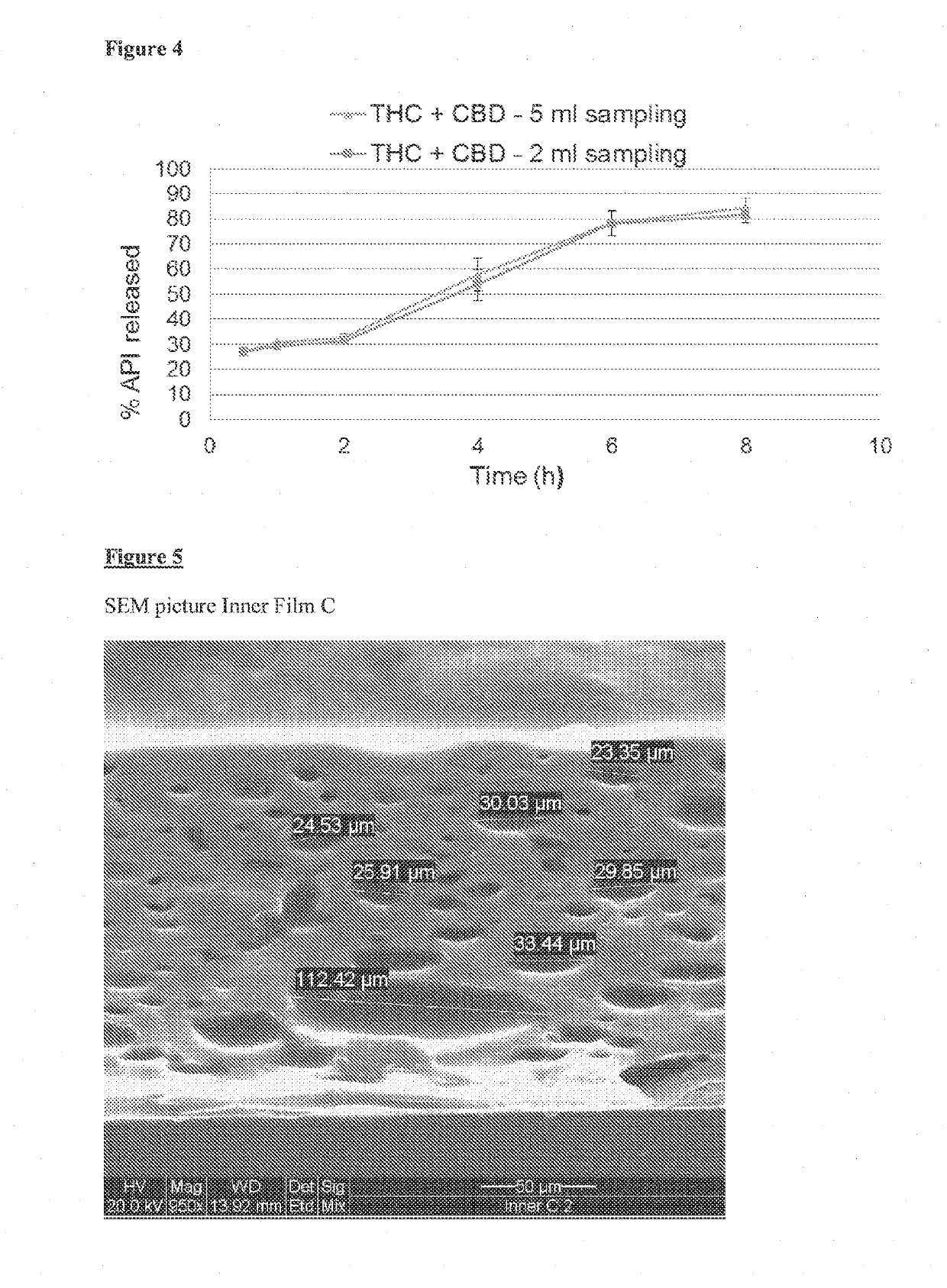
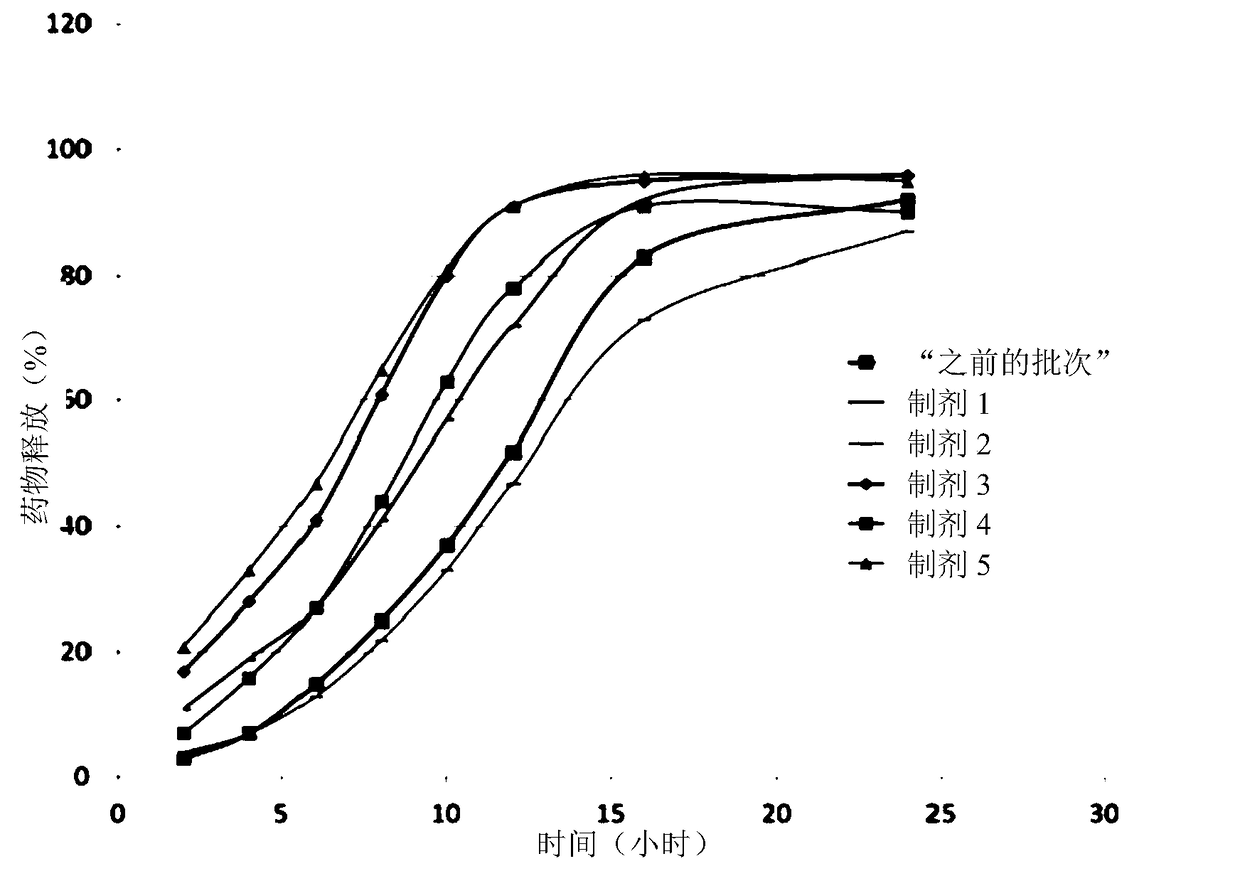
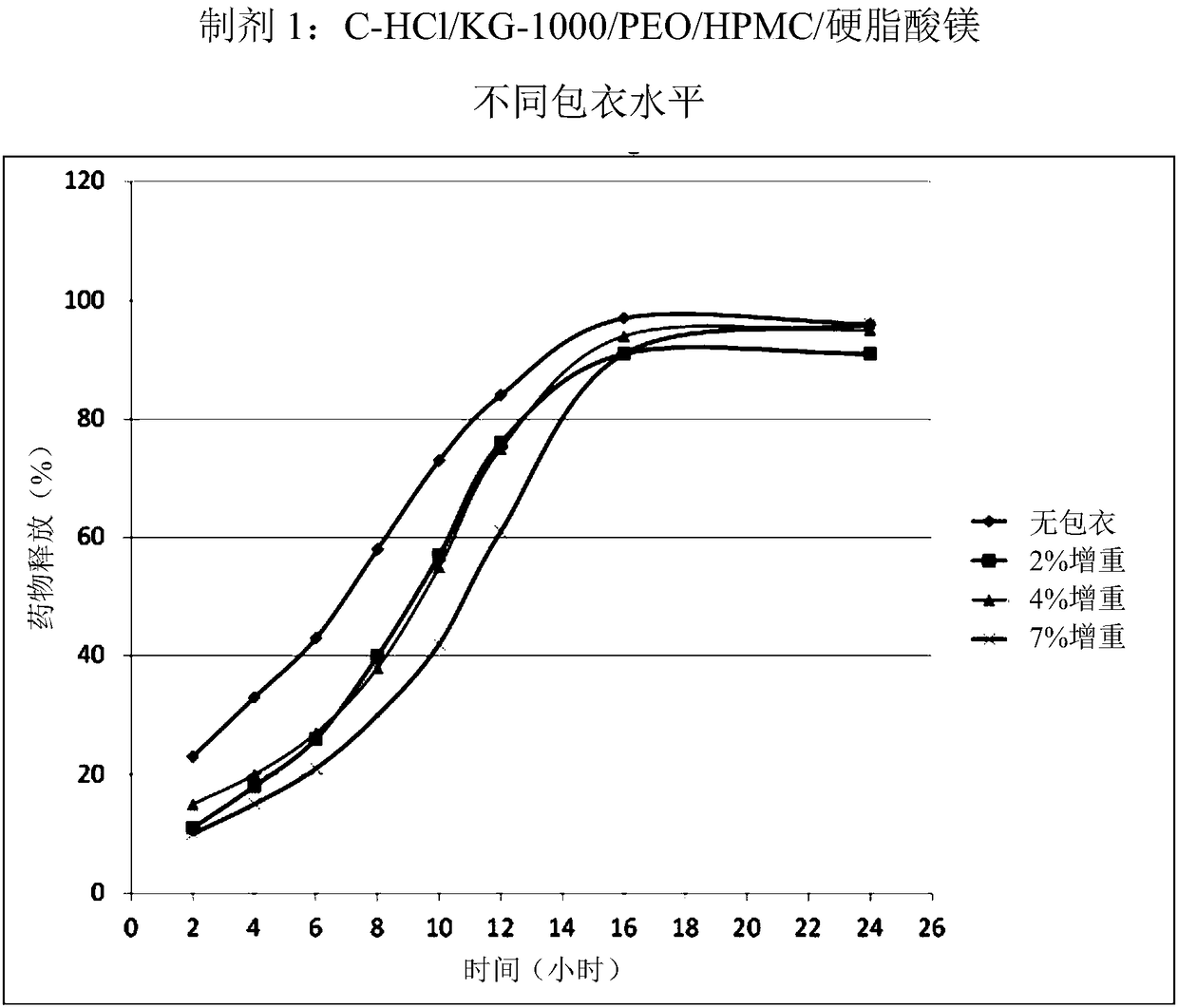
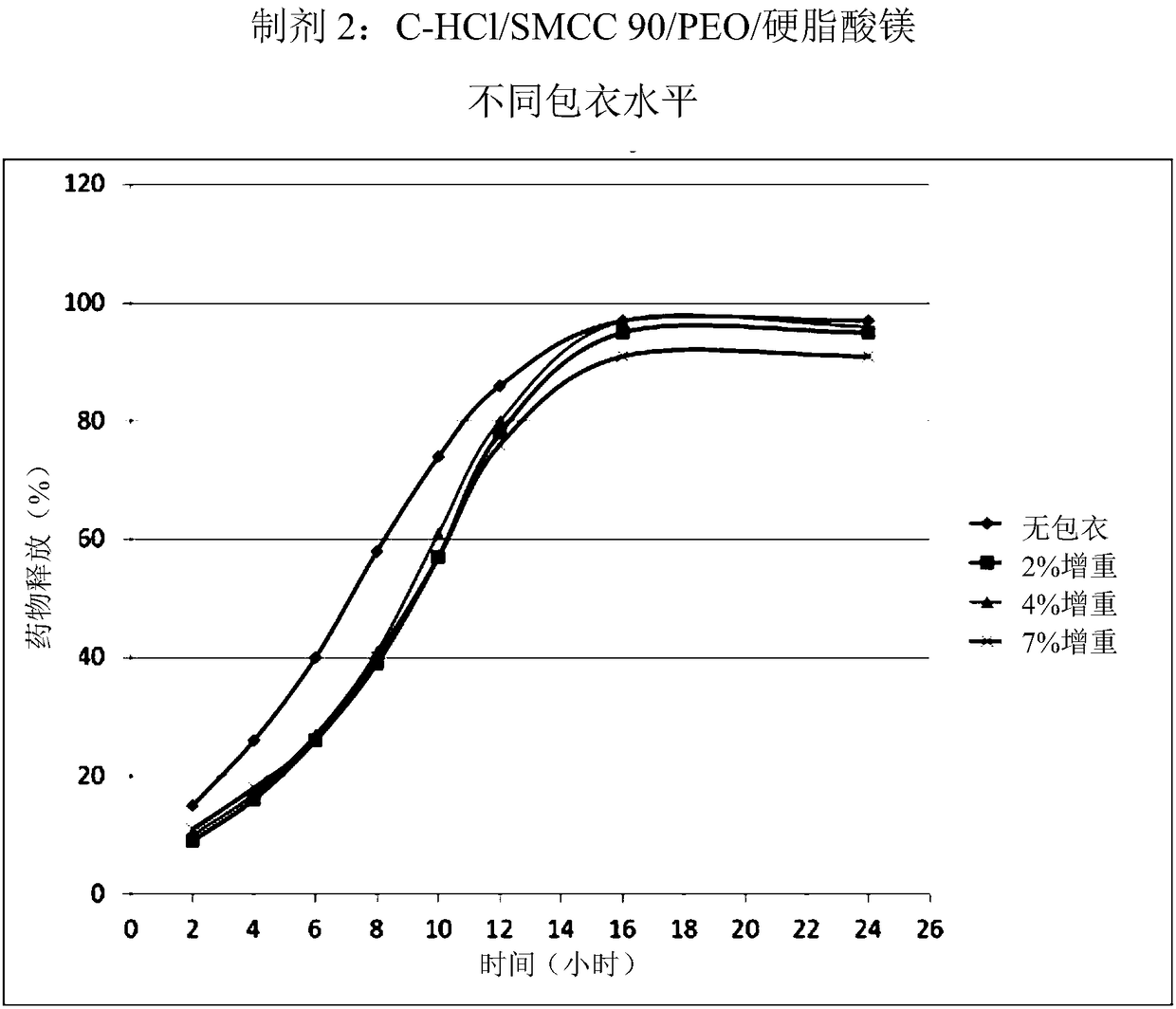
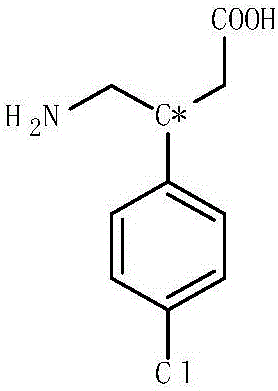
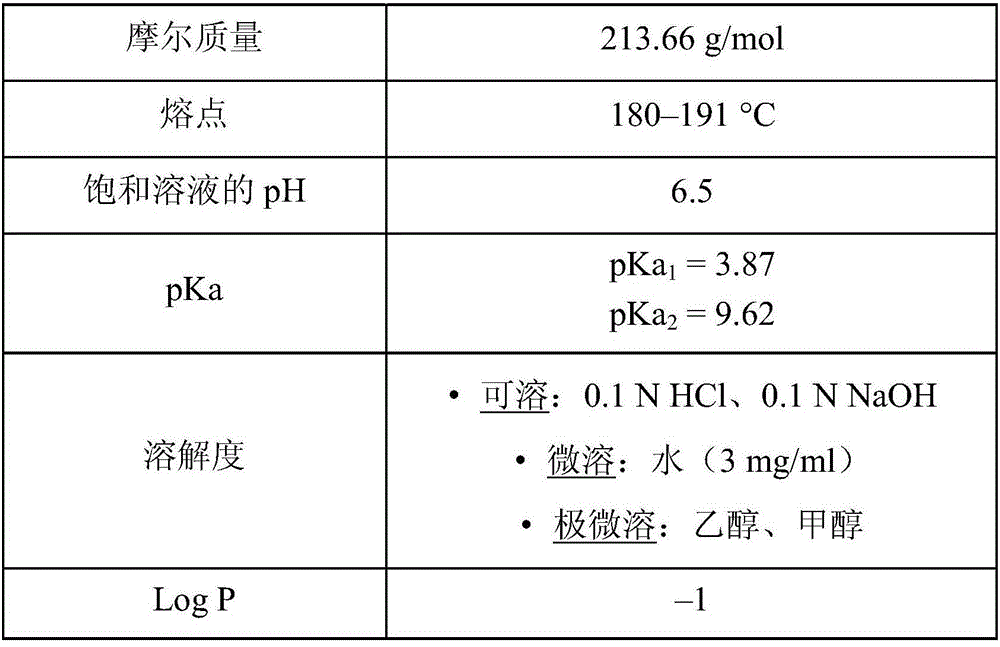
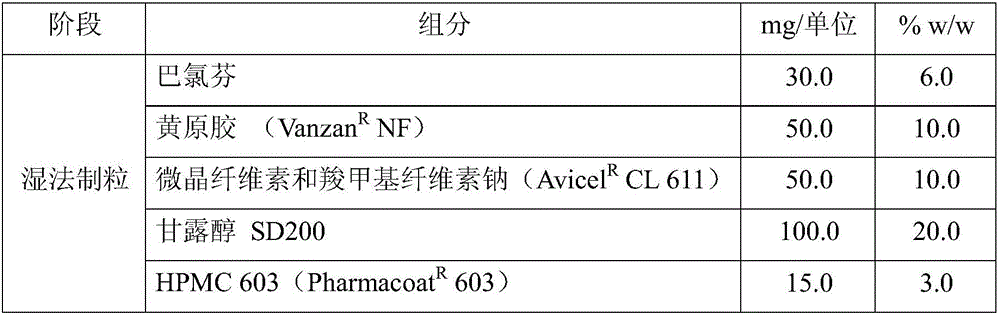
Patents
Literature
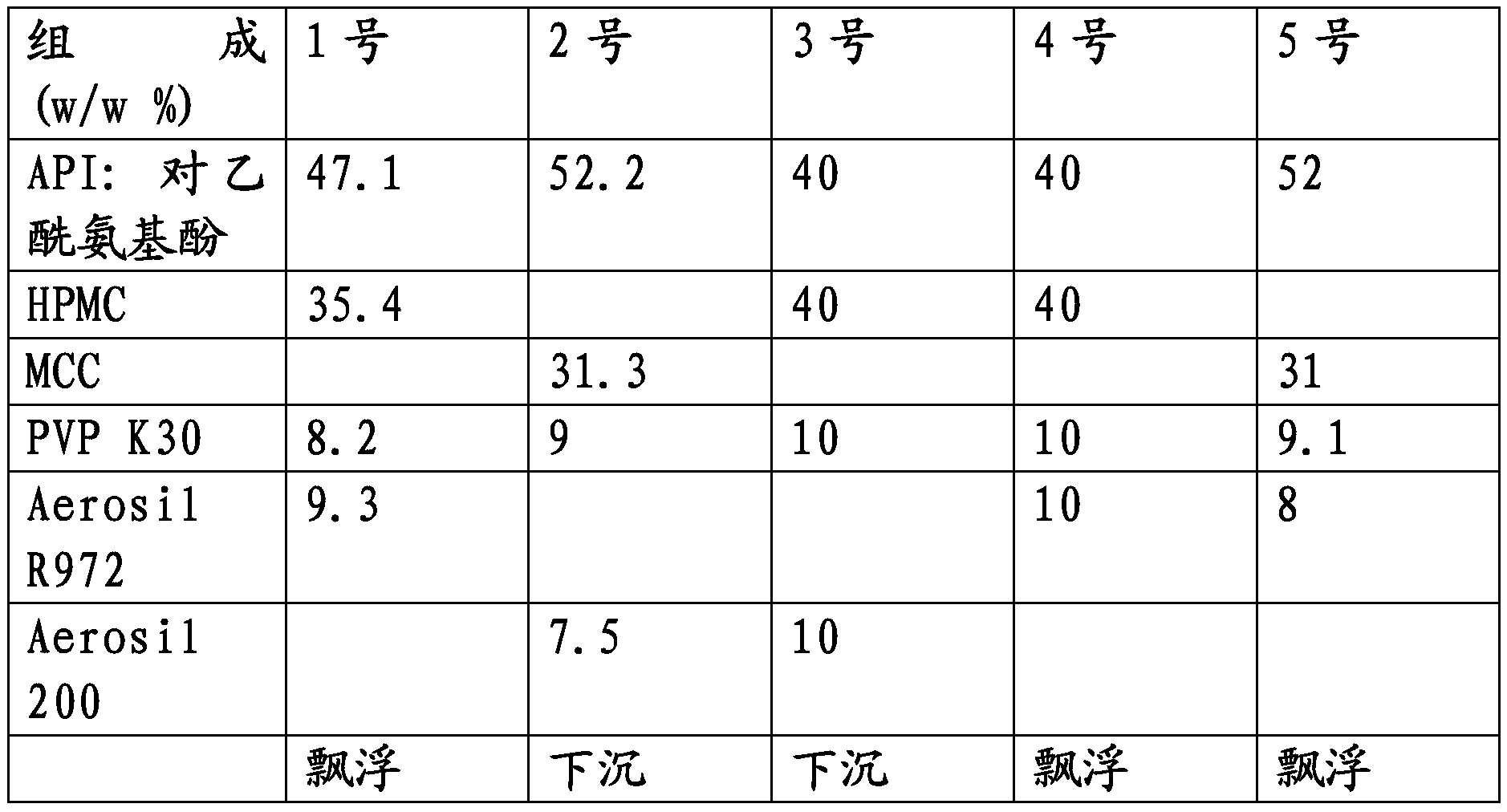
30 results about "Gastro retentive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel pharmaceutical dosage forms and method for producing same
InactiveUS20050202090A1Improve solubilityQuick fixPowder deliveryPharmaceutical product form changeGastro retentiveFast release
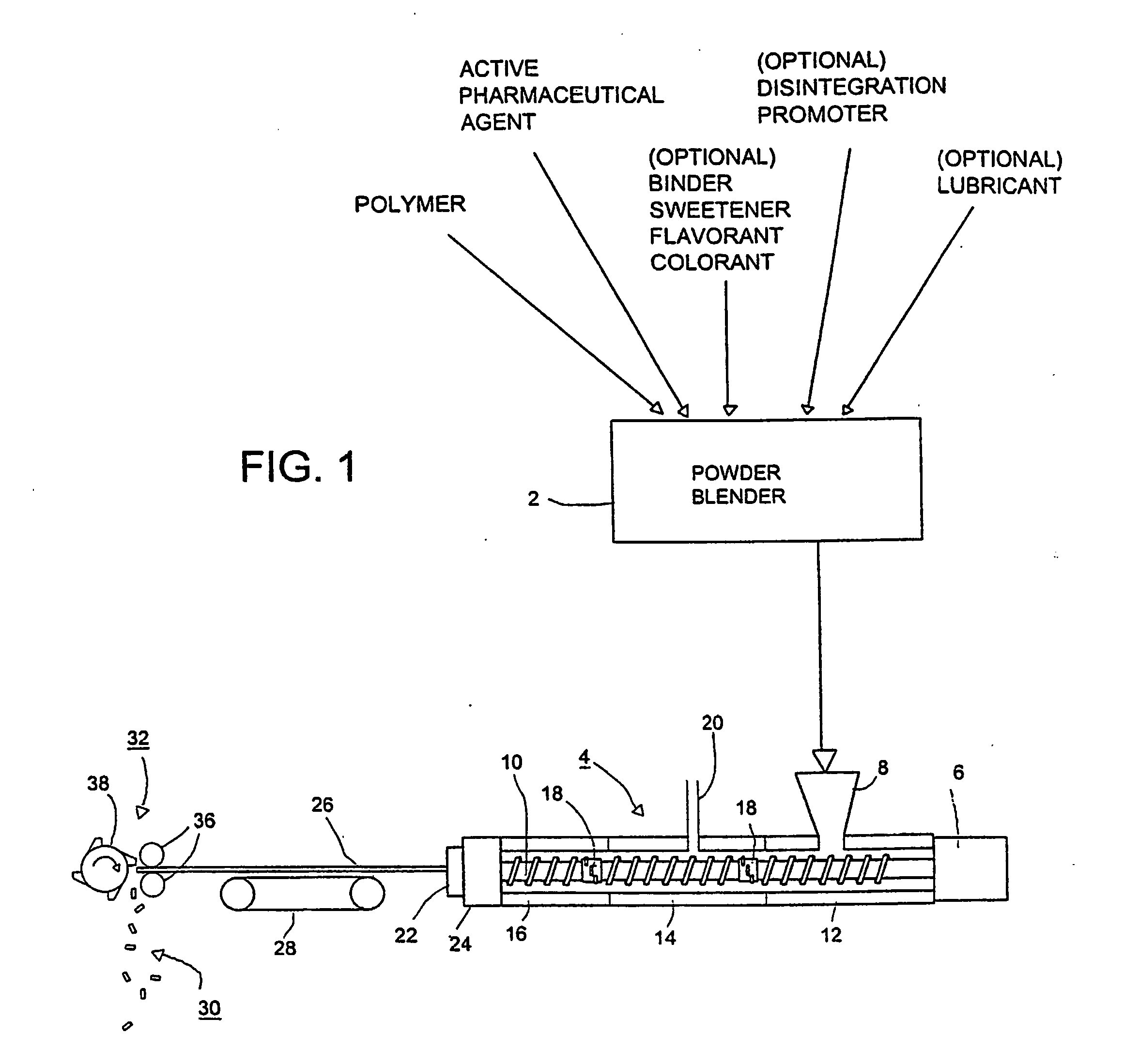
Pharmaceutical dosage forms are produced by injection molding a mixture of an agent and a polymer under pressure, in the presence of a gas or supercritical fluid. Rapid release of the pressure causes the mixture to form a microcellular or supermicrocellular solid. The release of pressure takes place in the mold. The process is especially useful for producing durable flash-dissolve and gastro-retentive tablets.
Owner:SMITHKLINE BECKMAN CORP
Process for making multiparticulate gastroretentive dosage forms
The instant invention relates to a process for making inherent low density particles, comprising the steps of (i) providing a powder mixture comprising a swelling agent; (ii) granulating the powder of step (i) with a granulating solution comprising a lipophilic agent into granules and (iii) drying the granules of step (ii). The instant invention further relates to multiparticulate oral gastro-retentive dosage forms comprising the inherent low density particles obtainable by the process.
Owner:MELIATYS
Gastro-Retentive System for the Delivery of Macromolecules
InactiveUS20090304768A1Envelopes/bags making machineryPeptide/protein ingredientsGastro retentivePathology
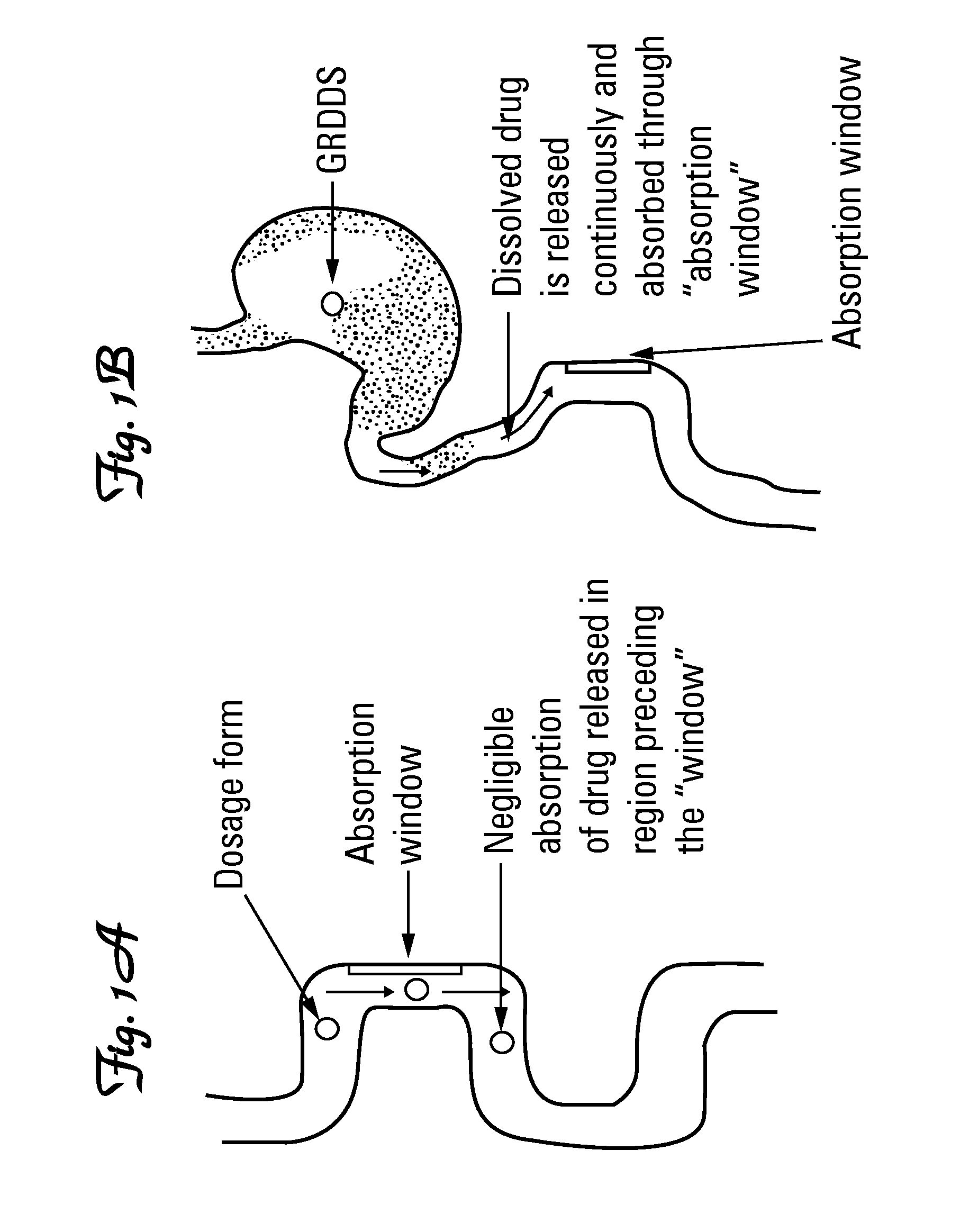
The present invention provides a gastro-retentive delivery assembly (GRDA) comprising a folded multi-layered device comprising a macromolecule-containing compartment bordered by enveloping layers and one or more enforcing strips, the device being adapted to unfold when in a subject's stomach, whereupon unfolding, the macromolecule is released from said device via at least one aperture in an enveloping layer. The invention also provides a method for gastroretentive delivery of macromolecules via the GRDA of the invention; a method of preparing the GRDA of the invention as well as a method for treating a subject for a pathological condition, making use of the GRDA of the invention.
Owner:INTEC PHARMA
Extended release gastro-retentive oral drug delivery system for valsartan
An extended release gastro-retentive drug delivery system of Valsartan. The drug delivery system contains a release portion containing the Valsartan, a gastro-retentive portion for retaining the drug delivery system in the stomach and an optional secondary portion for delivering a secondary pulse of Valsartan. In another embodiment, there is provided a swellable unfolding membrane comprising Valsartan for sustained administration of Valsartan to the upper GI tract of a patient.
Owner:NOVARTIS PHARM CORP
Modified gastroretentive drug delivery system for amine drugs
InactiveUS20110287096A1High dissolution rateOrganic active ingredientsPill deliveryGastro retentiveBioavailability
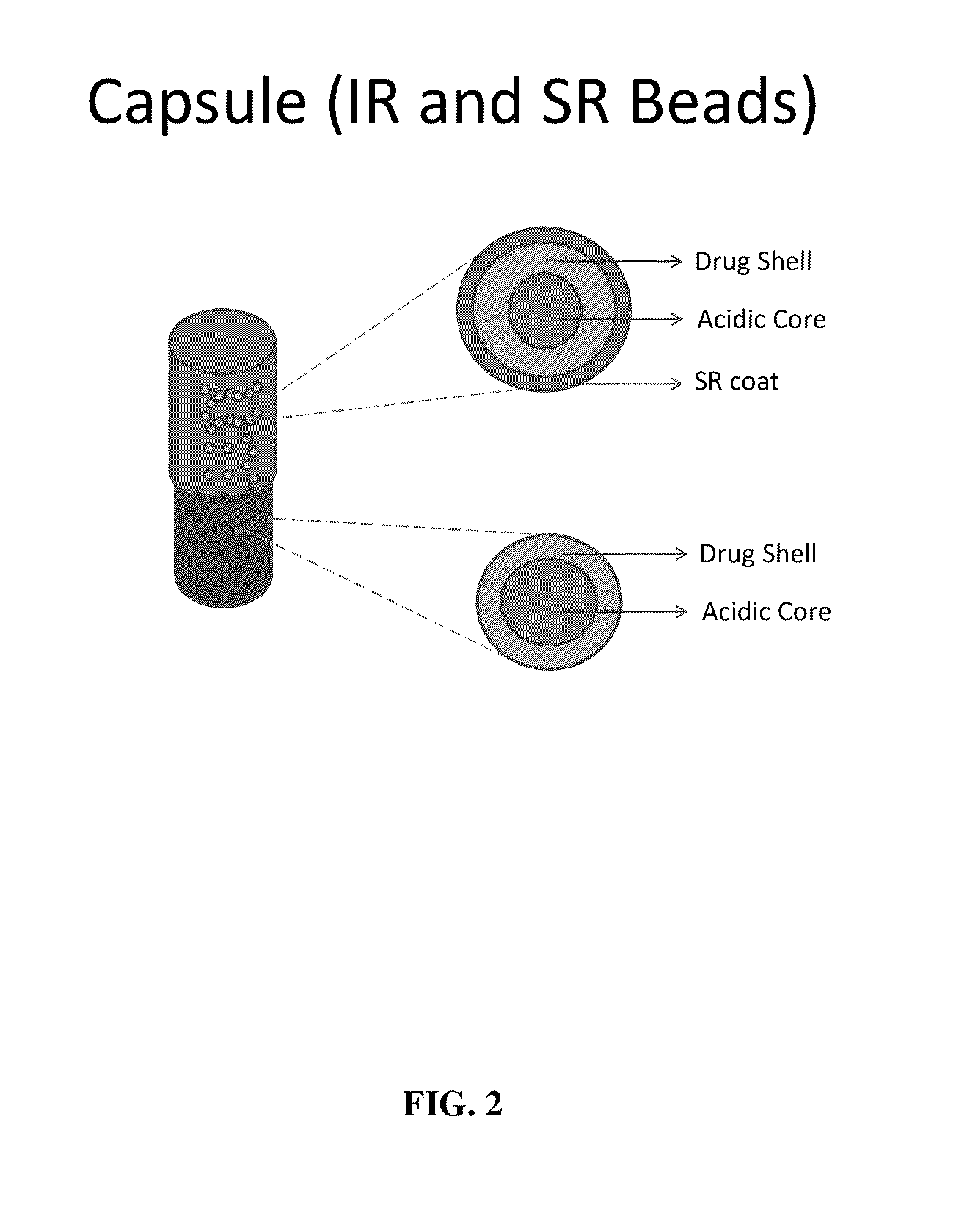
Oral dosage forms for basic amine drugs, the dosage forms having a gastro-retentive component and a non gastro-retentive component. These dosage forms are capable of providing both IR and SR release rates for these drugs. In addition, they provide for release of the drug in the stomach and / or intestine of a mammal to which such dosage forms are administered. Such dosage forms include tablets and capsules. Such dosage forms provide improved bioavailability of otherwise poorly bioavailable basic amine drugs.
Owner:ABON PHARMA
Novel gastro-retentive dosage forms
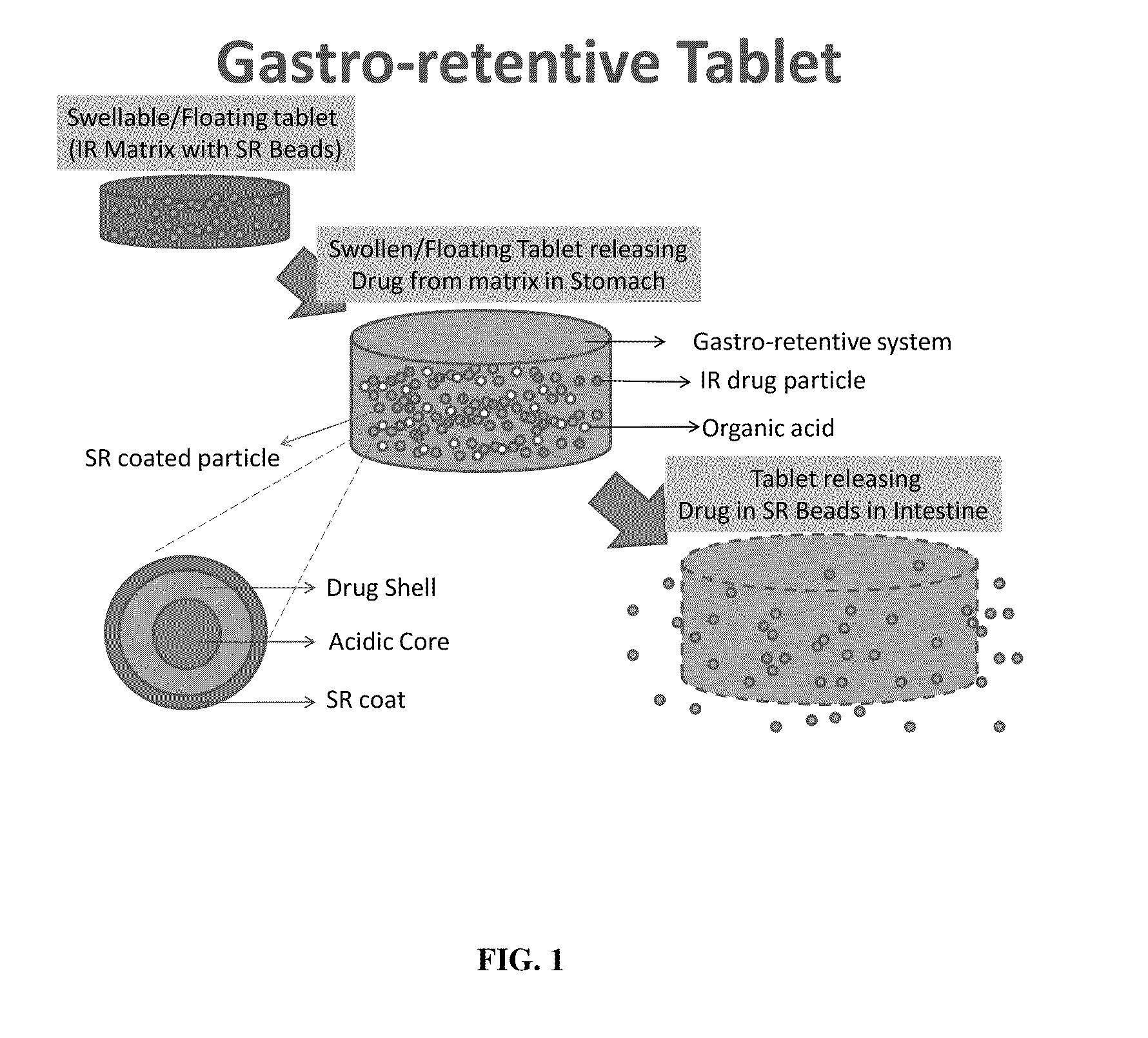
A gastro-retentive pharmaceutical dosage form comprising a therapeutically effective amount of at least one opioid, at least one form of acetaminophen, optionally an opioid antagonist and at least one pharmaceutically acceptable excipient, which dosage form is retained in the stomach for at least four hours and is suitable for once daily or twice daily administration. Also provided is a method of treating pain by administering the dosage form to a patient in need thereof. The acetaminophen is either in slow release form or in immediate release form or as combination of both.
Owner:GRUNENTHAL GMBH
Expandable gastroretentive dosage form
ActiveUS20150342877A1Volume maximizationMaximize weightBiocideMedical devicesGastro retentiveGastric fluid
An oral gastro-retentive delivery device is provided which unfolds rapidly upon contact with gastric juice. The device is configured in a collapsed configuration for oral intake and unfolding for gastric retention for a predetermined period of time and eventually reducing in size for passage through the rest of the GI track.
Owner:CLEXIO BIOSCIENCES LTD
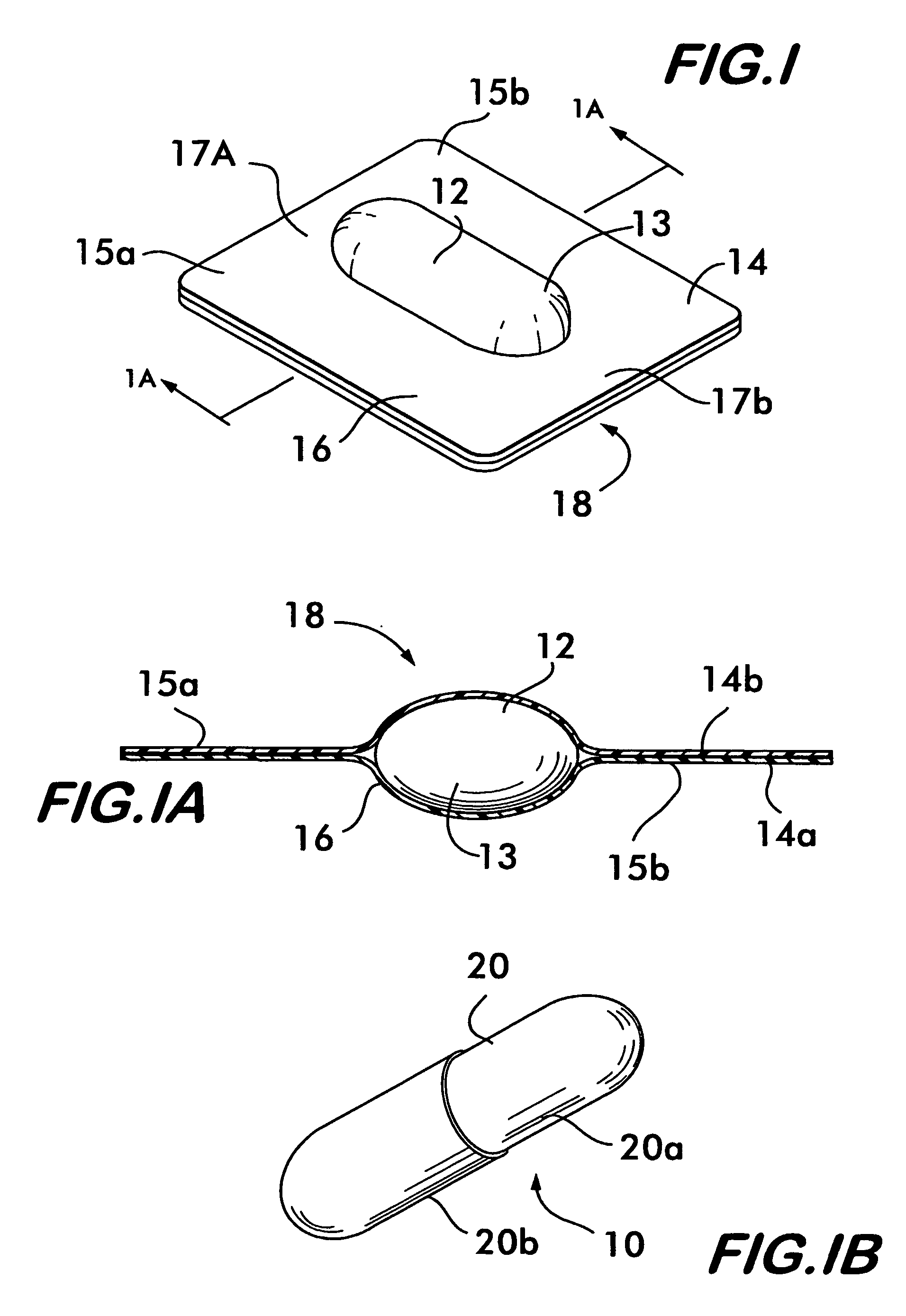
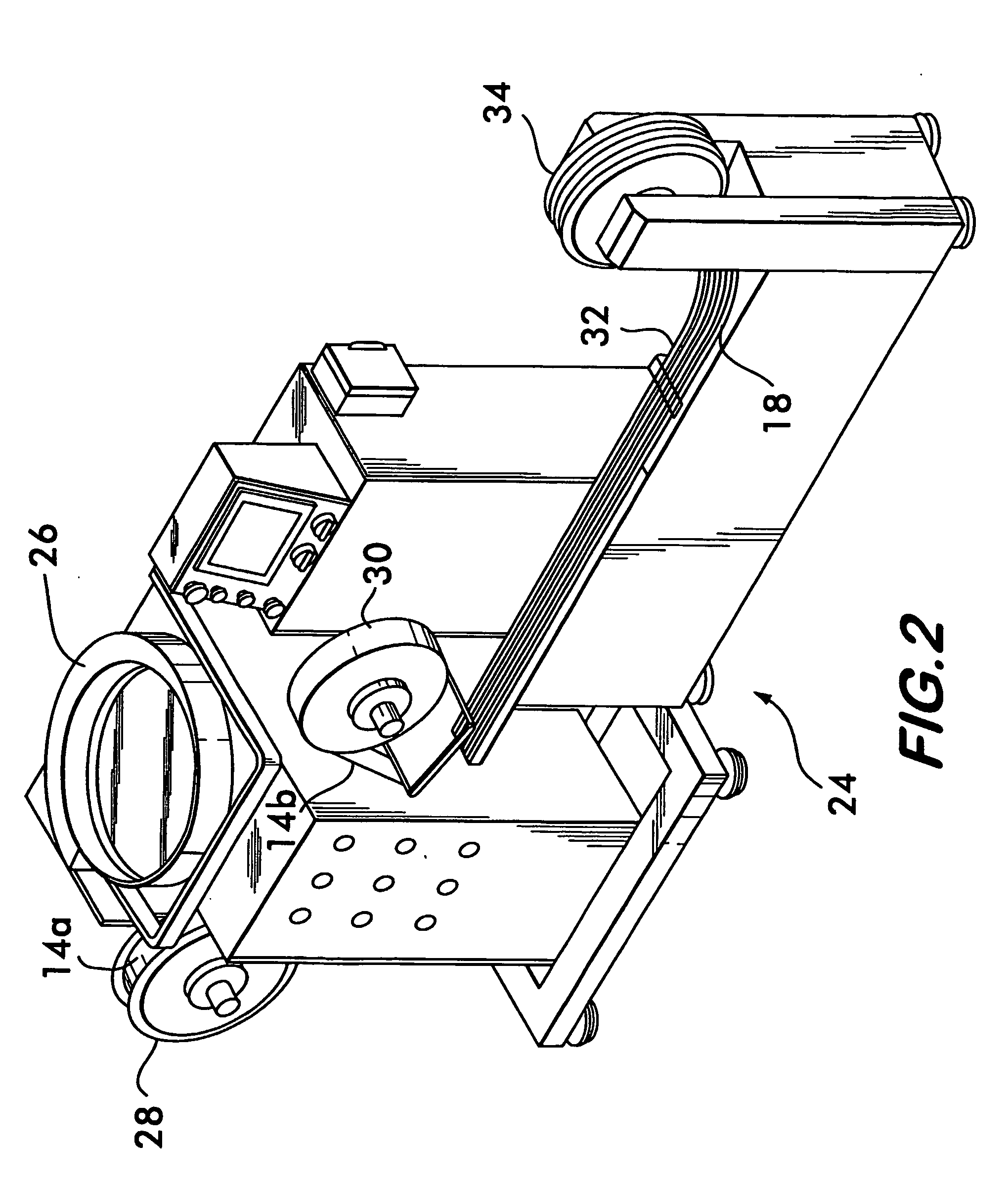
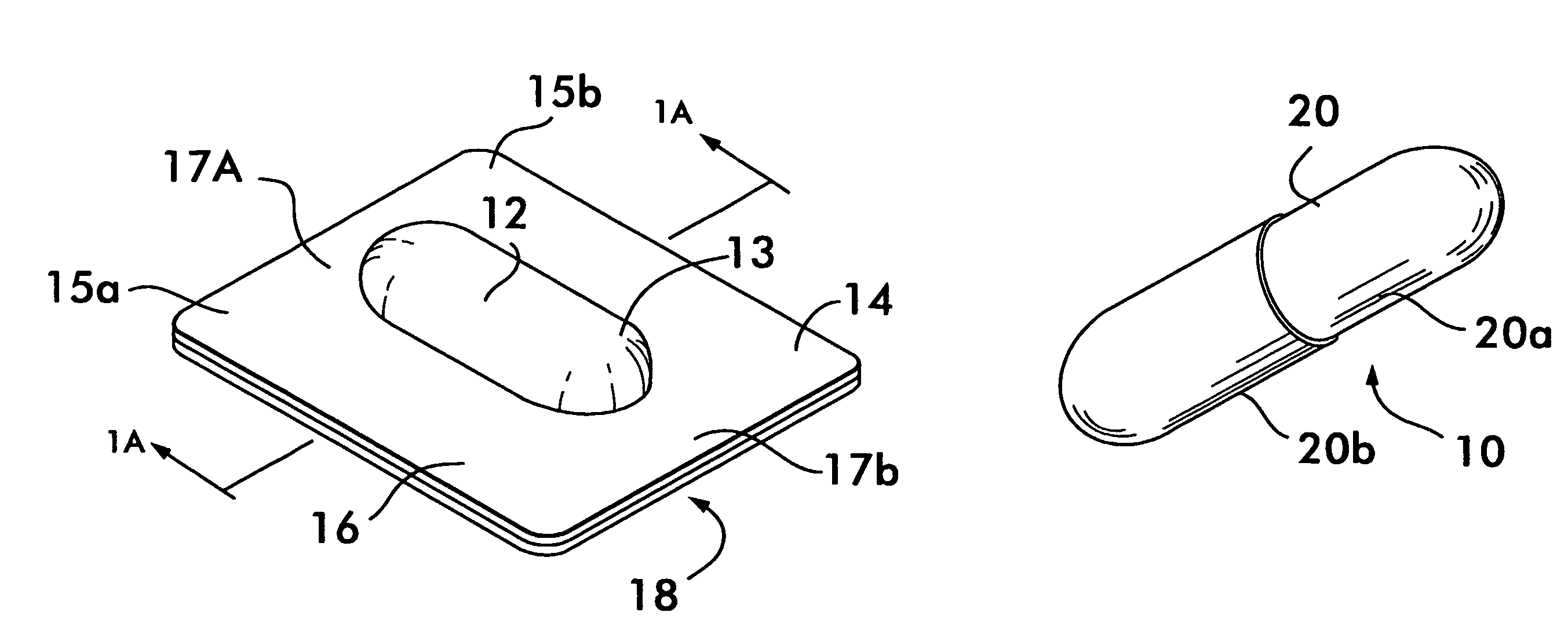
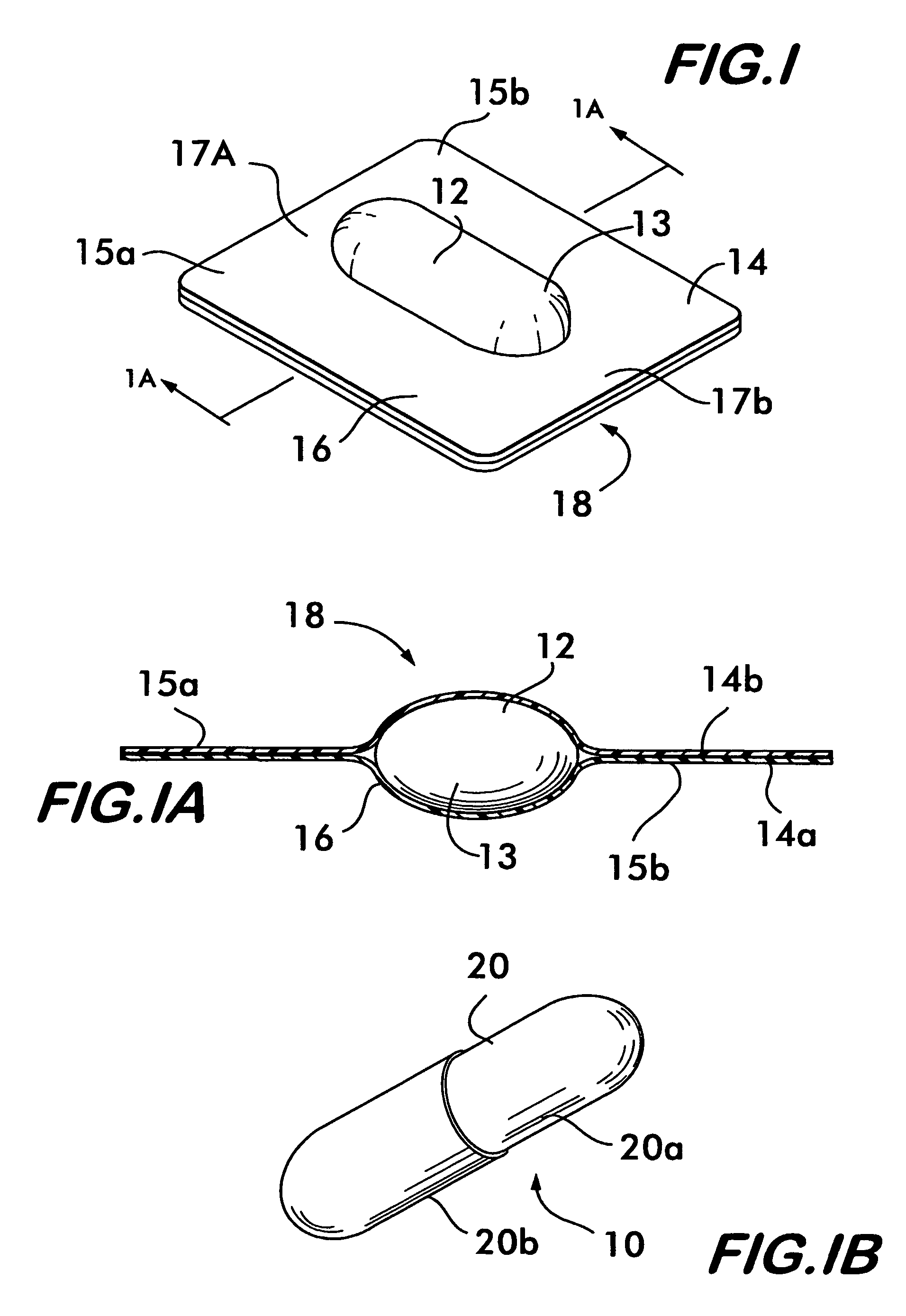
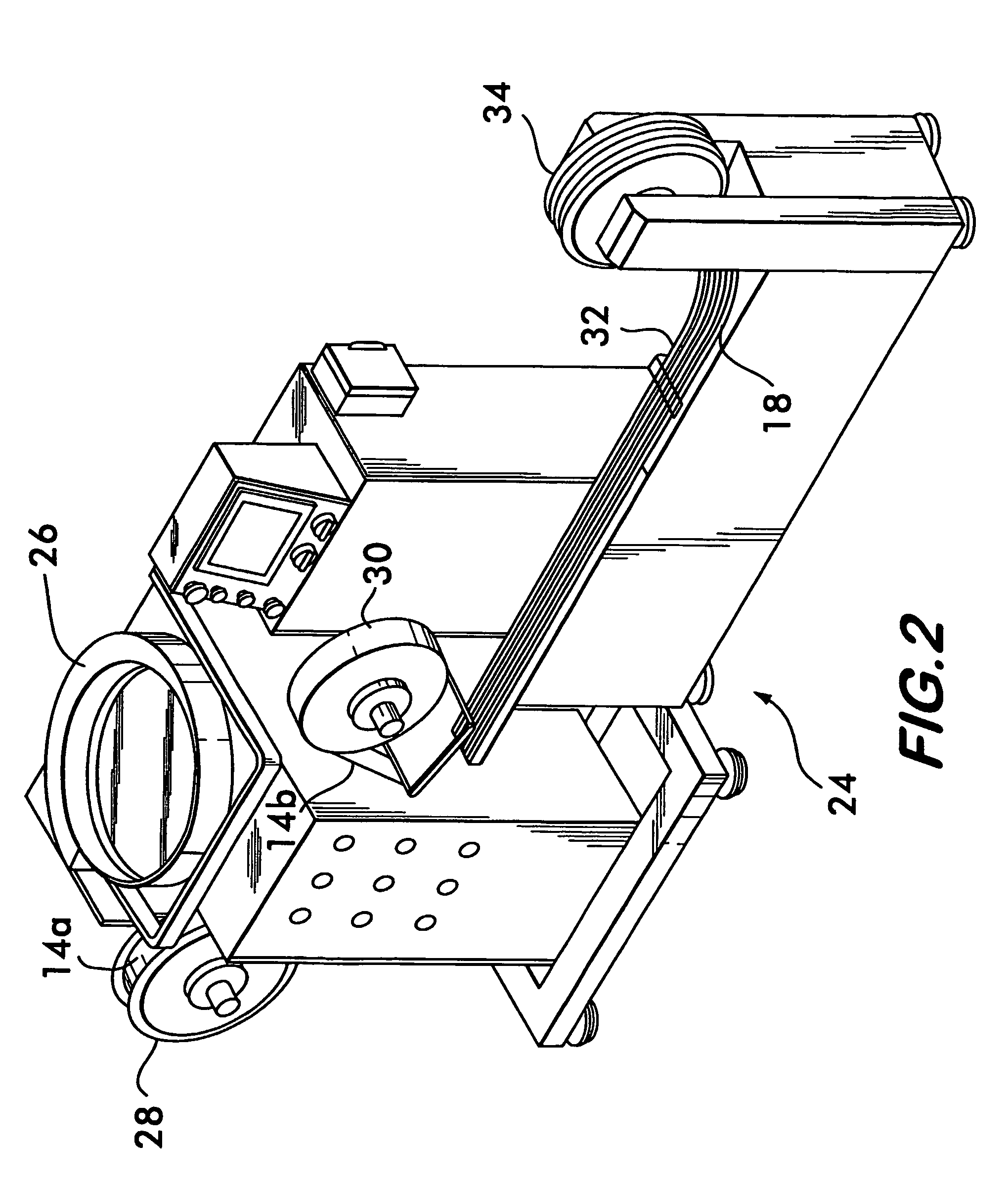
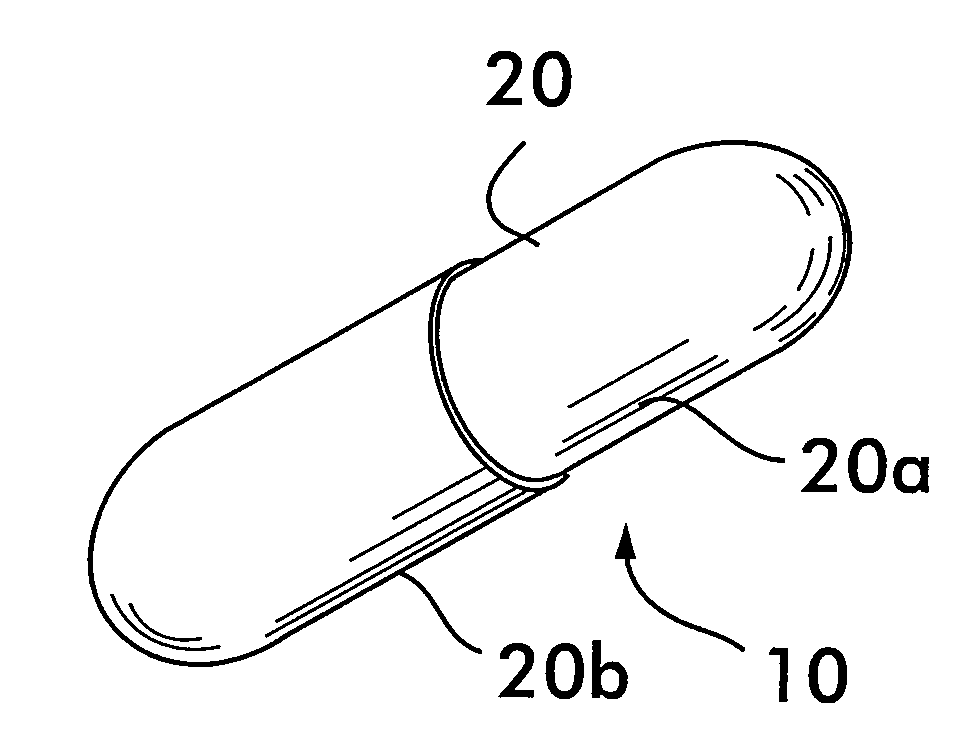
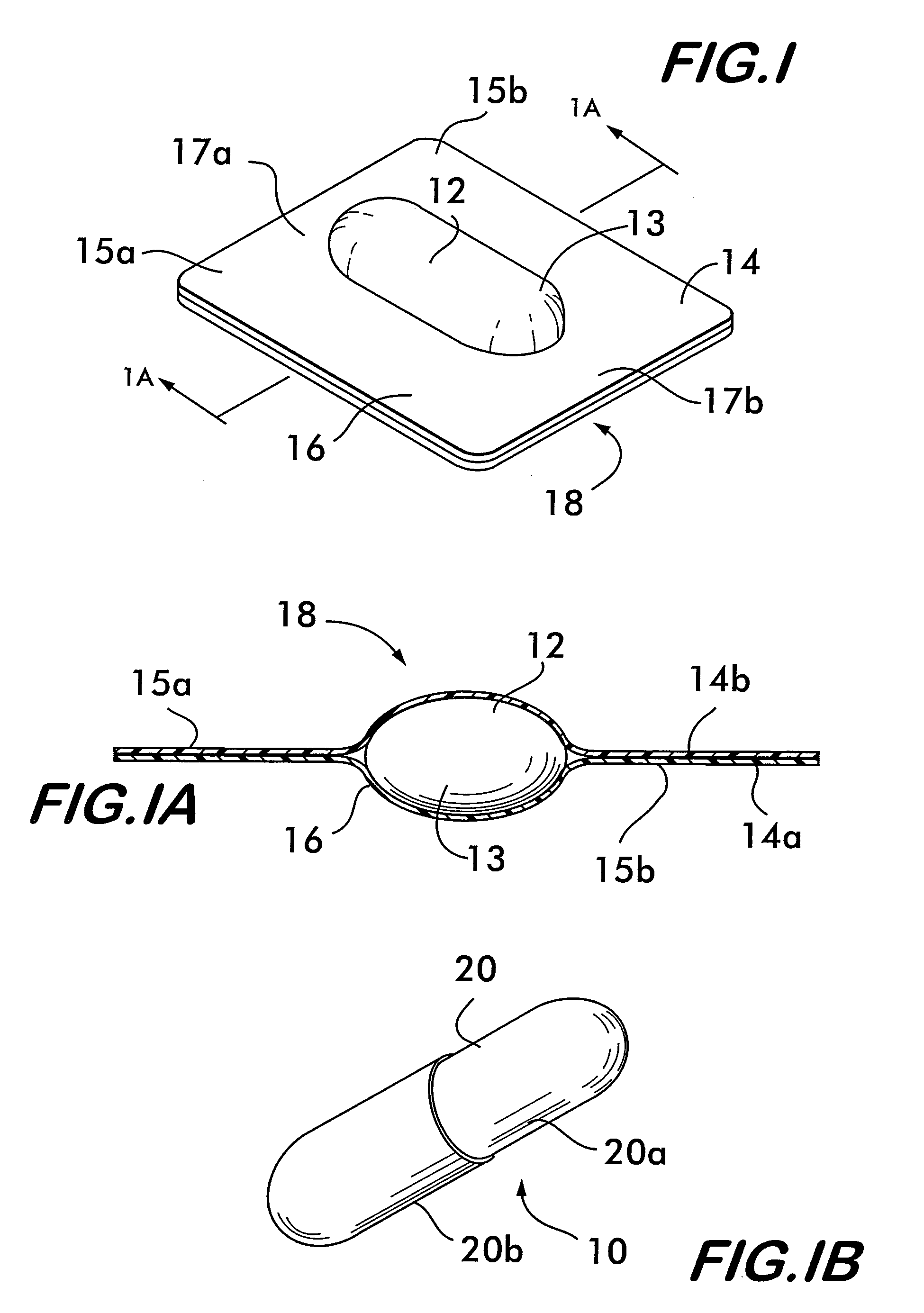
Process and machine for automated manufacture of gastro-retentive capsules
An automated process and apparatus for making a gastro-retentive device (10). The method includes the steps of providing a pouch assembly having an ingredient section within a membrane; folding the membrane to form a folded pouch assembly; inserting the folded pouch assembly into a first capsule section (20a) to form a pouch / first capsule assembly, and inserting the pouch / first capsule assembly into a second capsule section (20b). The process can further include the steps of providing a strip (32) of multiple pouch assemblies (18) and cutting a single pouch assembly from the strip (32). Also provided is an apparatus (38) for carrying out the above method which includes a tooling block (44) having a passageway (62) configured for slidable movement of the pouch assembly (18) therein, and a tooling pocket (60) extending from a top surface of the tooling block to the passageway and which receives the pouch assembly. A ram (86) is provided for pushing the pouch assembly through the tooling pocket into the passageway, wherein the pouch assembly is folded and encapsulated.
Owner:MERRION RES I
Expandable gastroretentive dosage form
ActiveUS10485758B2Volume maximizationMaximize weightMedical devicesPill deliveryGastro retentiveGastric juices
An oral gastro-retentive delivery device is provided which unfolds rapidly upon contact with gastric juice. The device is configured in a collapsed configuration for oral intake and unfolding for gastric retention for a predetermined period of time and eventually reducing in size for passage through the rest of the GI track.
Owner:CLEXIO BIOSCIENCES LTD
Tolperisone controlled release tablet
InactiveUS20100249423A1Organic active ingredientsOrganic chemistryGastro retentiveControlled Release Tablet
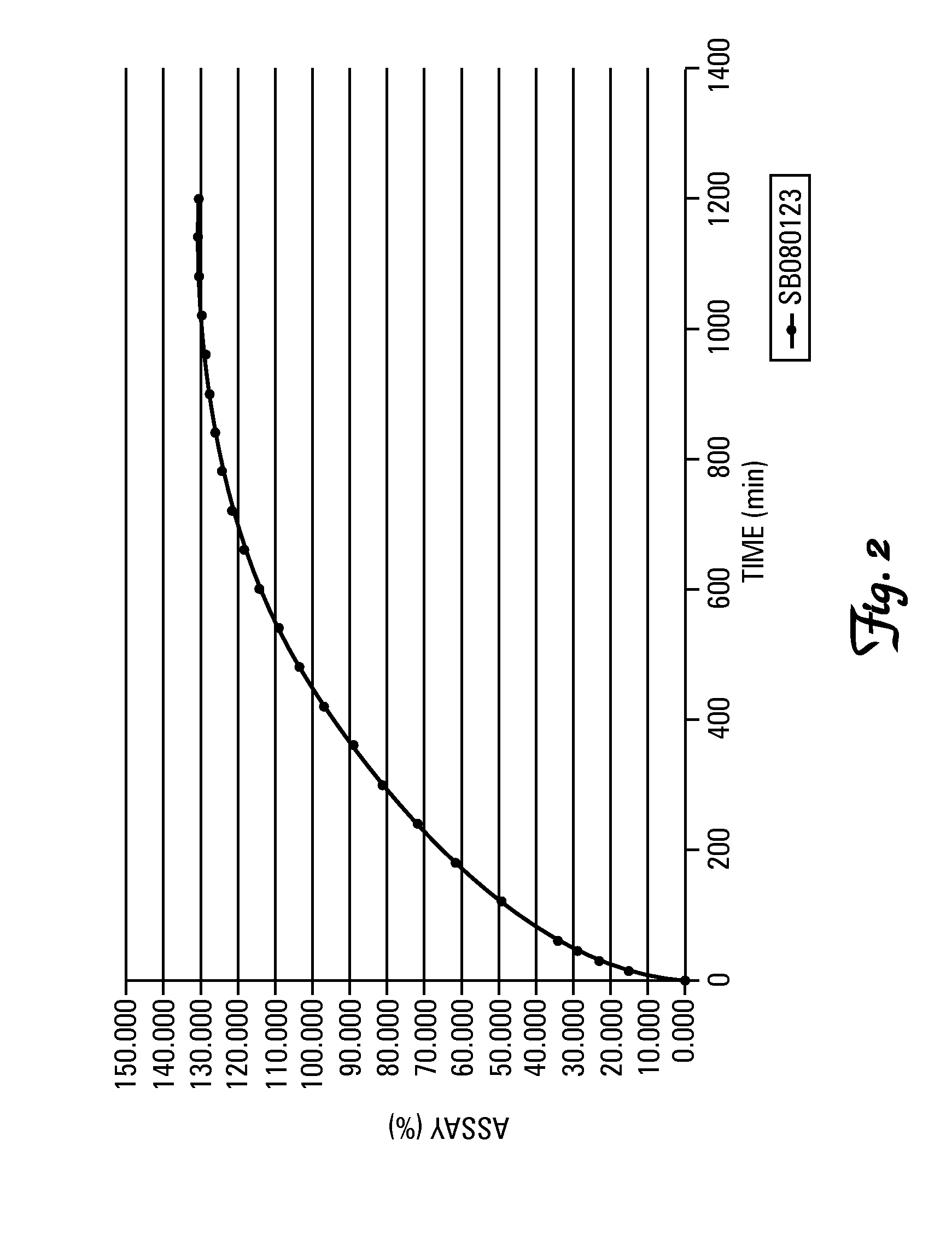
A GRDDS (Gastro Retentive Drug Delivery System) containing tolperisone and having a 2-methyl-1-(4-methylphenyl)-propenone (4-MMPPO) fraction of less than 1.5 ppm for the extended release of the active substance tolperisone while avoiding the formation of 4-MMPPO in the gastrointestinal tract.
Owner:SANOCHEMIA PHARMA AG
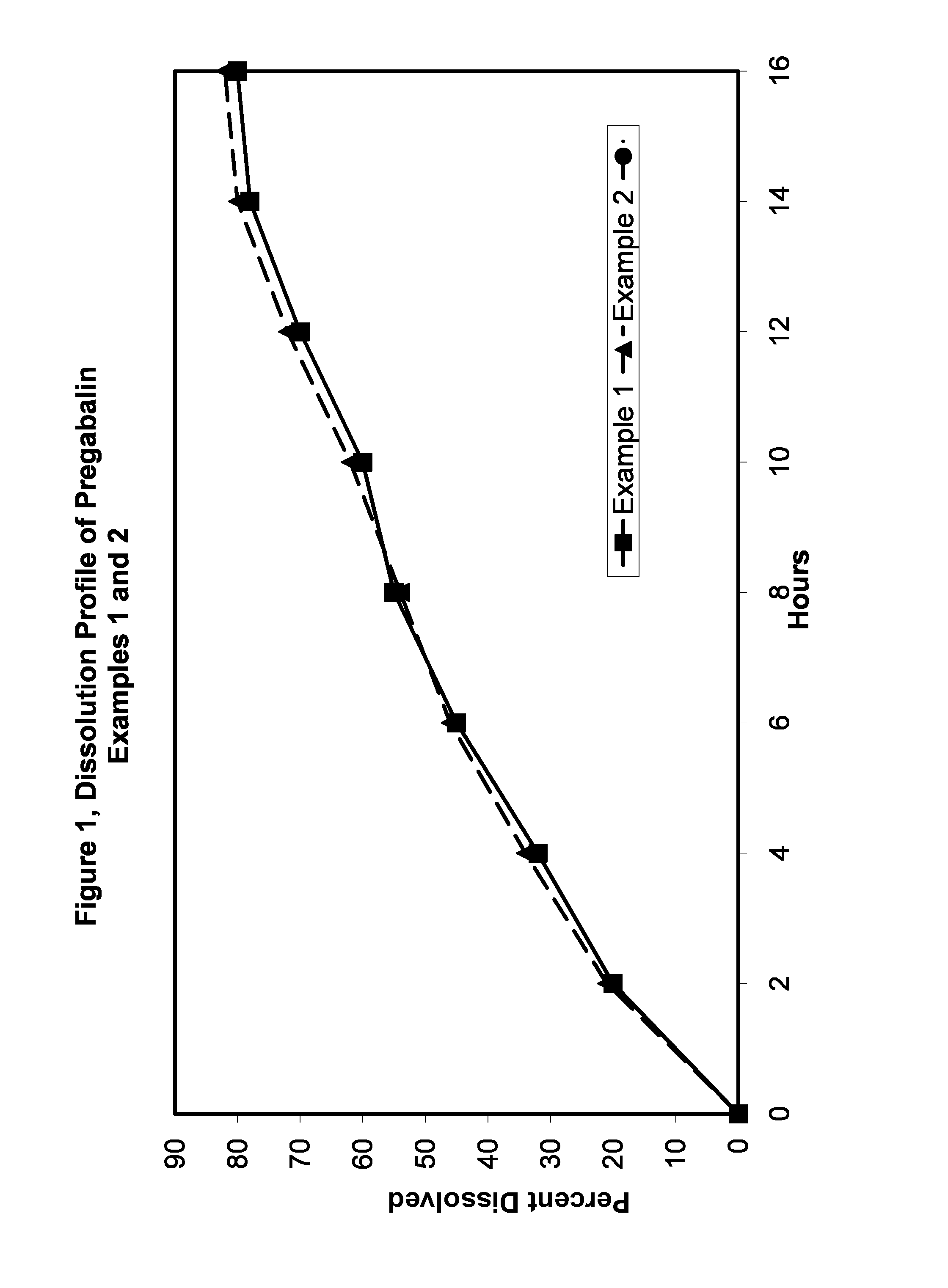
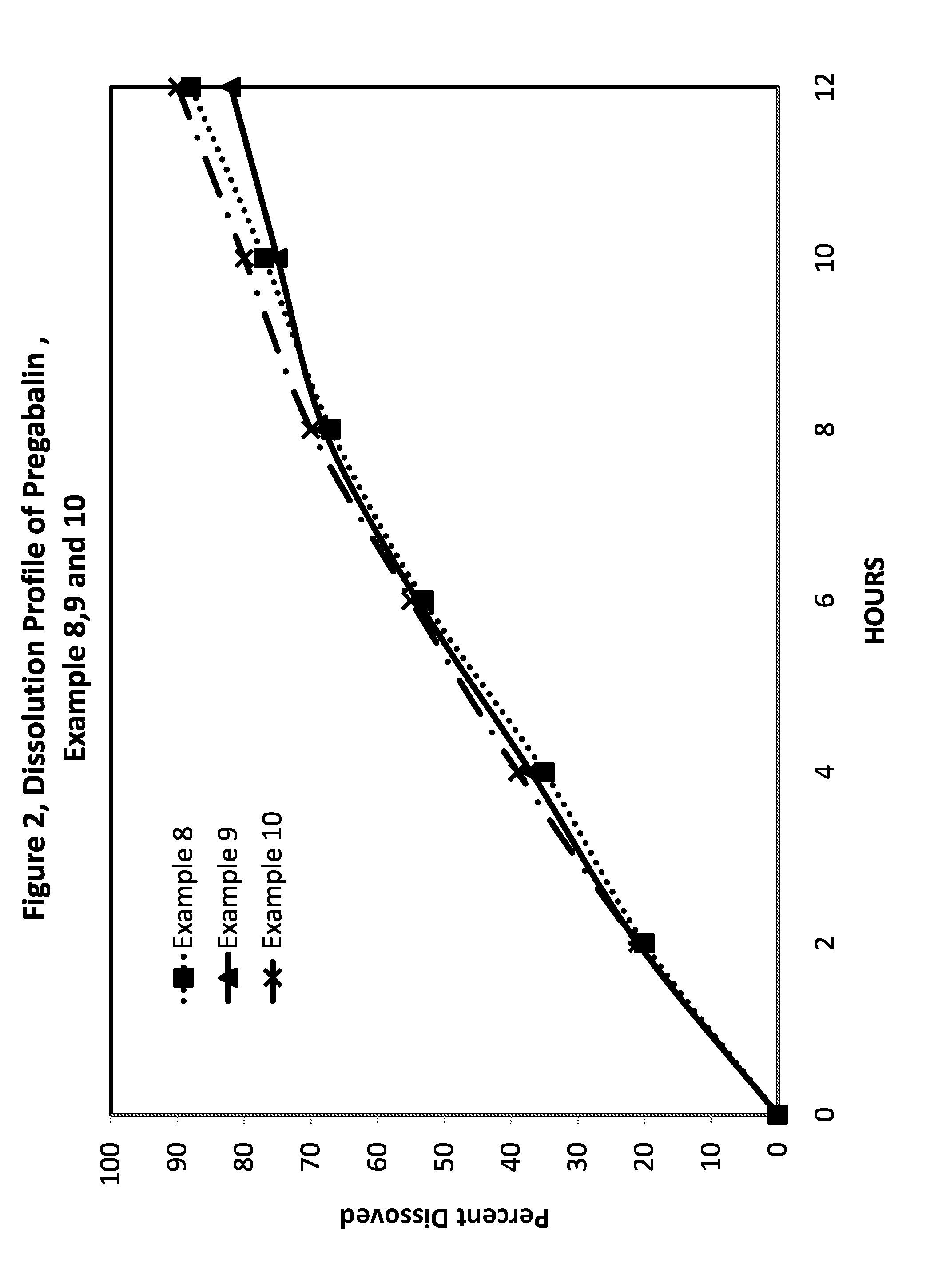
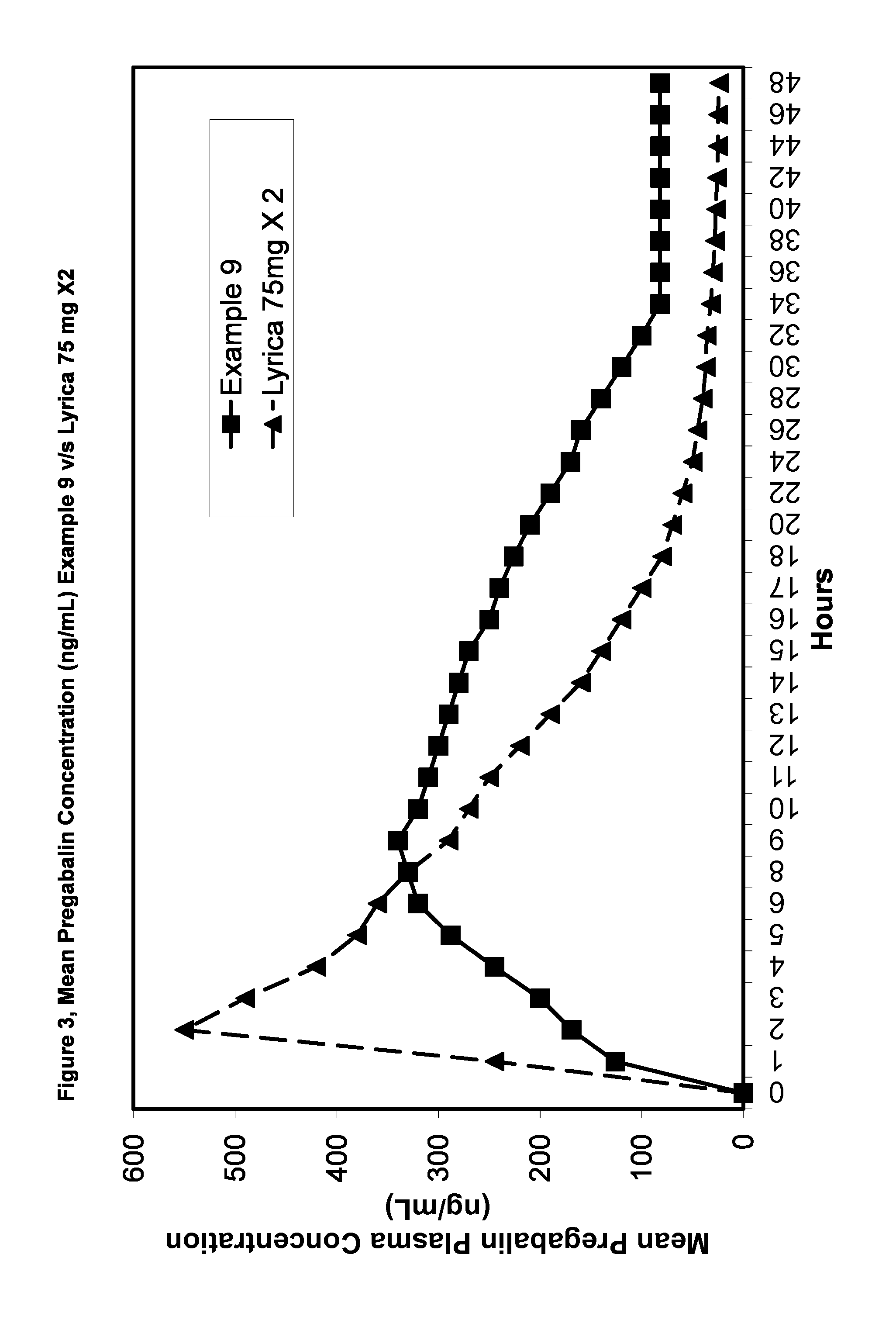
Pharmaceutical compositions containing pregabalin
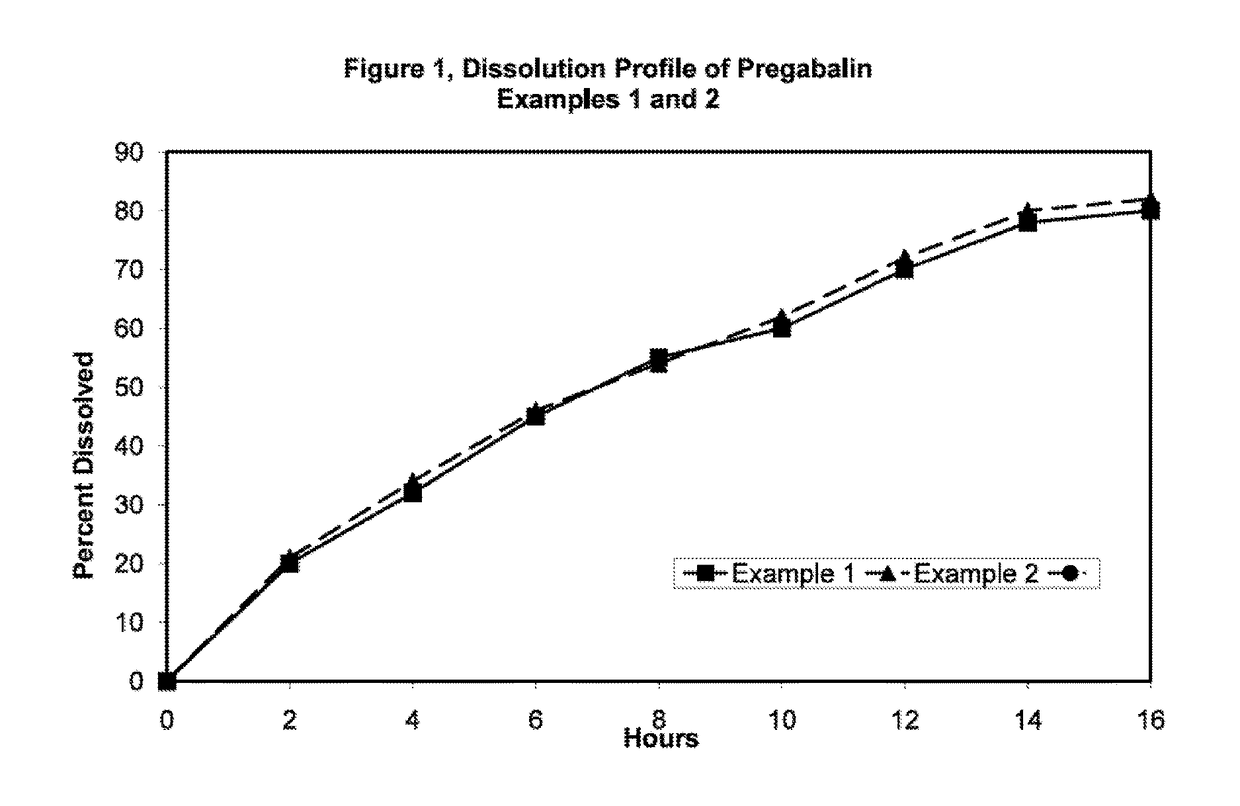
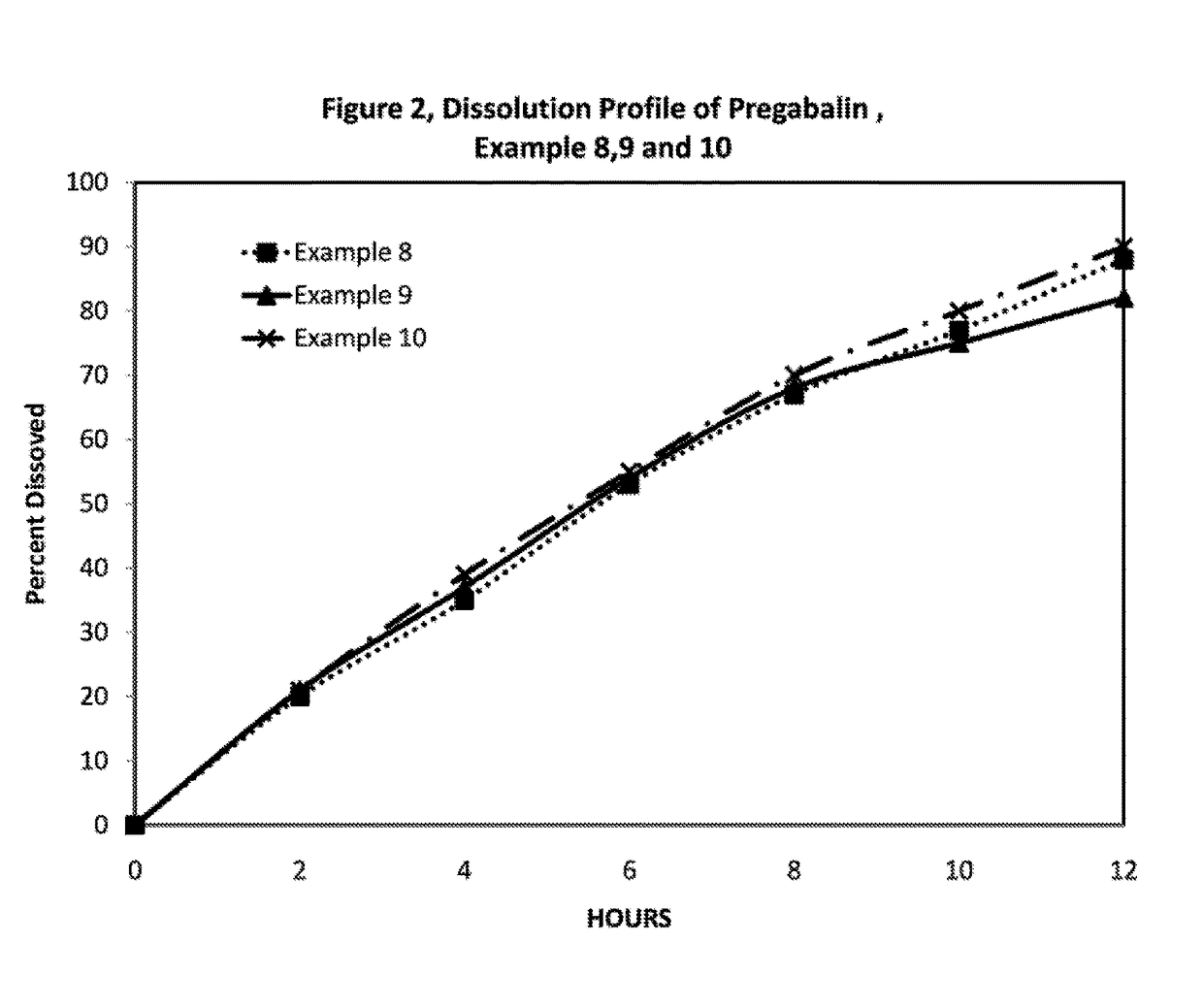
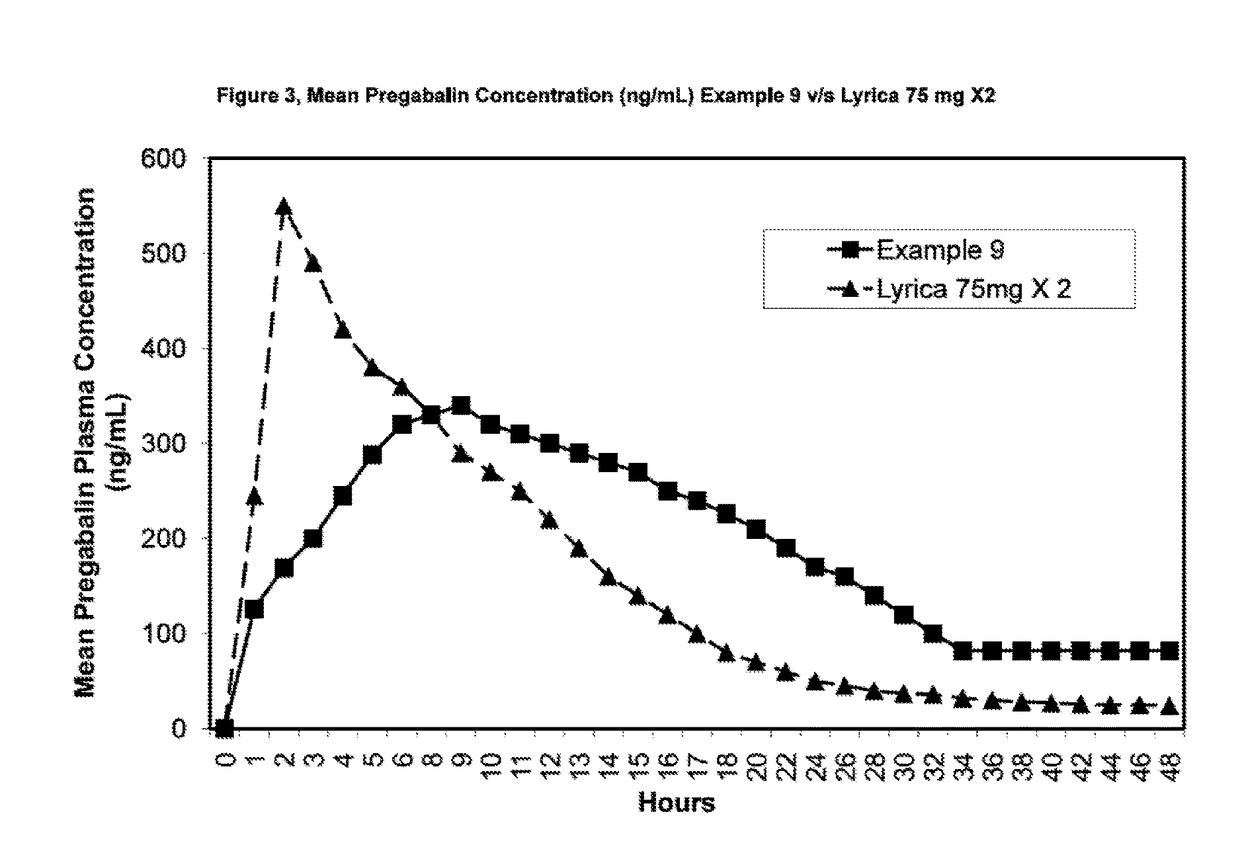
InactiveUS20110135723A1Widens the absorption windowBiocideOrganic active ingredientsPregabalinGastro retentive
The present invention provides pharmaceutical composition comprising pregabalin that is useful for once daily oral dosing. The present invention further relates to pharmaceutical composition comprising pregabalin that is useful for once daily oral dosing comprising pregabalin and on or more water insoluble components. The present invention preferably relates to a gastro-retentive tablet comprising pregabalin and one or more water insoluble component wherein water insoluble component preferably comprises of combination of ethylcellulose and hydrogenated castor oil.
Owner:MICRO LABS
Novel gastro-retentive dosage forms
InactiveUS20120009261A1Reduce doseSubstantial patient complianceBiocidePowder deliveryDrugGastro retentive
A gastro-retentive pharmaceutical dosage form of a therapeutically effective amount of at least one GABA Analog, at least one opioid, and at least one pharmaceutically acceptable excipient wherein the said dosage form is retained in the stomach for at least four hours and is suitable for formulating for once daily or twice daily administration. Further provided is a method of treating a disorder by administering to a patient in need thereof, a gastro-retentive pharmaceutical dosage form of a therapeutically effective amount of at least one GABA Analog, at least one opioid, and at least one pharmaceutically acceptable excipient wherein the said dosage form is retained in the stomach for at least four hours and is suitable for once daily or twice daily administration. The opioid is either in slow release form or in immediate release form.
Owner:GRUNENTHAL GMBH
Process and machine for automated manufacture of gastro-retentive devices
An automated process and apparatus for making a gastro-retentive device (10). The method includes the steps of providing a pouch assembly having an ingredient section within a membrane; folding the membrane to form a folded pouch assembly; inserting the folded pouch assembly into a first capsule section (20a) to form a pouch / first capsule assembly, and inserting the pouch / first capsule assembly into a second capsule section (20b). The process can further include the steps of providing a strip (32) of multiple pouch assemblies (18) and cutting a single pouch assembly from the strip (32).Also provided is an apparatus (38) for carrying out the above method which includes a tooling block (44) having a passageway (62) configured for slidable movement of the pouch assembly (18) therein, and a tooling pocket (60) extending from a top surface of the tooling block to the passageway and which receives the pouch assembly. A ram (86) is provided for pushing the pouch assembly through the tooling pocket into the passageway, wherein the pouch assembly is folded and encapsulated.
Owner:MERRION RES III LTD
Gastro-retentive formulations
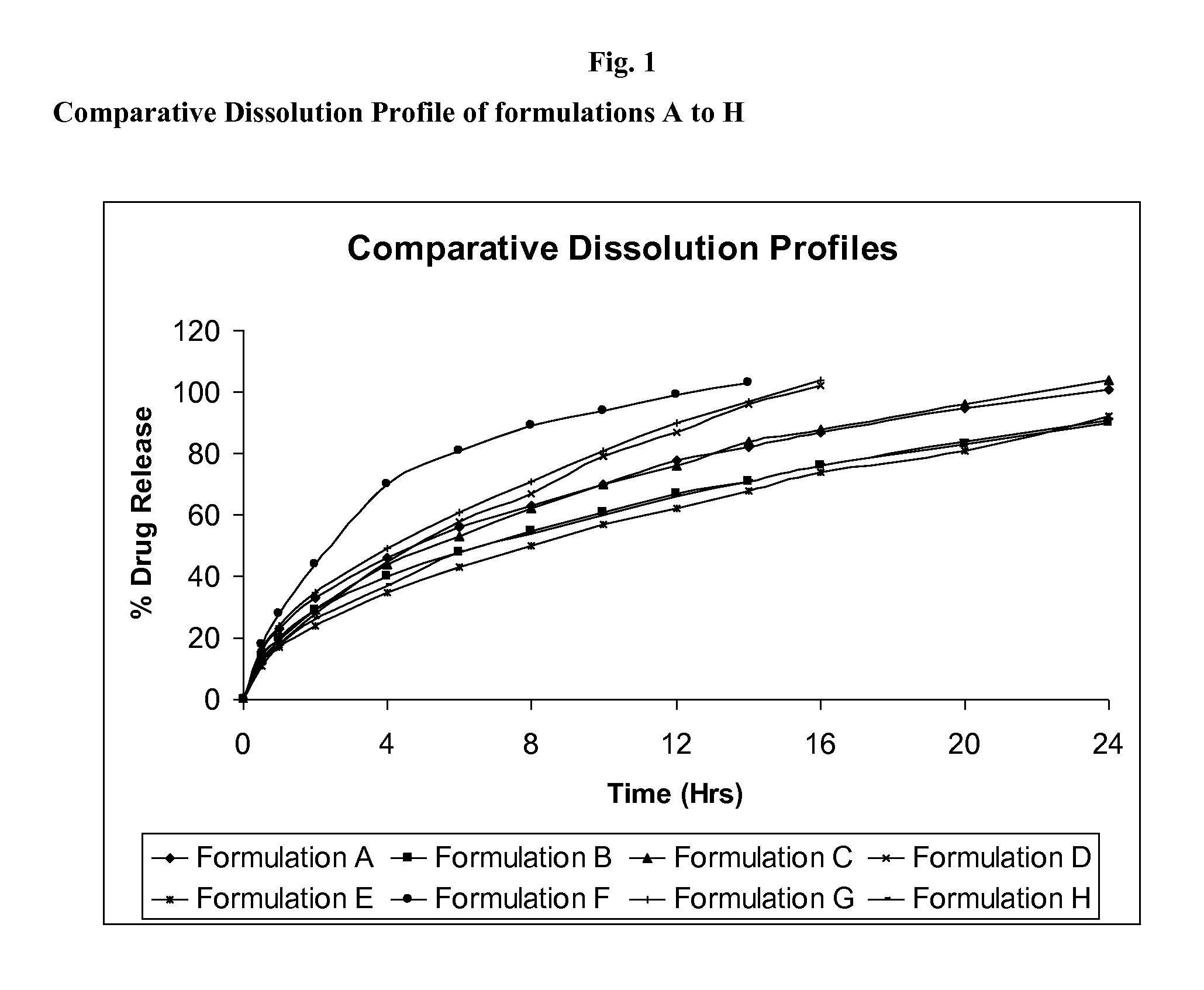
The present invention relates to pharmaceutical compositions of the poorly soluble drugs, and pharmaceutically acceptable salts thereof, in a controlled-release gastric retained oral dosage form. Such compositions are formulated so as to deliver the majority of the incorporated drug into the stomach and upper gastrointestinal tract, with restricted drug delivery in the lower gastrointestinal tract. The dosage forms have multiple layers including an active layer with a first swellable polymer with raltegravir incorporated therein and a non-active layer with a second swellable polymer having a similar molecular weight or a higher molecular weight as the swellable polymer in the active layer.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical gastro-retentive solid oral dosage form of nilotinib
The present invention relates to a pharmaceutical gastro-retentive solid oral dosage form comprising nilotinib as the active ingredient. The invention is further related to methods of preparing said dosage form.
Owner:RANBAXY LAB LTD
Expandable gastroretentive dosage form
ActiveUS20190365646A1Volume maximizationMaximize weightMedical devicesPill deliveryGastro retentiveGastric juices
An oral gastro-retentive delivery device is provided which unfolds rapidly upon contact with gastric juice. The device is configured in a collapsed configuration for oral intake and unfolding for gastric retention for a predetermined period of time and eventually reducing in size for passage through the rest of the GI track.
Owner:CLEXIO BIOSCIENCES LTD
Process for making multiparticulate gastroretentive dosage forms
The instant: invention relates to a process for making inherent Sow density particles., comprising the steps of (i) providing a powder mixture comprising a swelling agent; (ii) granulating the powder of step (i) with a granulating solution comprising a lipophilie agent into granules and (iii) drying the granules of step (ii). The instant invention further relates to multiparticulate oral gastro-retentive dosage forms comprising the inherent low density particles: obtainable by the process.
Owner:MELIATYS
Process and machine for automated manufacture of gastro-retentive devices
An improved automated process and apparatus for making a gastro-retentive device (10). The method includes the steps of providing a pouch assembly (18) having an ingredient section within a membrane; rotating the membrane to form a folded pouch assembly; inserting the folded pouch assembly into a first capsule section (20a) to form a pouch / first capsule assembly, and inserting the pouch / first capsule assembly into a second capsule section (20b). The process can further include the steps of providing a continuous strip (32) of multiple pouch assemblies (18) and cutting a single pouch assembly (18) from the strip (32).Also provided is an apparatus (100) for carrying out the above method which includes three wheels (102, 104, 106) for processing and moving the capsule sections (20a, 20b), a pouch load subassembly (166) for delivering a strip (32) of pouch assemblies (18) to a pouch load tooling mechanism (168), a pouch wrapping subassembly which, after receiving the pouches (18) from the pouch load tooling mechanism (168) wraps the flaps of the pouches which are then, via a pouch insert subassembly 200, inserted into the first capsule section (20a) in one of the wheels (104). The second capsule section (20b) is then combined with the first capsule section (20a) in the wheel (104) to complete the finished gastro-retentive device (10).
Owner:MERRION RES I
Gastro-retentive sustained-release oral dosage form of a bile acid sequestrant
InactiveCN105338958APrevent backflowAvoid damageOrganic active ingredientsPeptide/protein ingredientsDiseaseHistamine h2 receptor antagonist
Disclosed herein are novel compositions and methods for controlling the release of bile acid sequestrant to the stomach in order to treat or prevent upper GI tract disorders or disorders of the throat. The methods generally include administering to a patient in need thereof a therapeutically effective amount of a pharmaceutical composition comprising at least one bile acid sequestrant dispersed in a polymeric matrix. The bile acid sequestrant composition may be administered alone or in combination with at least one proton pump inhibitor, and optionally one or more agents chosen from antacids, histamine H2-receptor antagonists, gamma-aminobutyric acid-beta (GABA-B) agonists, prodrugs of GABA-B agonists, acid pump antagonists, protease inhibitors and GC-C agonists.
Owner:IRONWOOD PHARMA
Pharmaceutical gastro-retentive solid oral dosage form of nilotinib
The present invention relates to a pharmaceutical gastro-retentive solid oral dosage form comprising nilotinib as the active ingredient. The invention is further related to methods of preparing said dosage form.
Owner:RANBAXY LAB LTD
Gastro-retentive oral pharmaceutical compositions
The invention relates to a pharmaceutical matrix tablet which can be administered orally once or twice per day with gastro-retentive controlled release of Baclofen.
Owner:ETHYPHARM SA
Gelling agent-based dosage form
ActiveUS9452135B2Easily administered and swallowedPrevent extractionPowder deliveryOrganic active ingredientsLarge doseBULK ACTIVE INGREDIENT
The present invention generally relates to dosage forms for oral administration including one or more gelling agents. In particular, the present invention is directed to gelling agent-based dosage forms that are easily administered and taken, or swallowed. The present invention is also directed to gelling agent-based dosage forms that exhibit relatively low syneresis, are thermally stable, exhibit substantially constant active ingredient concentration, and / or exhibit one or more advantageous rheological properties. In particular, the present invention is directed to such gels containing one or more omega-3 fatty acids. The gelling agent-based dosage forms of the present invention are suitable for administration of a relatively large dose of active ingredient. The gelling agent-based dosage forms of the present invention are also suitable for administration of multiple active ingredients. Dosage forms of the present invention also provide tamper resistance and, thus, prevent recovery or diversion of active ingredients contained therein. The gelling agent-based dosage forms are also suitable for use as gastro-retentive and sustained release dosage forms.
Owner:PARTICLE DYNAMICS INT
Process for making gastroretentive dosage forms
The present invention relates to a novel process for making oral solid gastro-retentive forms, comprising the steps of providing a powder mixture comprising a hydrophobic powder, overgranulating this powder mixture with a granulating solution into an overgranulated paste, and drying said paste into a solid, as well as to pharmaceutical solid dosage forms which are retained in the stomach or upper gastrointestinal tract for a controlled delivery of a drug.
Owner:地中海大学
Gastro-retentive drug delivery system
InactiveUS20150202158A1Enhancing gastro-rentention and gastric residence timePromote absorptionOrganic active ingredientsNervous disorderGastro retentiveBULK ACTIVE INGREDIENT
The invention relates to floating drug delivery systems(FDDS) that provide solutions to the particular problems often encountered with floating drug delivery systems described in the art. On such generally recognized problem is the vulnerability of the systems, especially damage to the gas-filled compartment making it accessible to water so as to impair its buoyancy, ultimately resulting in insufficient gastric residence time. The invention, in an aspect, provides a self-repairing FDDS that maintains its floating capacity after damaging. The floating drug delivery systems of the invention, furthermore,allow for incorporation of high loads of active ingredients. The floating drug delivery systems can be designed in such a way that release of active ingredient from the system occurs entirely independent from the pH of the fluid surrounding the system. Furthermore, the procedure of manufacturing the floating drug delivery system of the invention is simple and straightforward, and therefore economically attractive.
Owner:APET HLDG
Oral gastroretentive formulations and uses thereof
InactiveUS20190224118A1Extended absorption timeNervous disorderHydroxy compound active ingredientsCannabinoidGastro retentive
Owner:INTEC PHARMA
Enteric-coated gastro-retentive oral dosage form in tablet form, and application and pharmaceutical composition thereof
PendingCN108057028APrevent backflowAvoid damageOrganic active ingredientsPeptide/protein ingredientsPhosphateColesevelam Hydrochloride
The invention relates to the field of medicine, and specifically discloses an enteric-coated gastro-retentive oral dosage form in tablet form, and applications and a pharmaceutical composition thereof. The oral dosage form includes: a. a bile acid chelating agent selected from colesevelam and colesevelam hydrochloride; b. a polymeric matrix comprising a poly(alkylene), the bile acid chelating agent being dispersed in the polymeric matrix; c. one or more fillers or compression agents selected from microcrystalline cellulose, lactose, starch, maltodextrin, and dicalcium phosphate, wherein the oral dosage form slowly releases the bile acid chelating agent into a stomach. The dosage form is capable of providing the bile acid chelating agent to the stomach in long-lasting and stable level, theconcentration of the bile acid chelating agent permitting the bile acid chelating agent to optimally combine with bile acids refluxing from a small intestine to the stomach, thereby avoiding damages of a gastric mucosa caused by bile acids, and preventing the bile acids in the stomach from refluxing to an esophagus and other parts of an upper digestive tract and a throat, to prevent further damage.
Owner:IRONWOOD PHARMA
Gastro-retentive oral pharmaceutical compositions
The invention relates to a pharmaceutical matrix tablet which can be administered orally once or twice per day with gastro-retentive controlled release of Baclofen.
Owner:ETHYPHARM SA
Hydrodynamically balancing oral drug delivery system
The present invention relates to an oral gastric retention drug delivery system comprising a highly porous matrix comprising at least one drug, a sugar, a gas generating component and optionally pharmaceutically acceptable adjuvant components. The pharmaceutical composition is either in the form of pills (multiparticulate or single unit dosage form), beads, granules or capsules, which are retained in the stomach and selectively released in the stomach and upper small intestine over a prolonged period of time.
Owner:RANBAXY LAB LTD
Novel gastro-retentive dosage forms
A gastro-retentive pharmaceutical dosage form of a therapeutically effective amount of at least one GABA Analog, at least one opioid, and at least one pharmaceutically acceptable excipient wherein the said dosage form is retained in the stomach for at least four hours and is suitable for formulating for once daily or twice daily administration. Further provided is a method of treating a disorder by administering to a patient in need thereof, a gastro-retentive pharmaceutical dosage form of a therapeutically effective amount of at least one GABA Analog, at least one opioid, and at least one pharmaceutically acceptable excipient wherein the said dosage form is retained in the stomach for at least four hours and is suitable for once daily or twice daily administration. The opioid is either in slow release form or in immediate release form.
Owner:GRUNENTHAL GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com