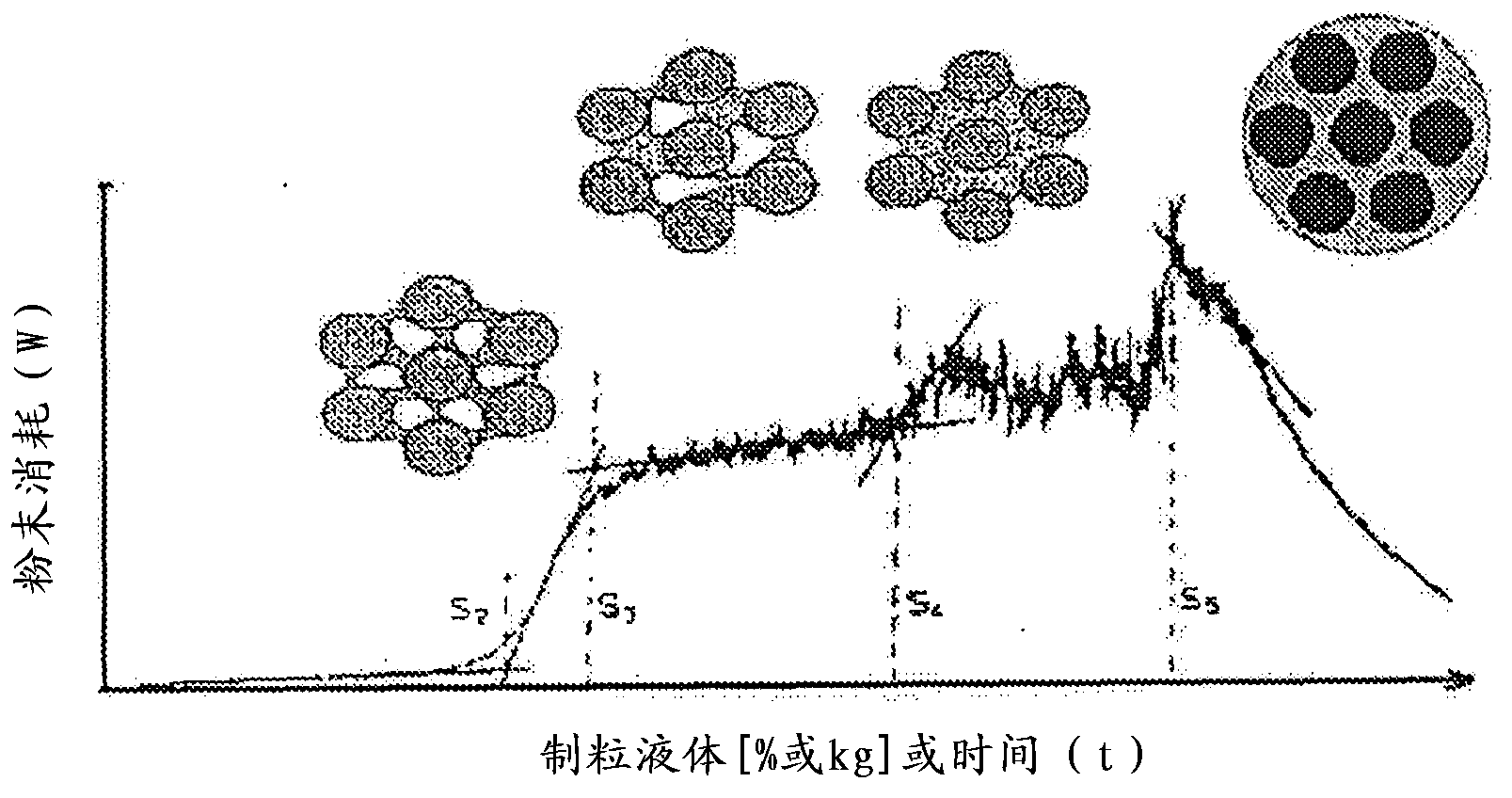
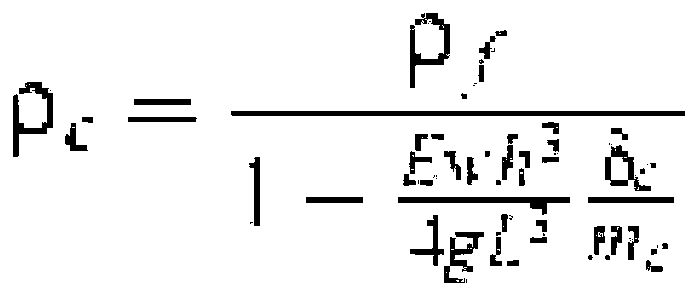Process for making multiparticulate gastroretentive dosage forms
A technology of multi-particles and granules, which is applied in the direction of medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc., and can solve problems such as inability to mix or obtain super-granular pastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Embodiment 1: the preparation of multiparticulate oral gastric retention dosage form of the present invention
[0111] The multiparticulate oral gastroretentive dosage form of the present invention can be prepared according to the following method, but not limited to the following examples.
[0112] In one aspect, a powder made of 55g of acetaminophen (active ingredient) together with 40g of HPMC and about 5g of PVPK30 was loaded into a planetary mixer and blended at 150rpm during 2min30sec. On the other hand, a granulation suspension was prepared by dissolving about 10 g of PVP K30 in 200 ml of an aqueous solution containing 15 g of Aerosil R972. The suspension was prepared by using an Ultra turax mixer.
[0113]Granulation was started at 100 rpm by adding the suspension to the powder at a rate of 10 ml / min. Granules were obtained after adding about 130 ml of the solution to the powder. The resulting granules are then dried in a ventilated oven at about 50°C until t...
Embodiment 2
[0115] Embodiment 2: buoyancy
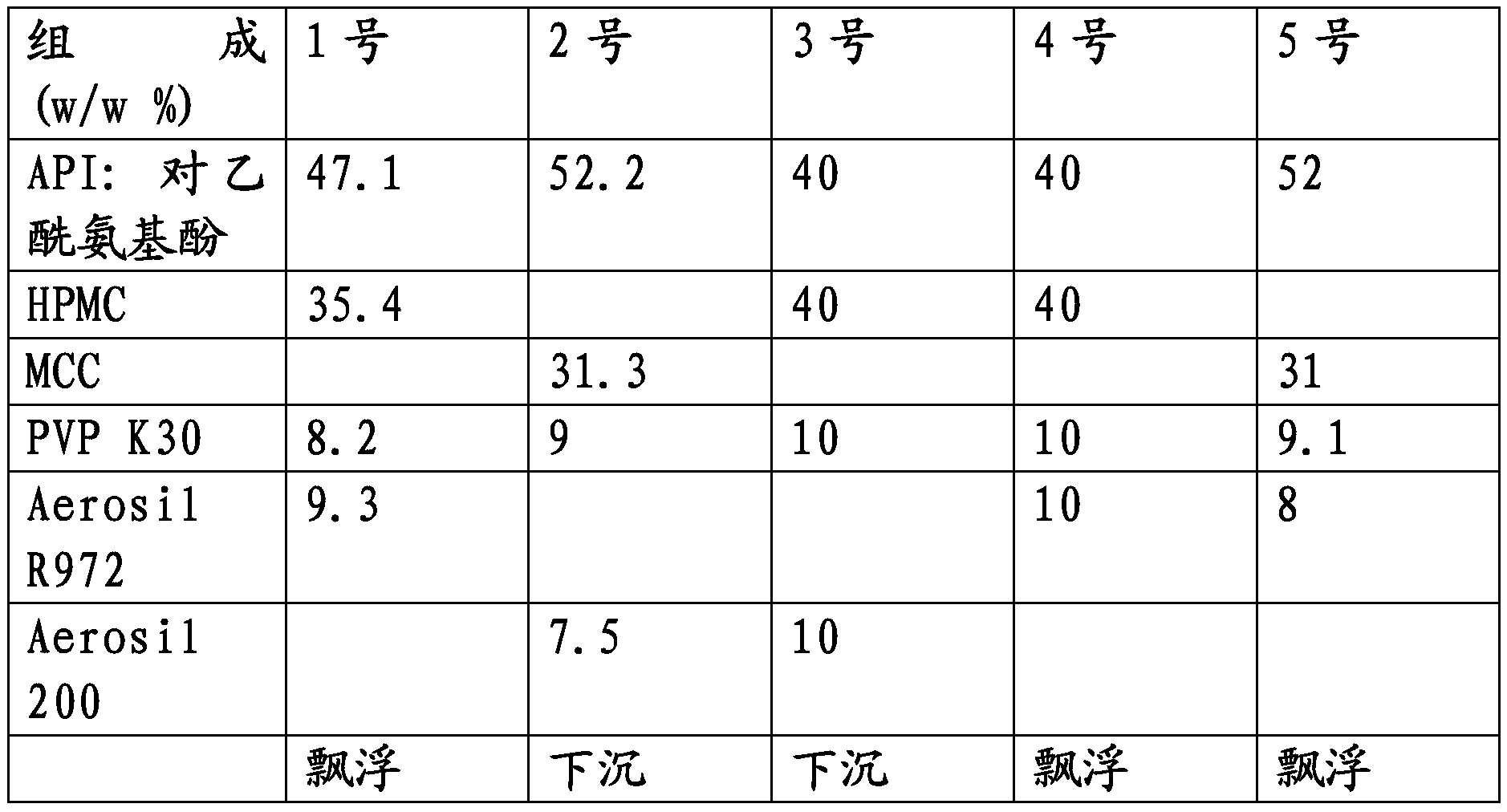
[0116] Five different types of granules of the following composition (based on the weight of the total composition) were tested for buoyancy. Types 1, 4 and 5 were prepared using a hydrophobic excipient (Aerosil R972), while types 2 and 4 were prepared using a hydrophilic excipient (Aerosil 200). The results are shown in the table below.
[0117]
[0118] The results thus show that the buoyant properties of the particles cannot be attributed to the mere presence of the swelling agent (ie HPMC or MCC), as the dosage forms comprising hydrophilic materials do not buoyant. Thus, the use of hydrophobic materials results in an inherently low density of particles.
[0119] Furthermore, it appears that the use of HPMC produces dosage forms with lower densities than those prepared using MCC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com