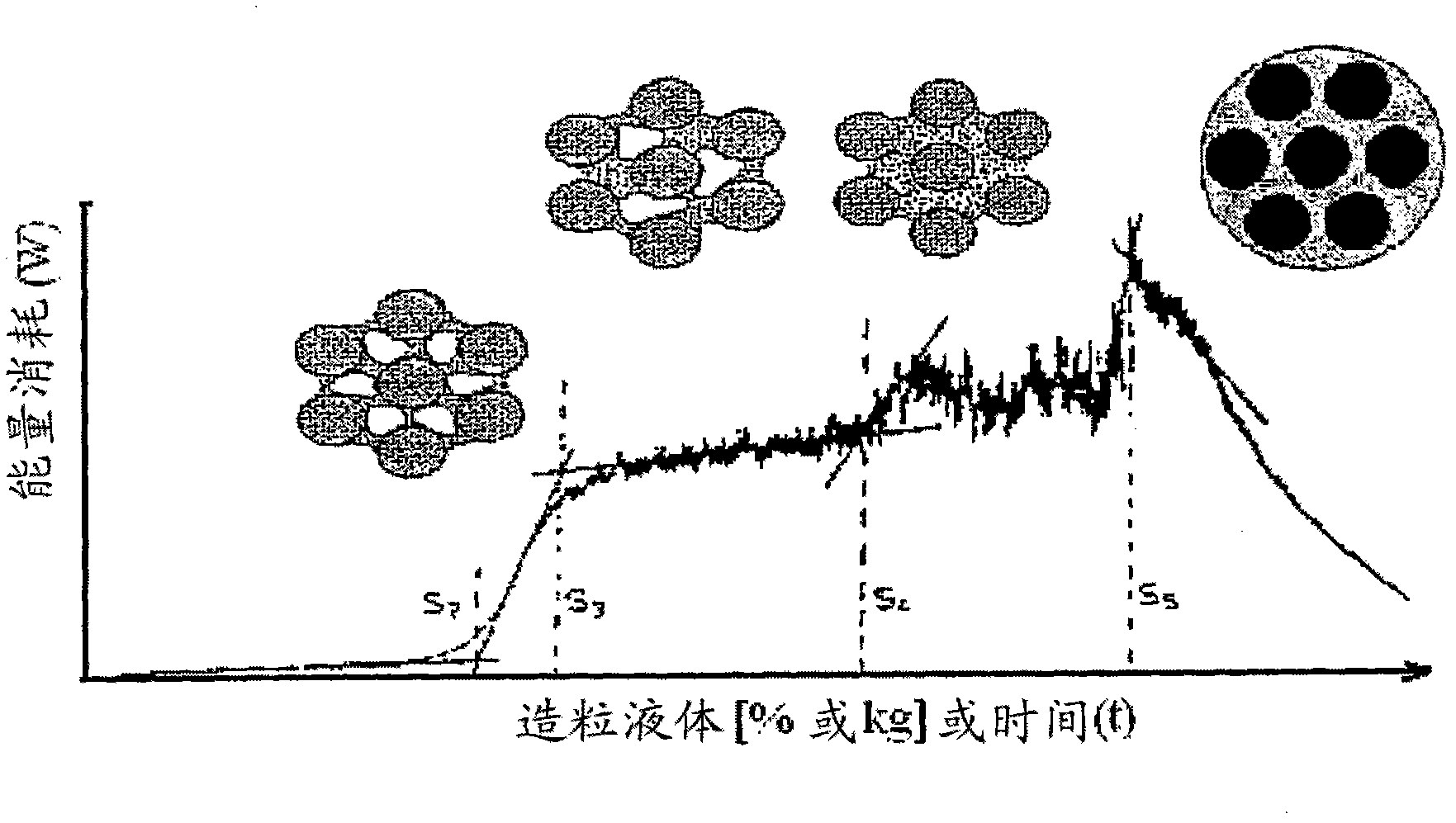
Process for making gastroretentive dosage forms
A gastroretentive and dosage form technology, which is applied in the field of preparation of gastroretentive dosage forms, can solve the problems of inapplicable active ingredients, inability to provide loading rate, difficult to implement and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0119] Preparation of solid dosage forms according to the invention
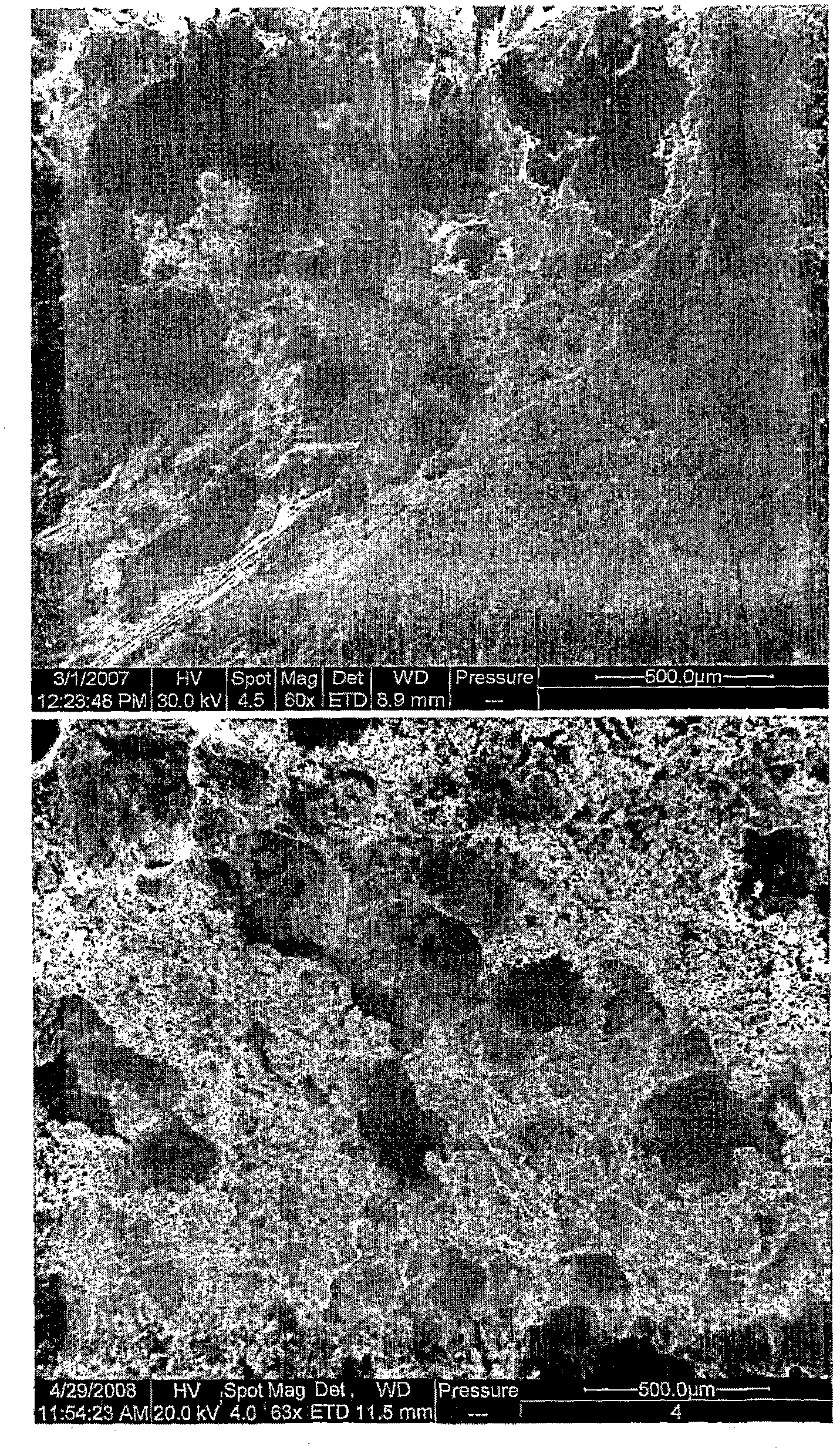
[0120]Two different solid dosage forms (100 gram batches) of the following compositions were prepared according to the invention. Dosage Form No. 1 includes a hydrophilic API and Dosage Form No. 2 includes a hydrophobic API. Enlarged pictures of dosage forms No. 1 and No. 2 of the described structure are in image 3 given in.
[0121]
[0122]
77.5%
[0123] The API powder was loaded into a shear mixer 4M8 granulator together with other excipients and mixed at 150 rpm for 2 minutes and 30 seconds. Granulation was initiated by adding water at a rate of 10 ml / min at 1000 rpm. Over-granulation was achieved after introducing 80 ml of water to form a solution:powder ratio of 0.8, and the resulting paste was further kneaded at 1500 rpm for 2 minutes and 30 seconds until it showed 4 to 8% torque resistance. The resulting material is then molded in a paraffin oil lubricat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com