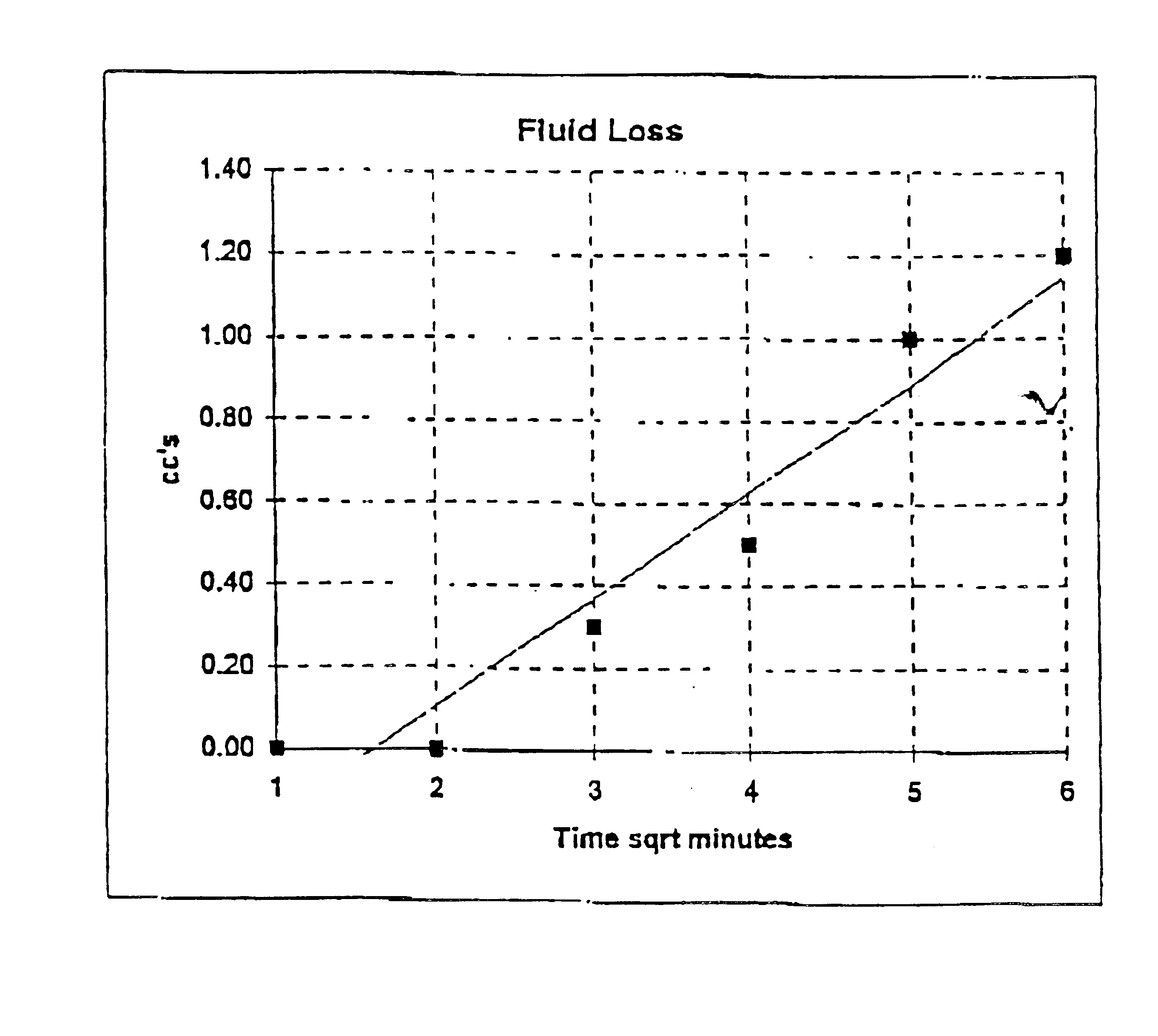
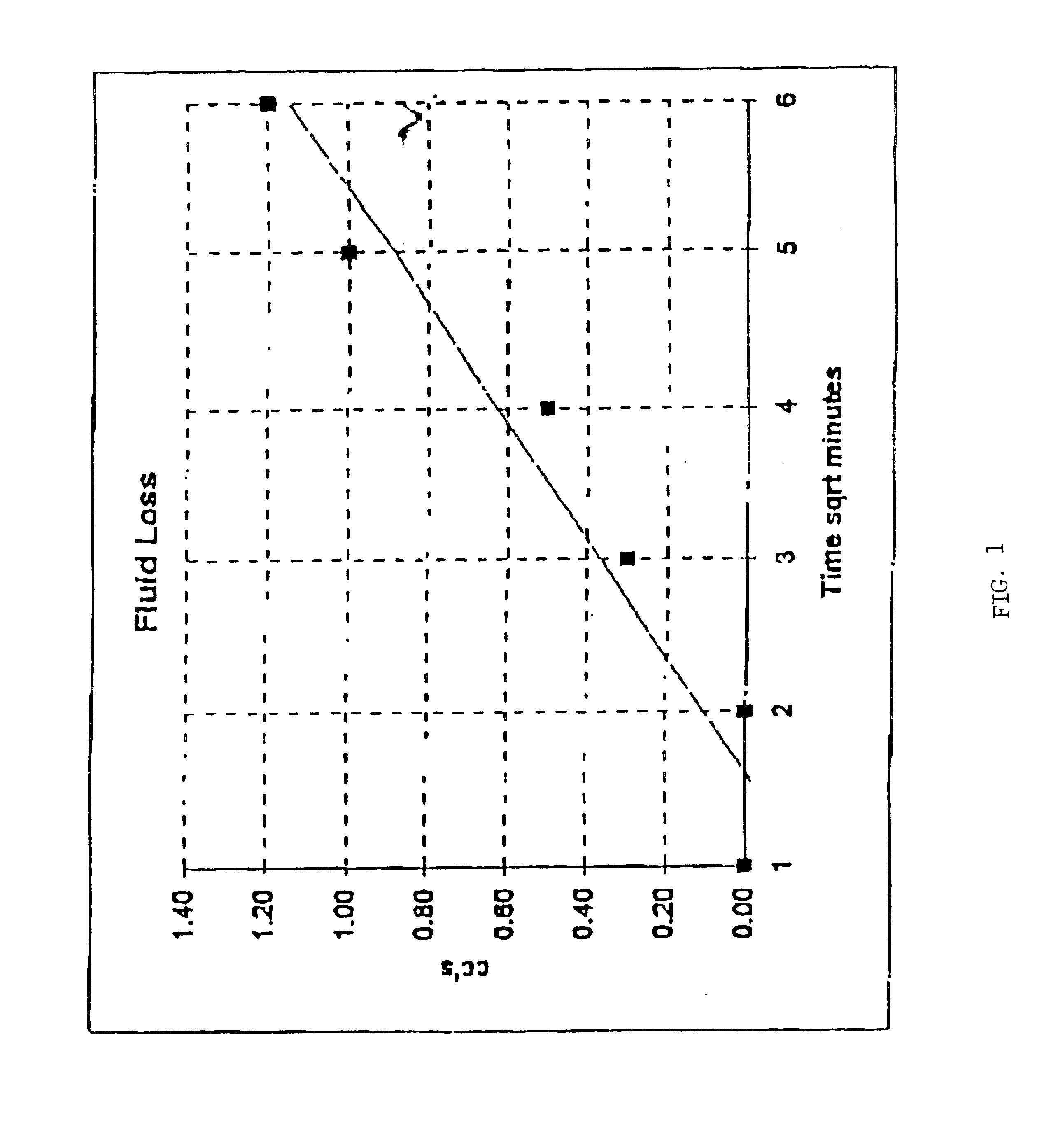
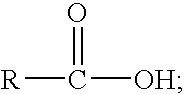
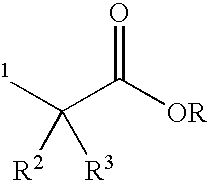
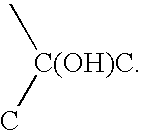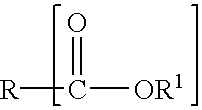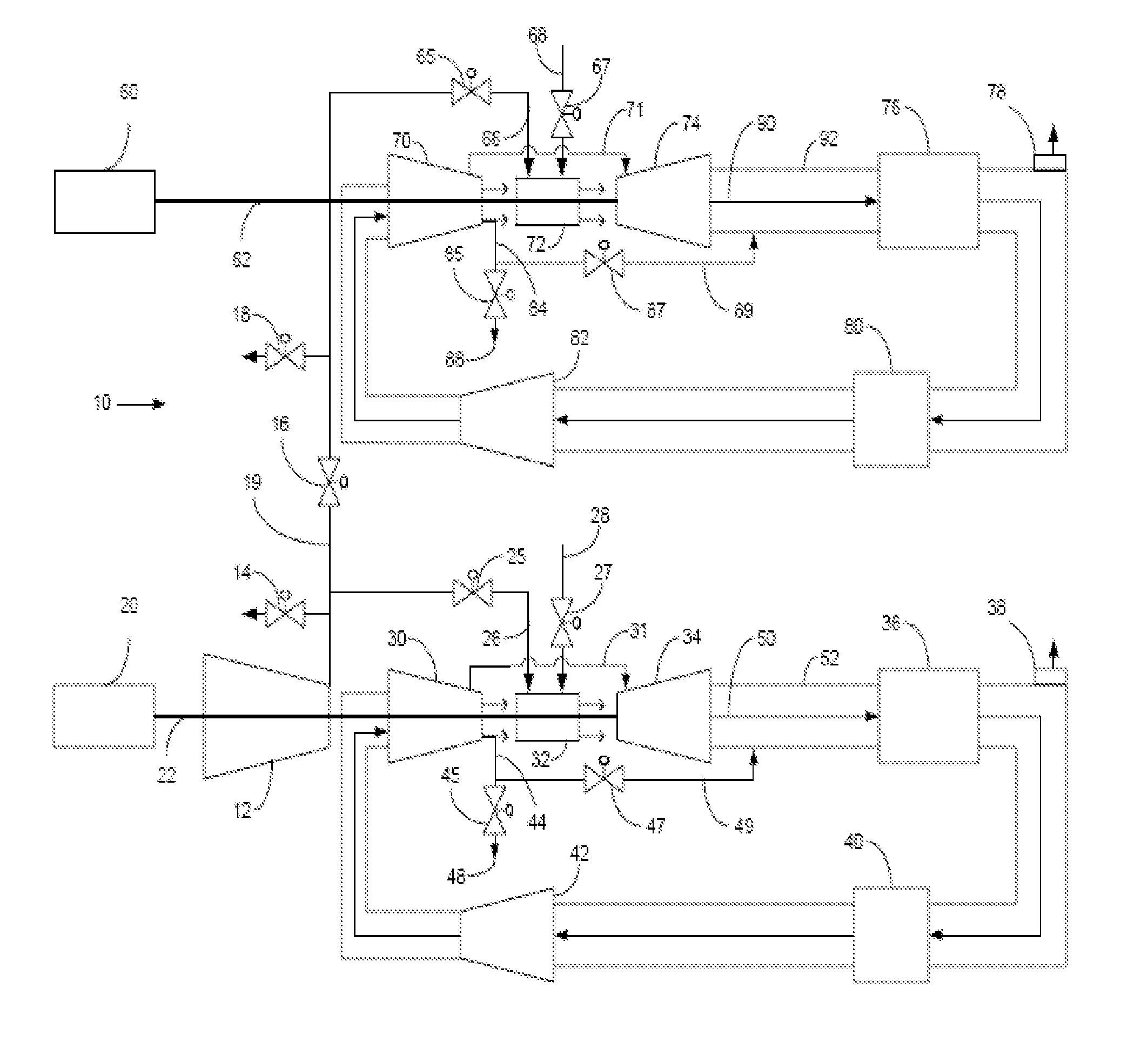Patents
Literature
1628 results about "Mixture formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
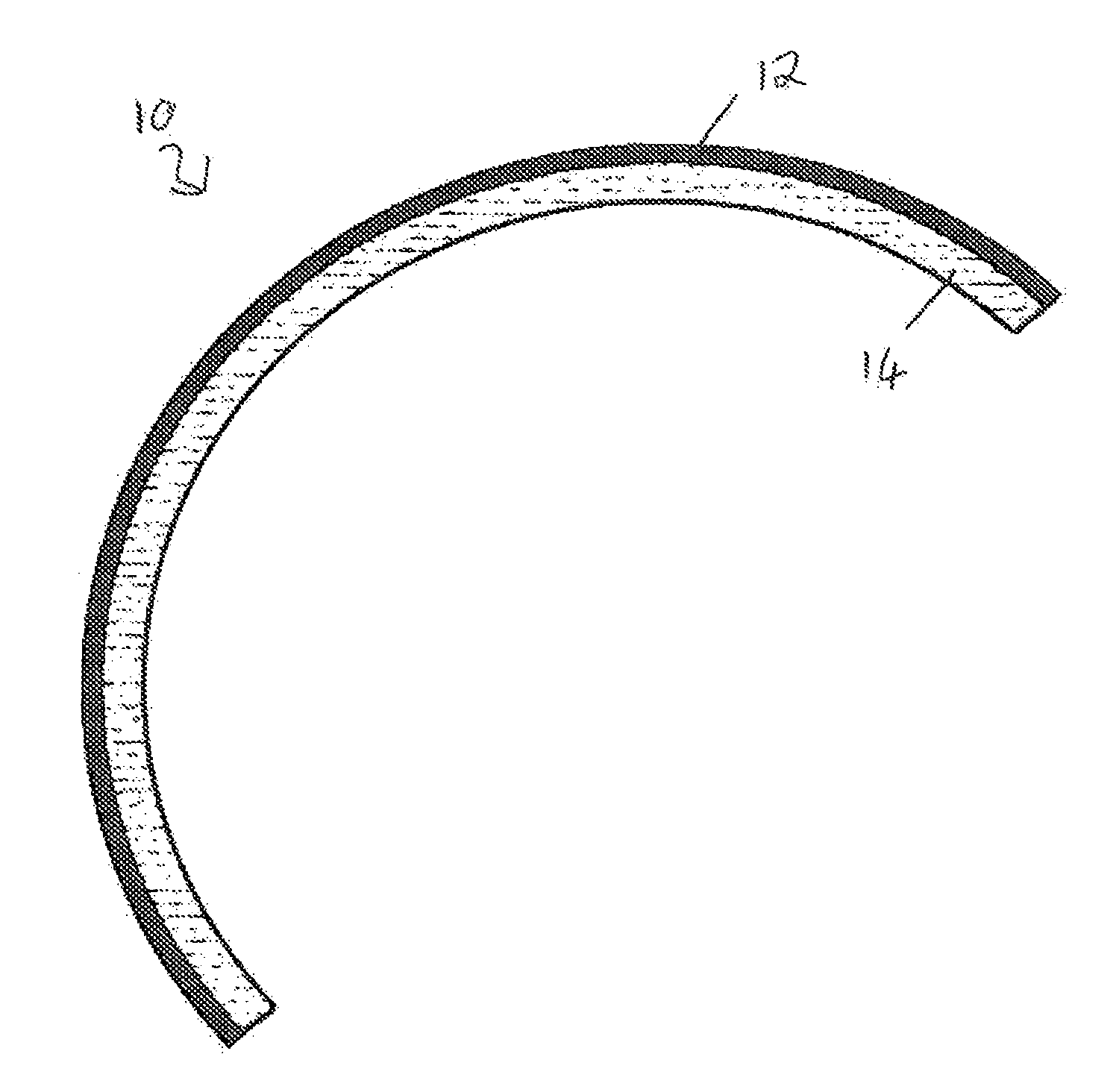
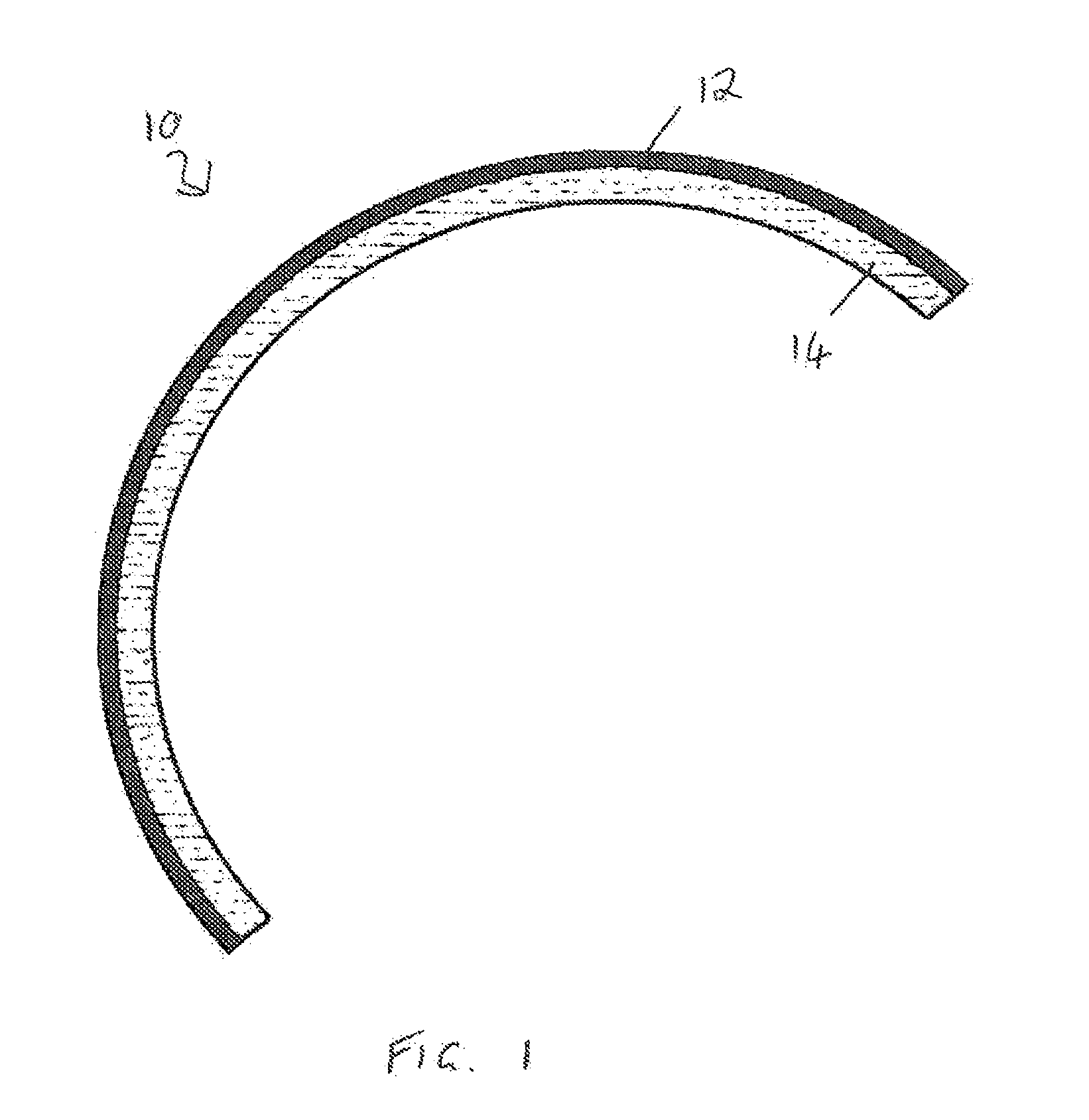
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Atomic layer deposition of metal oxynitride layers as gate dielectrics
InactiveUS20060051925A1Semiconductor/solid-state device manufacturingSemiconductor devicesGate dielectricHafnium
A metal oxynitride layer formed by atomic layer deposition of a plurality of reacted monolayers, the monolayers comprising at least one each of a metal, an oxide and a nitride. The metal oxynitride layer is formed from zirconium oxynitride, hafnium oxynitride, tantalum oxynitride, or mixtures thereof. The metal oxynitride layer is used in gate dielectrics as a replacement material for silicon dioxide. A semiconductor device structure having a gate dielectric formed from a metal oxynitride layer is also disclosed.
Owner:AHN KIE Y +1
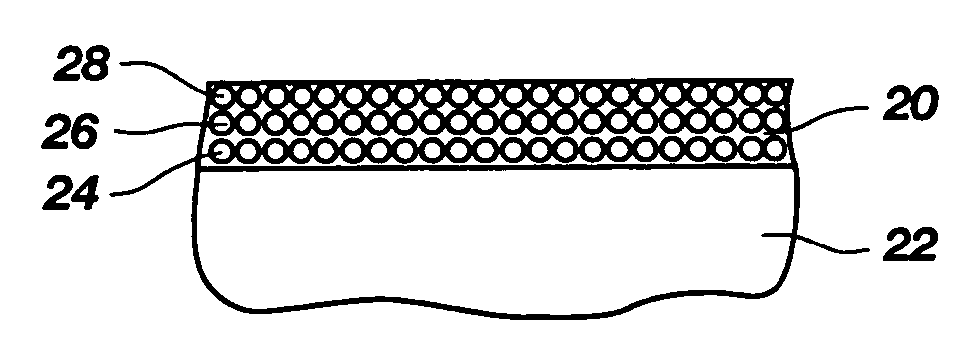
Electrical conductors formed from mixtures of metal powders and metallo-organic decomposition compounds
The present invention relates to a thick film formed of a mixture of metal powders and metallo-organic decomposition (MOD) compounds in an organic liquid vehicle and a process for advantageously applying them to a substrate by silk screening or other printing technology. The mixtures preferably contain metal flake with a ratio of the maximum dimension to the minimum dimension of between 5 and 50. The vehicle may include a colloidal metal powder with a diameter of about 10 to about 40 nanometers. The concentration of the colloidal metal in the suspension can range from about 10 to about 50% by weight. The MOD compound begins to decompose at a temperature of approximately about 200 DEG C. to promote consolidation of the metal constituents and bonding to the substrate which is complete at temperatures less than 450 DEG C. in a time less than six minutes. The mixtures can be applied by silk screening, stencilling, gravure or lithography to a polymer-based circuit board substrate for producing rigid and flexible printed wiring boards in a single operation with negligible generation of hazardous wastes. The same mixtures can be used in place of solder to assemble circuits by bonding electrical components to conductors as well as to make the conductors themselves.
Owner:PARELEC
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
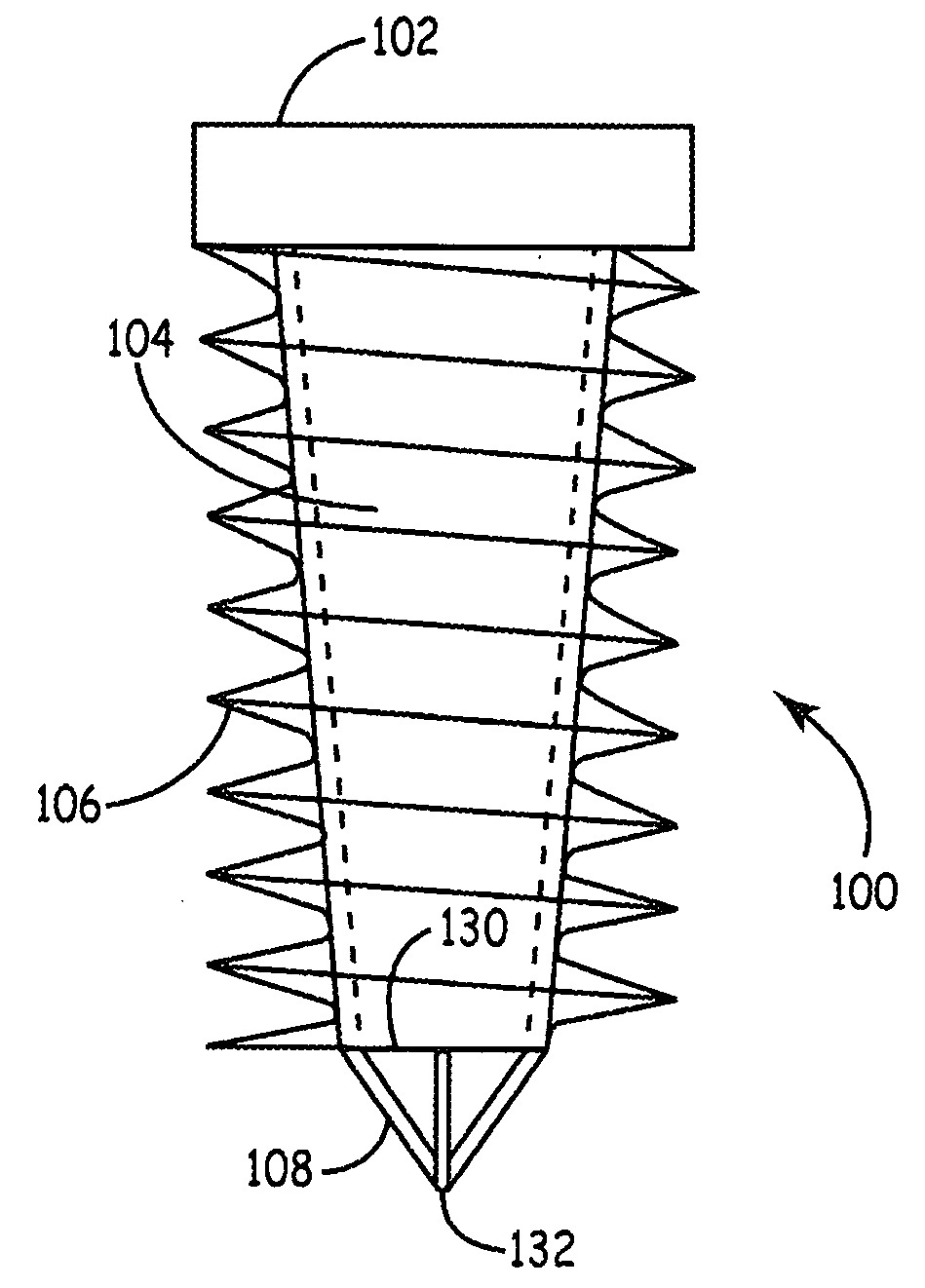
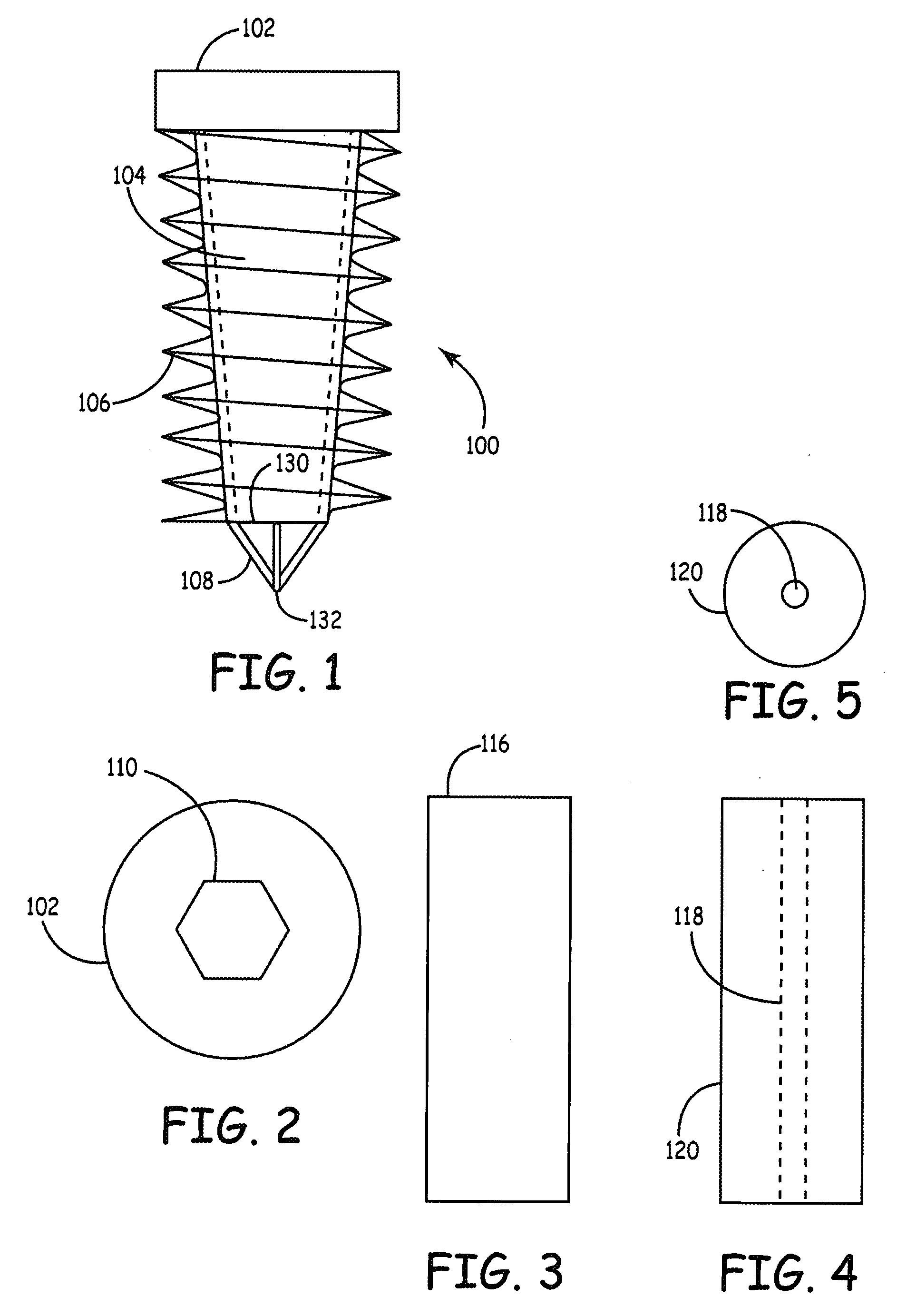
Bone screws and particular applications to sacroiliac joint fusion
Procedures for the fusion of the sacroiliac joint advantageously make use of an implant selected to distract the joint upon insertion and to maintain or increase tension upon insertion. The implant can have a varying structure along its length. In some method described herein for fusing the sacroiliac joint using an implant, an implant is screwed into the sacroiliac joint between the sacrum bone and the iliac bone. The implant comprises a shaft, a tool engagement flange at top end of the shaft, a pointed tip comprising no more than about 20 percent of the length of the screw, and threads spiraling around the shaft. For screws of particular interest, the volume displacement perpendicular to the shaft increases at least about 5 percent from a point adjacent the tip to a point near the top of the shaft. Some of the desirable screw designs can be used in other orthopedic application, especially situations involving varying bone hardness. Useful filler material can be formed from a blend of bone powder and bioactive agents.
Owner:ILION MEDICAL
Method for rapid forming of a ceramic work piece
InactiveUS6217816B1Rapid productionPrevent dislocationAdditive manufacturing apparatusFeeding arrangmentsHeat fusionLaser beams
An inorganic binder and a dissolving agent are put into ceramic powder. They are mixed to form a plastic green mixture. Then the said mixture is formed into a thin green layer. Preferably, this thin green layer will be preheated and dried such that the thin green layer will be hardened due to the bonding effect of the inorganic binder. A portion of the thin green layer exposed under a directed high-energy beam is sintered, preferably by a laser beam, to cause ceramic molecules to bond together locally due to heat fusion. By controlling the scanning path of the high-energy beam, a two-dimensional thin cross section of the ceramic part in arbitrary form can be produced. A second thin ceramic layer can be built onto the first thin ceramic layer and bonded to it by the same method. After multiple repetitions of this procedure a three dimensional ceramic part can be fabricated layer upon layer. The green portion, which is not scanned by the high-energy beam, will be removed with suitable method. A ceramic part can be rapidly produced in this way.
Owner:NAT SCI COUNCIL
Thermally stable diamond polycrystalline diamond constructions
ActiveUS20060060390A1Improve thermal stabilityGood adhesionPigmenting treatmentDrill bitsDiamond crystalPolycrystalline diamond
Thermally stable diamond constructions comprise a diamond body having a plurality of bonded diamond crystals, a plurality of interstitial regions disposed among the crystals, and a substrate attached to the body. The body includes a working surface and a side surface extending away from the working surface to the substrate. The body comprises a first region adjacent the side surface that is substantially free of a catalyst material and that extends a partial depth into the diamond body. The first region can further extend to at least a portion of the working surface and a partial depth therefrom into the diamond body. The diamond body can be formed from natural diamond grains and / or a mixture of natural and synthetic diamond grains. A surface of the diamond body is treated to provide the first region, and before treatment is finished to an approximate final dimension.
Owner:SMITH INT INC
Method of Forming a Polymer Component
ActiveUS20110153025A1Reduce oxidationPrevent oxidationMedical devicesPretreated surfacesCross-linkArticular surfaces
This invention relates to a method of forming a polymer component and comprises blending polymer particles with antioxidant to form a mixture in which the antioxidant coats the polymer particles, irradiating the mixture to cross-link the polymer particles therein and forming the irradiated mixture into a consolidated component. The invention also relates to a method of forming an articular surface for a prosthesis and a prosthesis having a polymer articular bearing surface wherein at least one pre-determined portion of the bearing surface is provided with cross-linked polymer bonds.
Owner:JOINTMEDICA LTD
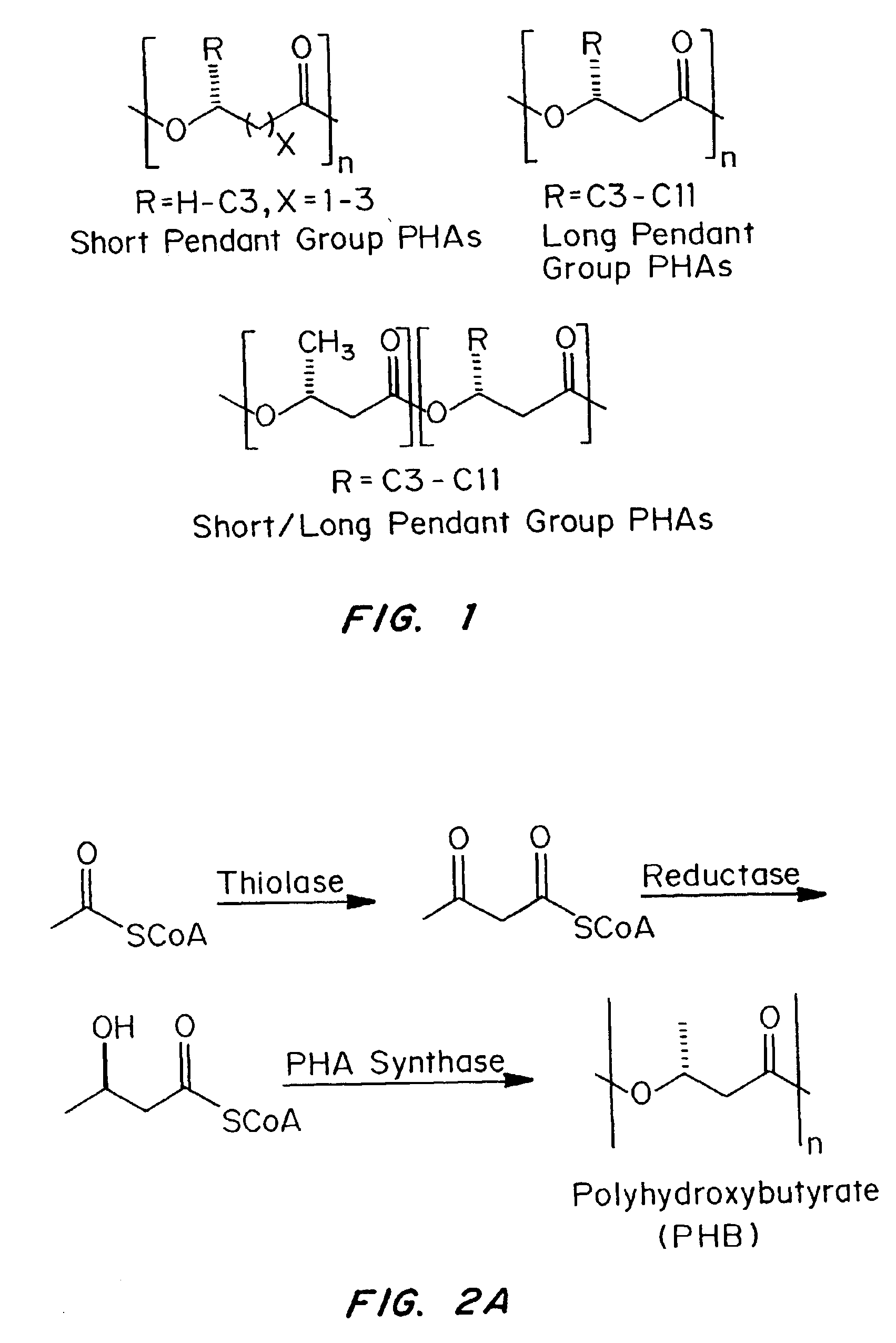
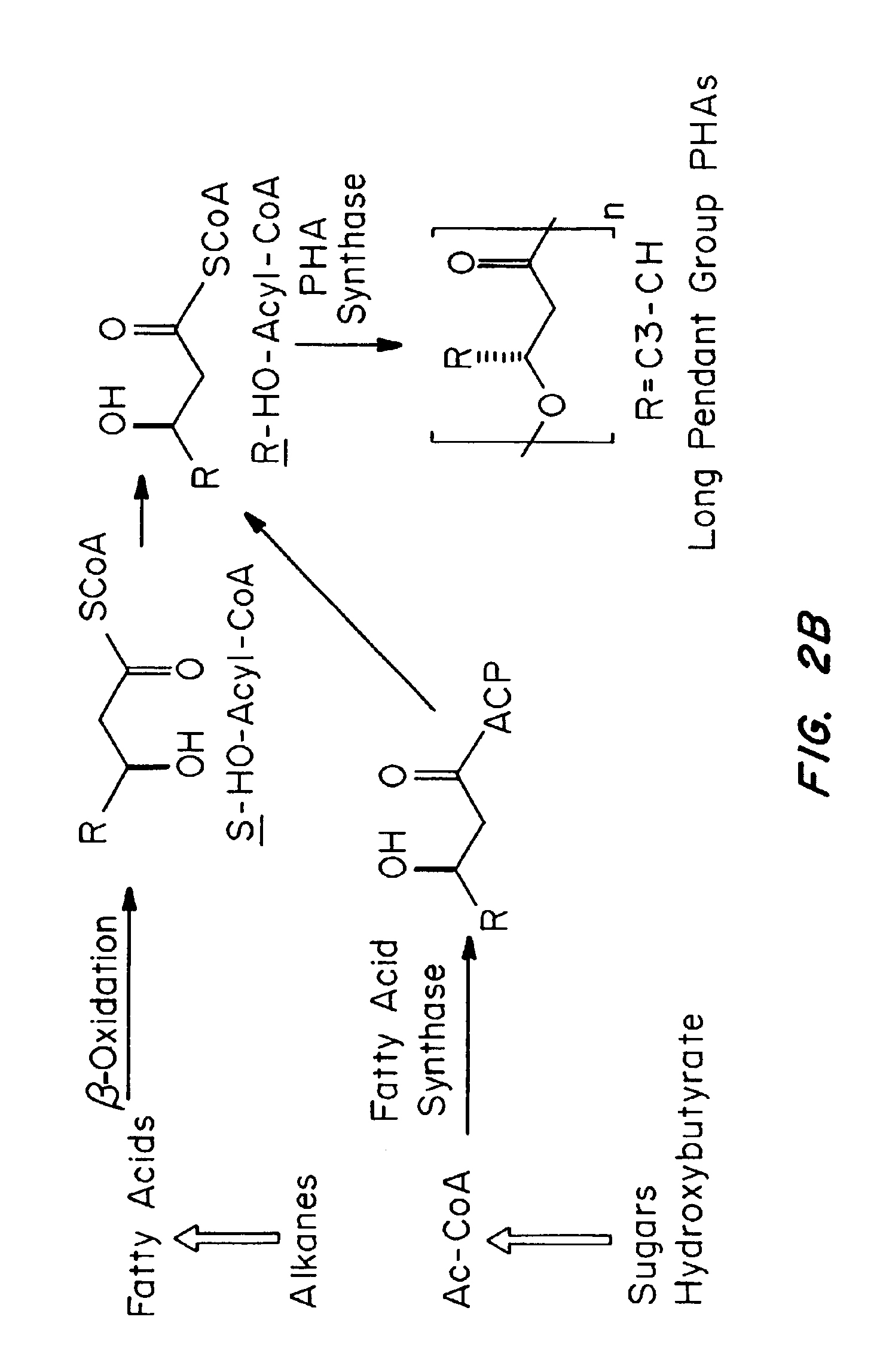
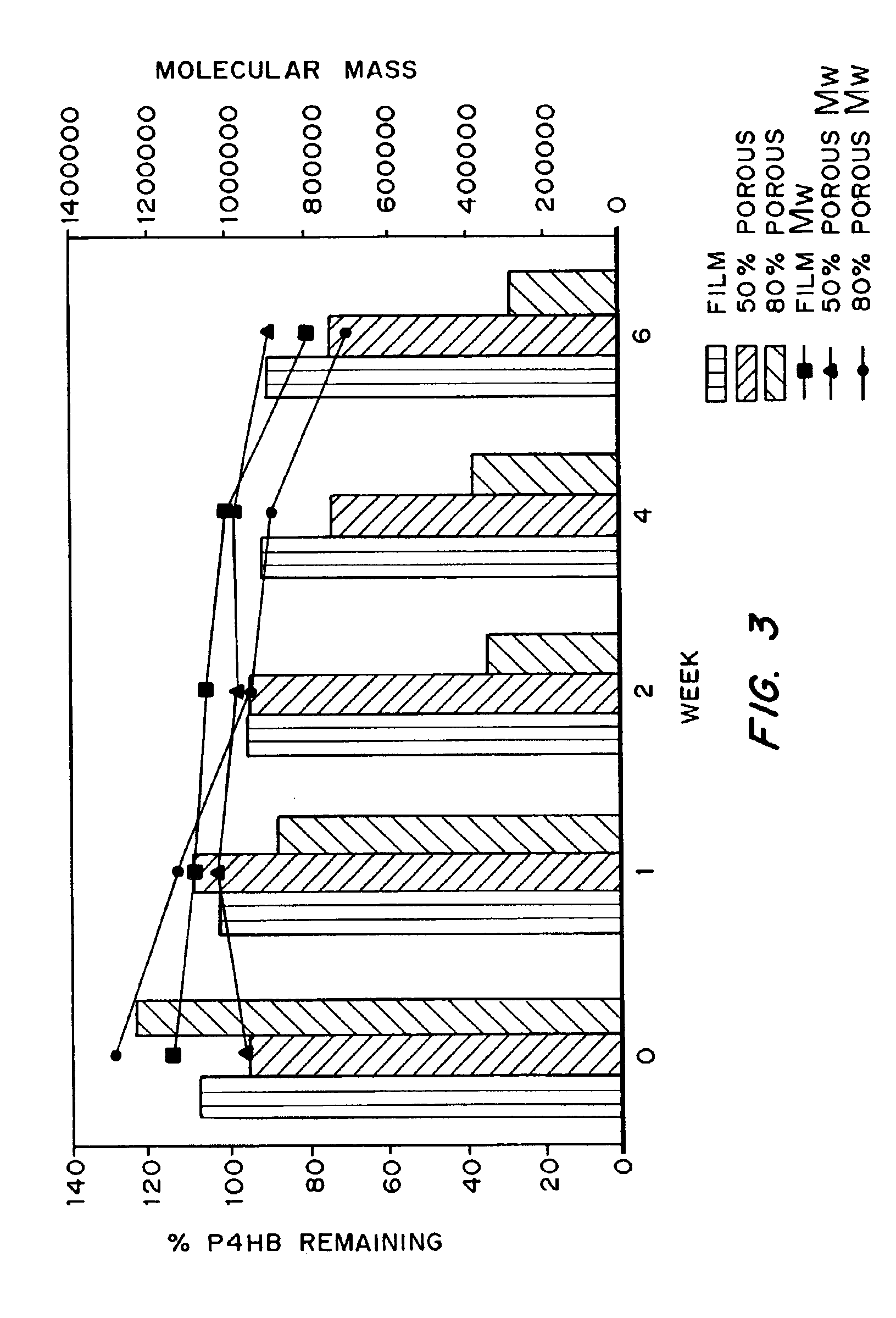
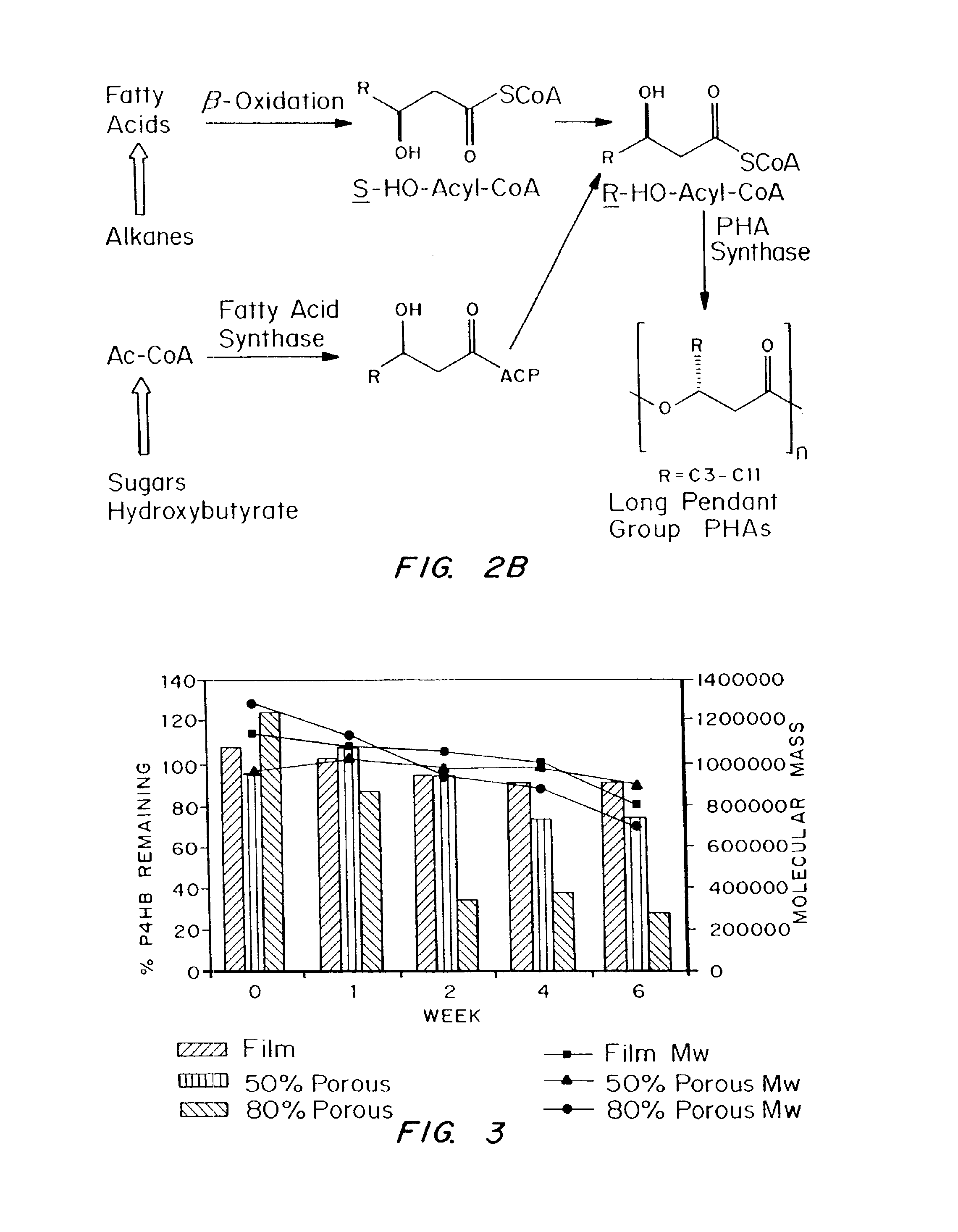
Polyhydroxyalkanoate compositions having controlled degradation rates
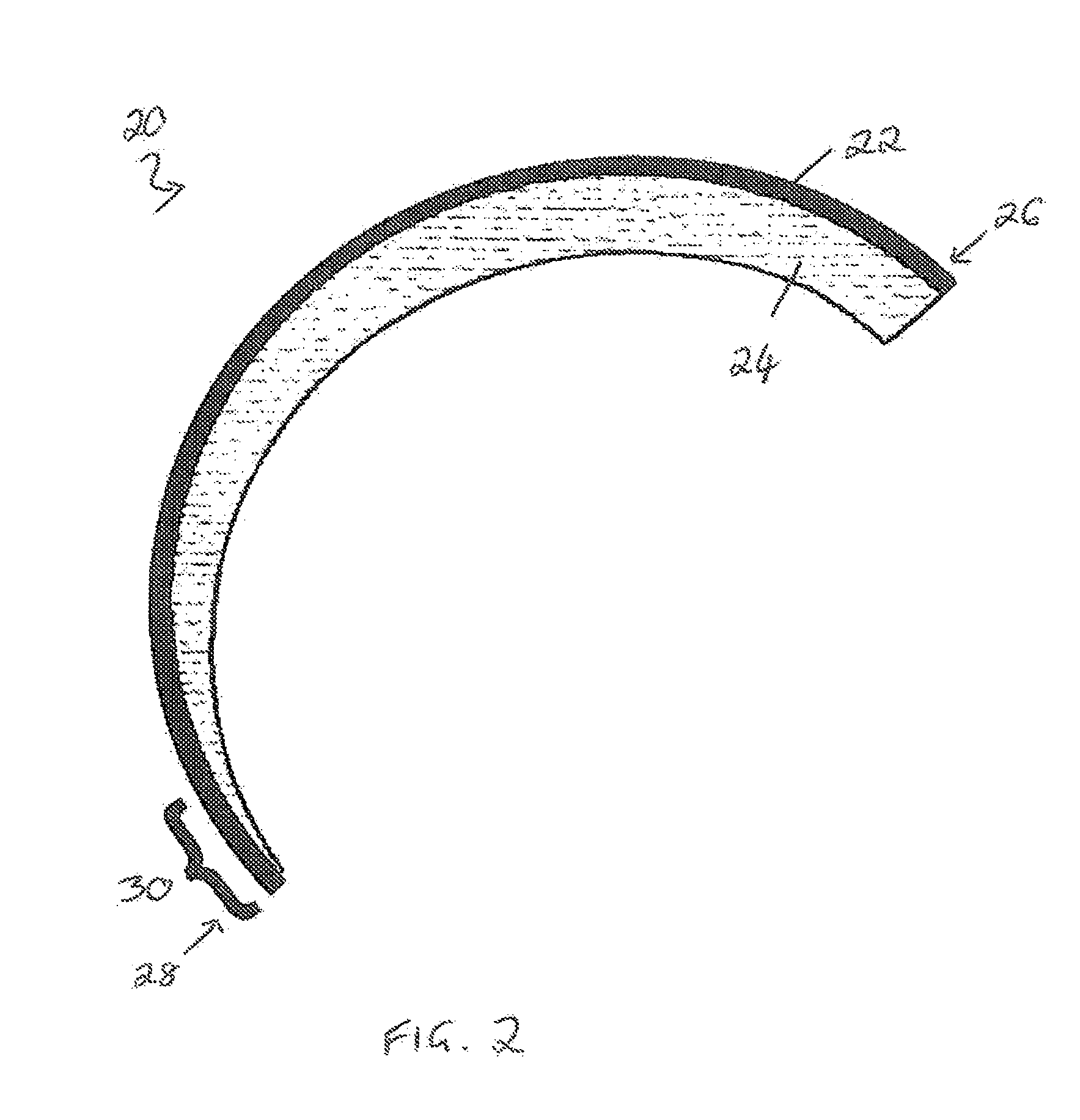
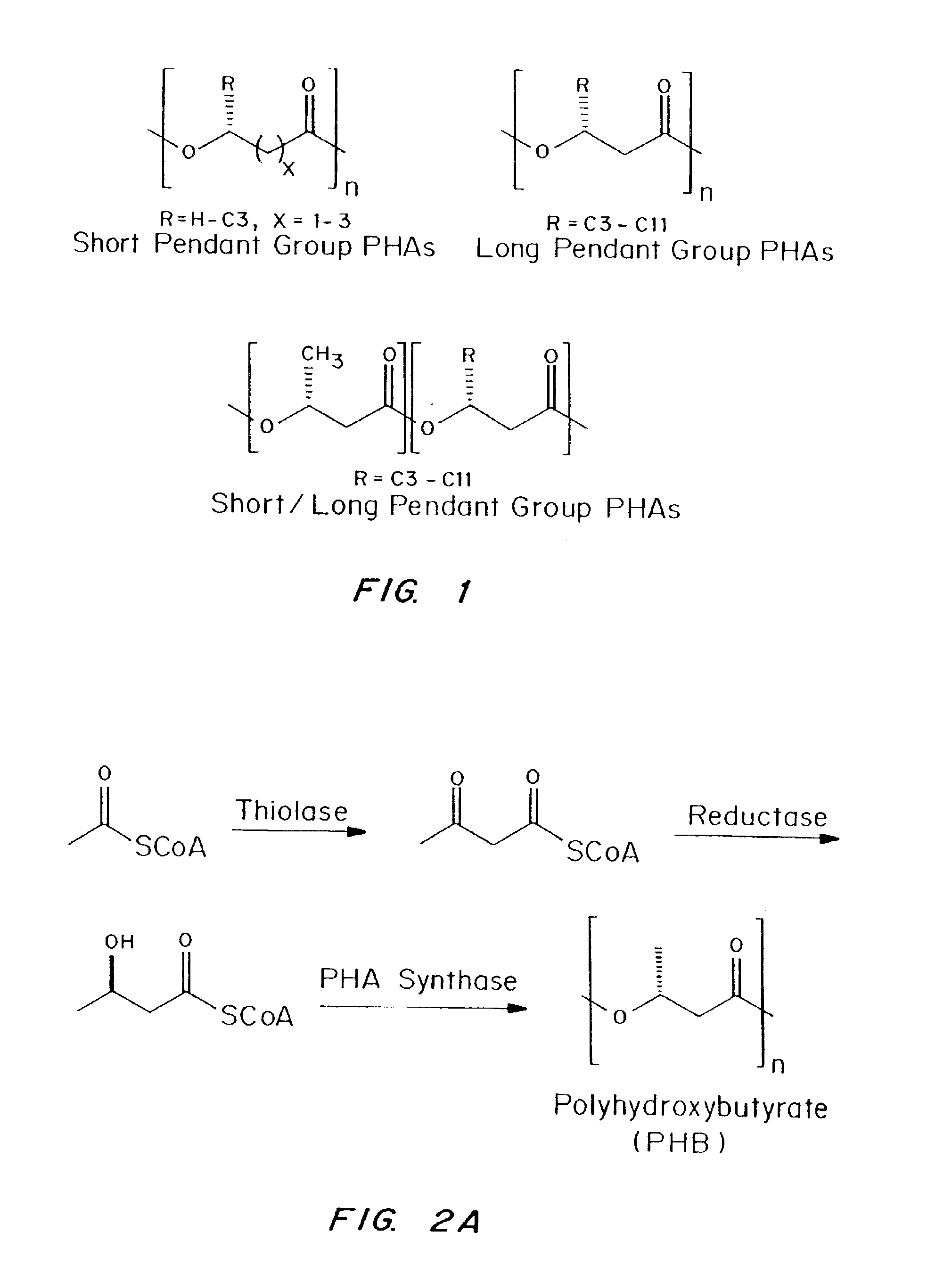
Biocompatible polyhydroxyalkanoate compositions with controlled degradation rates have been developed. In one embodiment, the polyhydroxyalkanoates contain additives to alter the degradation rates. In another embodiment, the polyhydroxyalkanoates are formed of mixtures of monomers or include pendant groups or modifications in their backbones to alter their degradation rates. In still another embodiment, the polyhydroxyalkanoates are chemically modified. Methods for manufacturing the devices which increase porosity or exposed surface area can be used to alter degradability. For example, as demonstrated by the examples, porous polyhydroxyalkanoates can be made using methods that creates pores, voids, or interstitial spacing, such as an emulsion or spray drying technique, or which incorporate leachable or lyophilizable particles within the polymer. Examples describe poly(4HB) compositions including foams, coatings, meshes, and microparticles. As demonstrated by the examples, these polyhydroxyalkanoate compositions have extremely favorable mechanical properties, as well as are biocompatible and degrade within desirable time frames under physioogical conditions. These polyhydroxyalkanoate materials provide a wider range of polyhydroxyalkanoate degradation rates than are currently available. Methods for processing these materials, particularly for therapeutic, prophylactic or diagnostic applications, or into devices which can be implanted or injected, are also described.
Owner:TEPHA INC
Sheets having a starch-based binding matrix
Compositions and methods for manufacturing sheets having a starch-bound matrix, optionally reinforced with fibers and optionally including an inorganic mineral filler. Suitable mixtures for forming the sheets are prepared by mixing together water, unmodified and ungelatinized starch granules, a cellulosic ether, optionally fibers, and optionally an inorganic mineral filler in the correct proportions to form a sheet having desired properties. The mixtures are formed into sheets by passing them between one or more sets of heated rollers to form green sheets. The heated rollers cause the cellulosic ether to form a skin on the outer surfaces of the sheet that prevents the starch granules from causing the sheet to adhere to the rollers upon gelation of the starch. The green sheets are passed between heated rollers to gelatinize the starch granules, and then to dry the sheet by removing a substantial portion of the water by evaporation. The starch and cellulosic ether form the binding matrix of the sheets with the fibers and optional inorganic filler dispersed throughout the binding matrix. The starch-bound sheets can be cut, rolled, pressed, scored, perforated, folded, and glued to fashion articles from the sheets much like paper or paperboard. The sheets are particularly useful in the mass production of containers, such as food and beverage containers.
Owner:E KHASHOGGI INDS
Gelled hydrocarbon compositions and methods for use thereof
InactiveUS6849581B1Improve stabilityAvoid stabilityOrganic detergent compounding agentsSurface-active detergent compositionsOrganic baseOrganic fluid
Gelled organic compositions and methods for using same. The gelled compositions may be liquid organic fluids, such as gelled liquid hydrocarbons, formed from a mixture of an organic-base fluid, a carboxylic acid, and one or more metal source compounds, such as a metal salt of carboxylic acid. The gelled compositions may be used in variety of applications including, but not limited to, oil field, pipeline and processing facility applications.
Owner:BJ SERVICES LLC +1
Composition and method for forming a sprayable materials cover
InactiveUS20050084334A1Improve performanceImprove adhesionSolid waste disposalLandfill technologiesWater dispersibleSlurry
An alternative cover for landfill may be formed from a slurry mixture of water, cementitious binder, adhesion enhancing admixture and fiber. These constituents may be mixed and applied to cover landfilled wastes, granular material piles or for soil erosion control. The cover will harden to minimize water infiltration, wind blown dust, odor and affinity to birds, flies and other insects. The water may include tap water, landfill leachate and wastewater. The binder may include Portland cement, blended cement, cement kiln dust, class C fly ash, and / or calcium sulphate hemihydrate. The adhesion enhancing admixture includes water-dispersible polymers. The fibers may comprise shredded paper or wood or plastic fibers.
Owner:CJS TECH
Organic electroluminescent element and lumiscent device or display including the same
InactiveUS20050158577A1Superior in luminanceImprove reliabilityDischarge tube luminescnet screensElectroluminescent light sourcesDisplay deviceHole transport layer
The present invention provides an organic electroluminescent element which is superior in luminance, reliability, and thermal stability and is capable of selectively emitting light with comparative long wavelengths such as red and good color purity and a light-emitting device or display device incorporated therewith. The organic electroluminescent element consists of a glass substrate (1), an anode (2), a hole transporting layer (10), an emitting layer (11), an electron transporting layer (12), and a cathode (3), which are sequentially laminated on top of the other. The emitting layer (11) is formed from a mixture composed of at least one species of the styryl compound represented by the general formula [I] below and a material with charge transporting capability. Y—CH═CH—X General formula [I](where X denotes an aryl group (such as phenyl group) which has a substituent group (such as cyano group and methyl group), and Y denotes a group having a skeleton of aminophenyl group or the like.)
Owner:JOLED INC
Polycrystalline diamond carbide composites
InactiveUS20070193782A1Improve fracture toughnessEasy to useDrill bitsOther chemical processesPolycrystalline diamondCarbide
Owner:SMITH INT INC
Solid composite electrolyte membrane and method of making
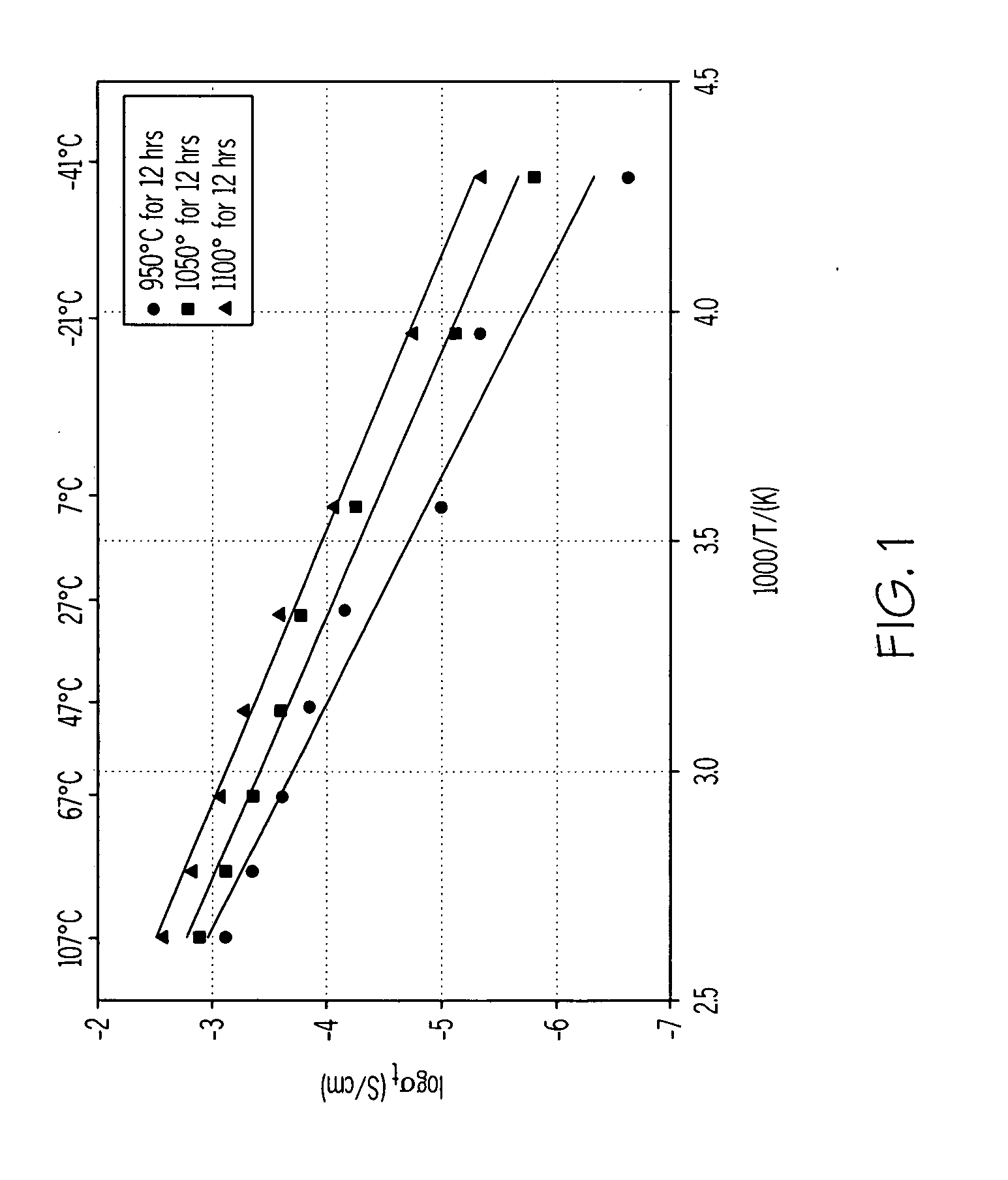
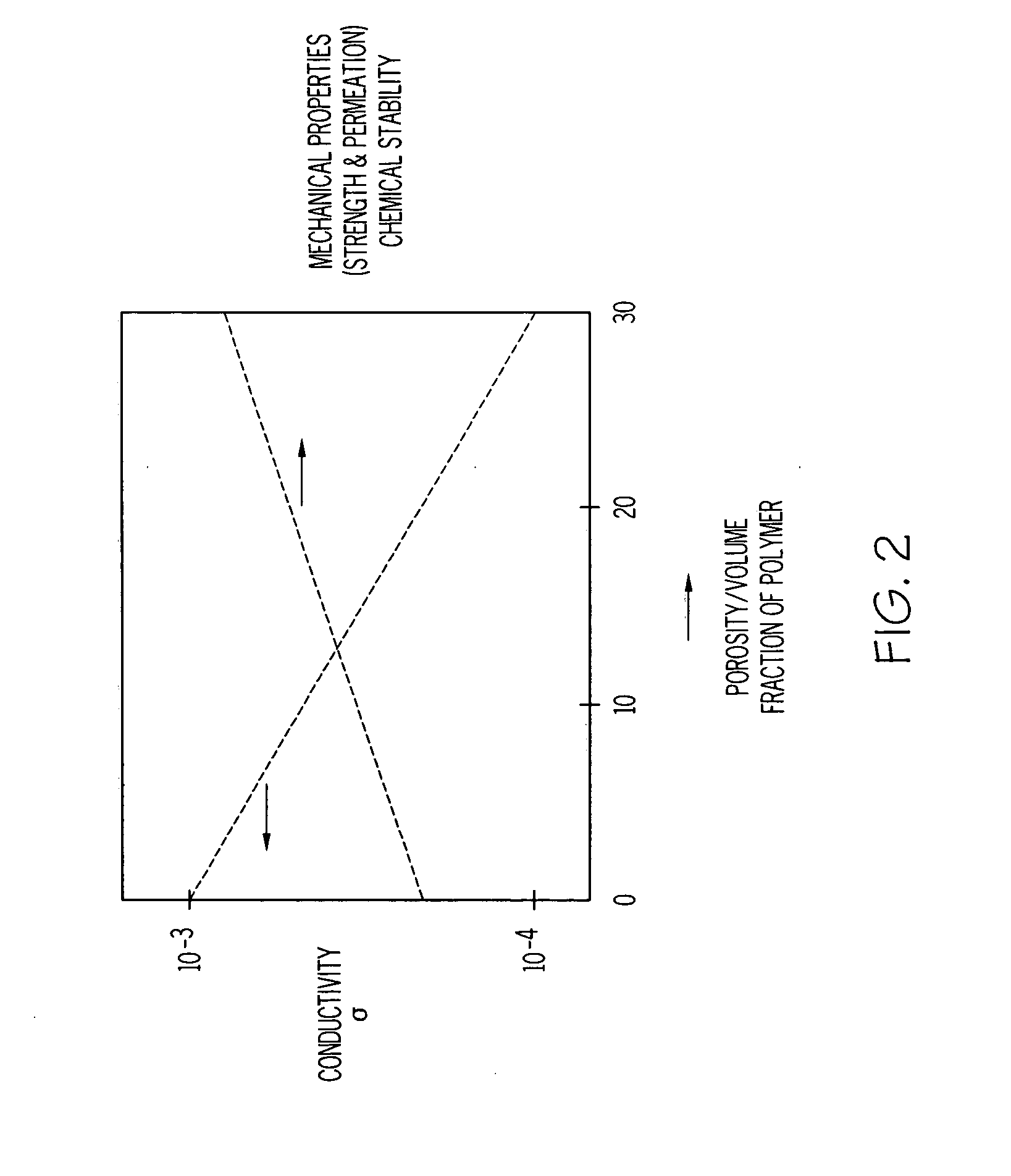
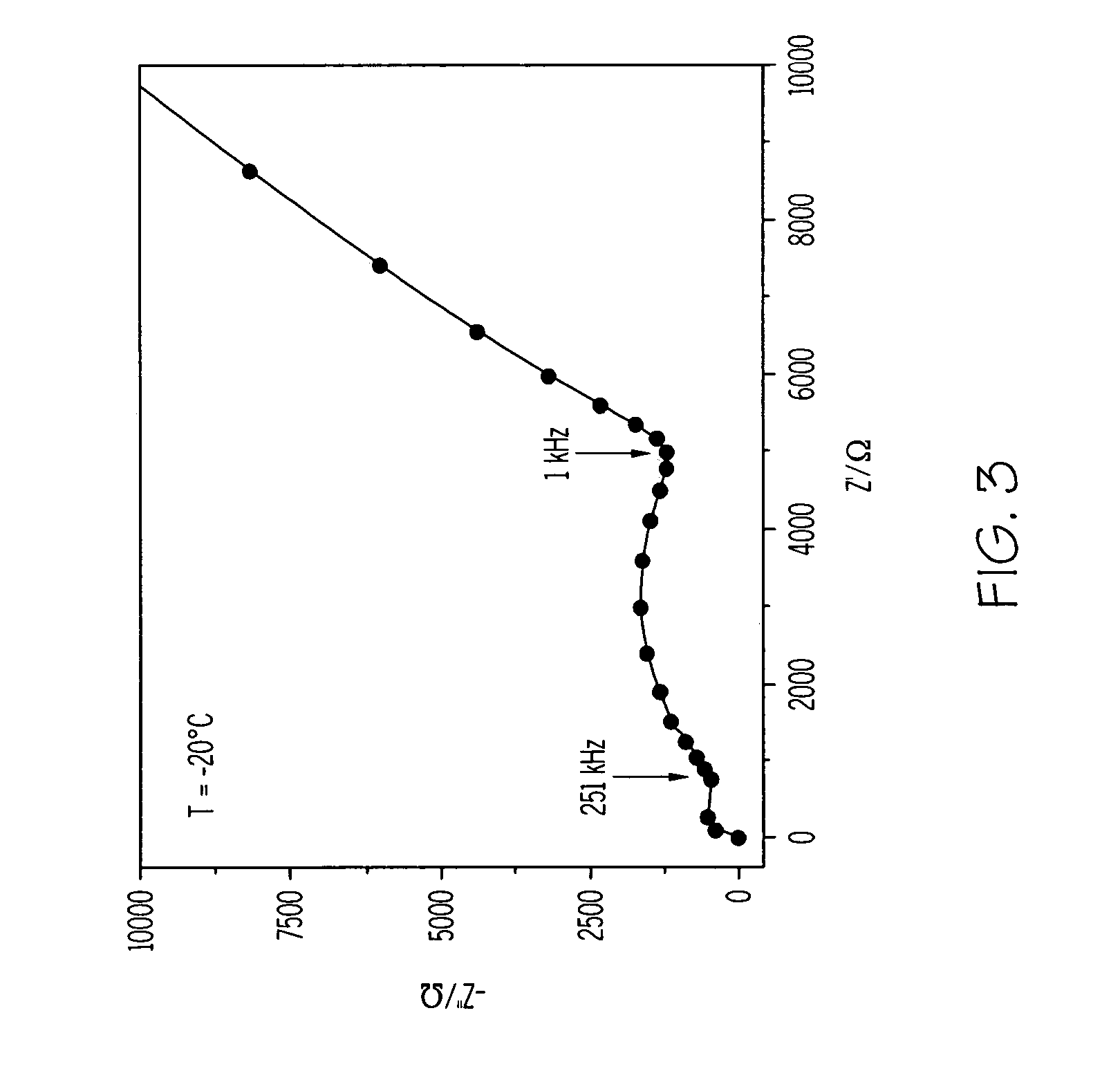
ActiveUS20070117026A1Improve conductivitySolid electrolytesSolid electrolyte cellsPorosityComposite electrolyte
A solid composite electrolyte membrane for use in a lithium battery is provided which exhibits a conductivity ranging from about 10−4 S cm−1 to about 10−3 S cm−1 at ambient temperature. The membrane is formed by providing a glass or glass-ceramic powder formed from a mixture of lithium carbonate, alumina, titanium dioxide, and ammonium dihydrogen phosphate. The powder is mixed with a conditioning agent and at least one solvent, followed by the addition of a binder and one or more plasticizers. The resulting slurry is cast into a tape which is then subjected to a binder burn-off and sintering process to form the membrane. The resulting membrane may be a glass-ceramic composite having a porosity ranging from 0 to 50%, or the membrane may be further infiltrated with a polymer to form a water-impermeable polymeric-ceramic composite membrane.
Owner:UNIV OF DAYTON THE
Air freshening compositions, articles comprising same and methods
An air freshening composition including porous carrier particles having a perfume composition entrapped therein, and a second component selected from an inert filer, hygroscopic agent, binder, coating material, moisture providing agent, and mixtures thereof. The composition may further comprise various optional components such as free perfumes, colorants, disintegrants, water swelling agents, porosity modifiers and mixtures thereof. A method for processing the compositions into a solid article includes the steps of entrapping a perfume in the porous carrier particles, heating and incorporating a carrier or binder material. The compositions may be further processed by optionally incorporating a powder inert filler and forming articles from the mixture. The articles may be formed from the compositions through prilling, extrusion, or compaction amongst other techniques. The air freshening articles and compositions will provide a sustained and controlled release of the perfume compositions over a long period of time without the use of a heat to activate the release. Articles of manufacture including the air freshening articles and various packaging are also disclosed.
Owner:THE PROCTER & GAMBLE COMPANY
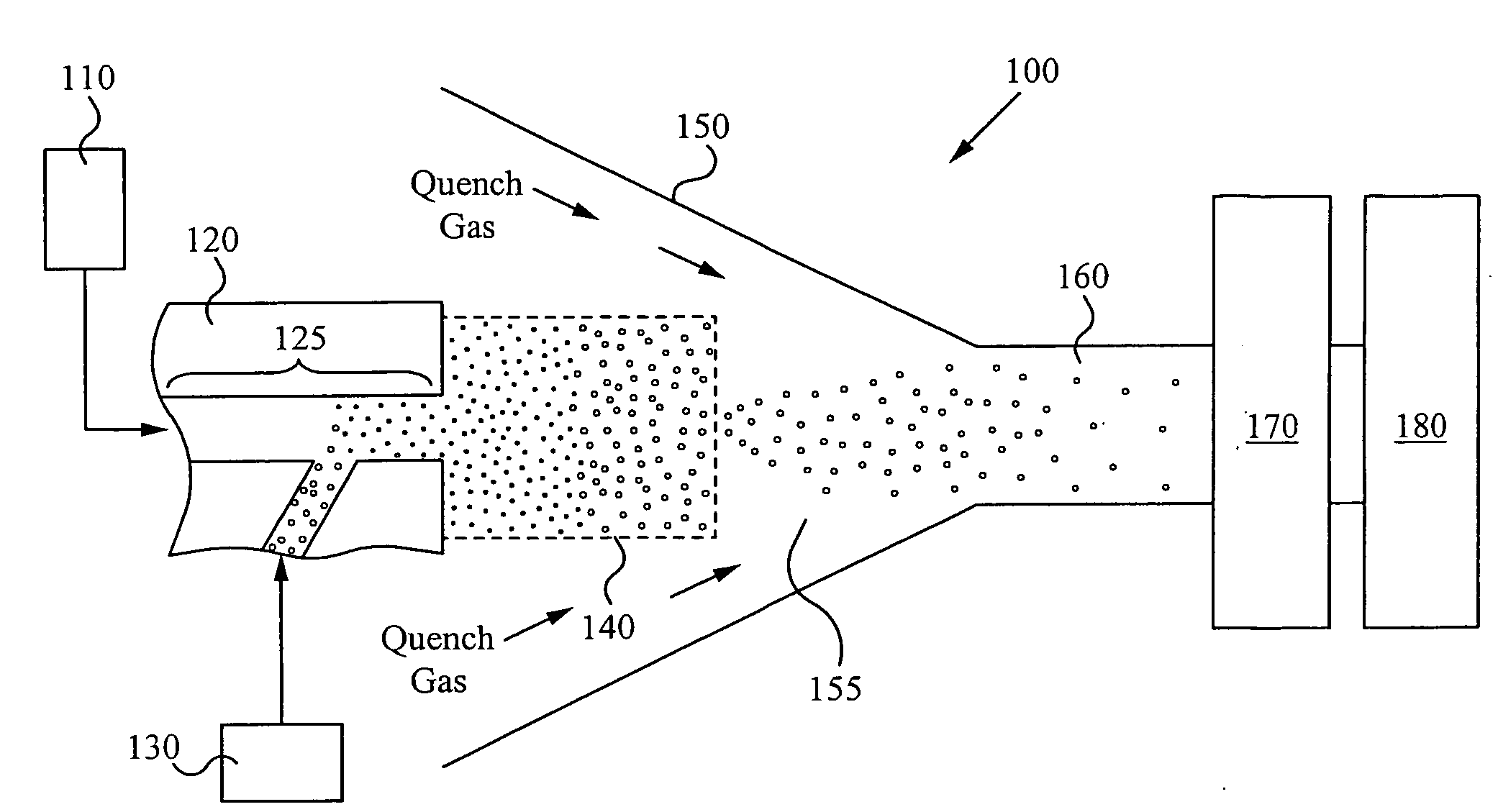
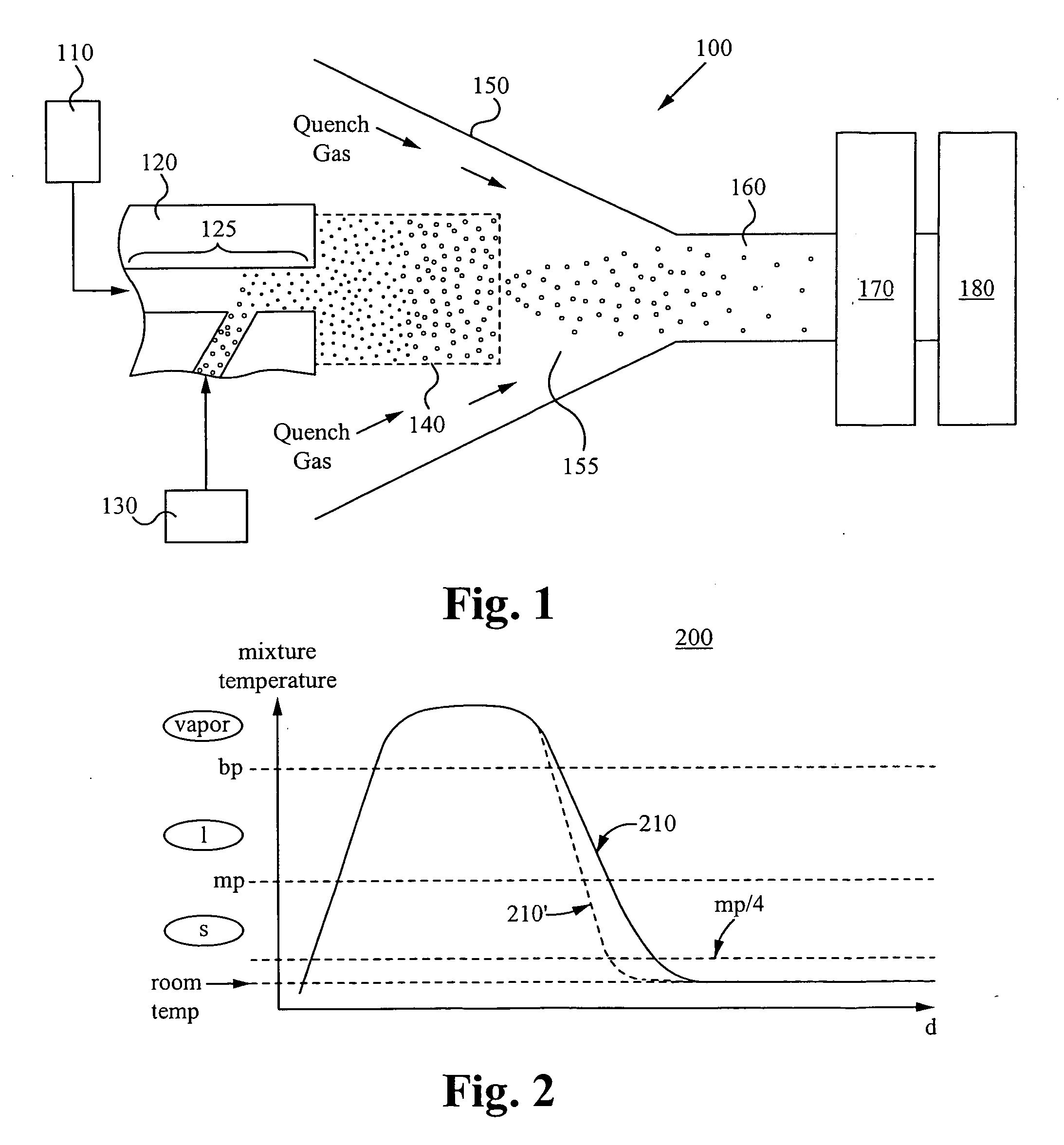
Method and apparatus for making uniform and ultrasmall nanoparticles
ActiveUS20080277270A1Catalyst activation/preparationDirect contact heat exchangersNanoparticleEngineering
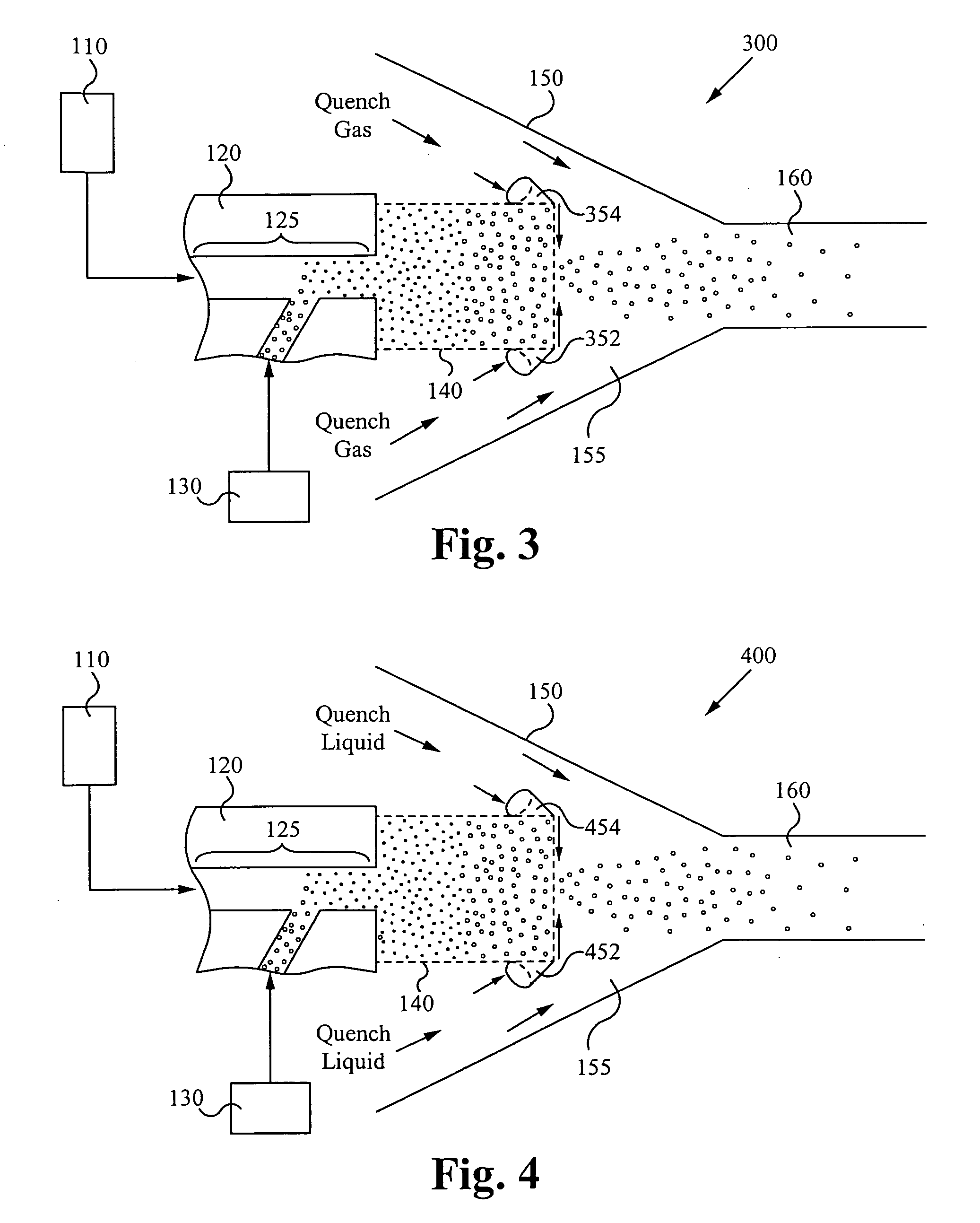
A system comprising: a plasma production chamber configured to produce a plasma; a reaction chamber vaporize a precursor material with the plasma to form a reactive mixture; a quench chamber having a frusto-conical surface and a quench region formed within the quench chamber between an ejection port of the reaction chamber and a cooled mixture outlet, wherein the quench region configured to receive the reactive mixture from the ejection port, to cool the reactive mixture to form a cooled mixture, and to supply the cooled mixture to the cooled mixture outlet; and a conditioning fluid injection ring disposed at the ejection port and configured to flow a conditioning fluid directly into the reactive mixture as the reactive mixture flows through the ejection port, thereby disturbing the flow of the reactive mixture, creating turbulence within the quench region and cooling the reactive mixture to form a cooled mixture comprising condensed nanoparticles.
Owner:SDC MATERIALS +1
Method for increasing the strength of a cellulosic product
InactiveUS7090745B2Increased amine contentSure easyNatural cellulose pulp/paperSpecial paperCellulosePolymer science
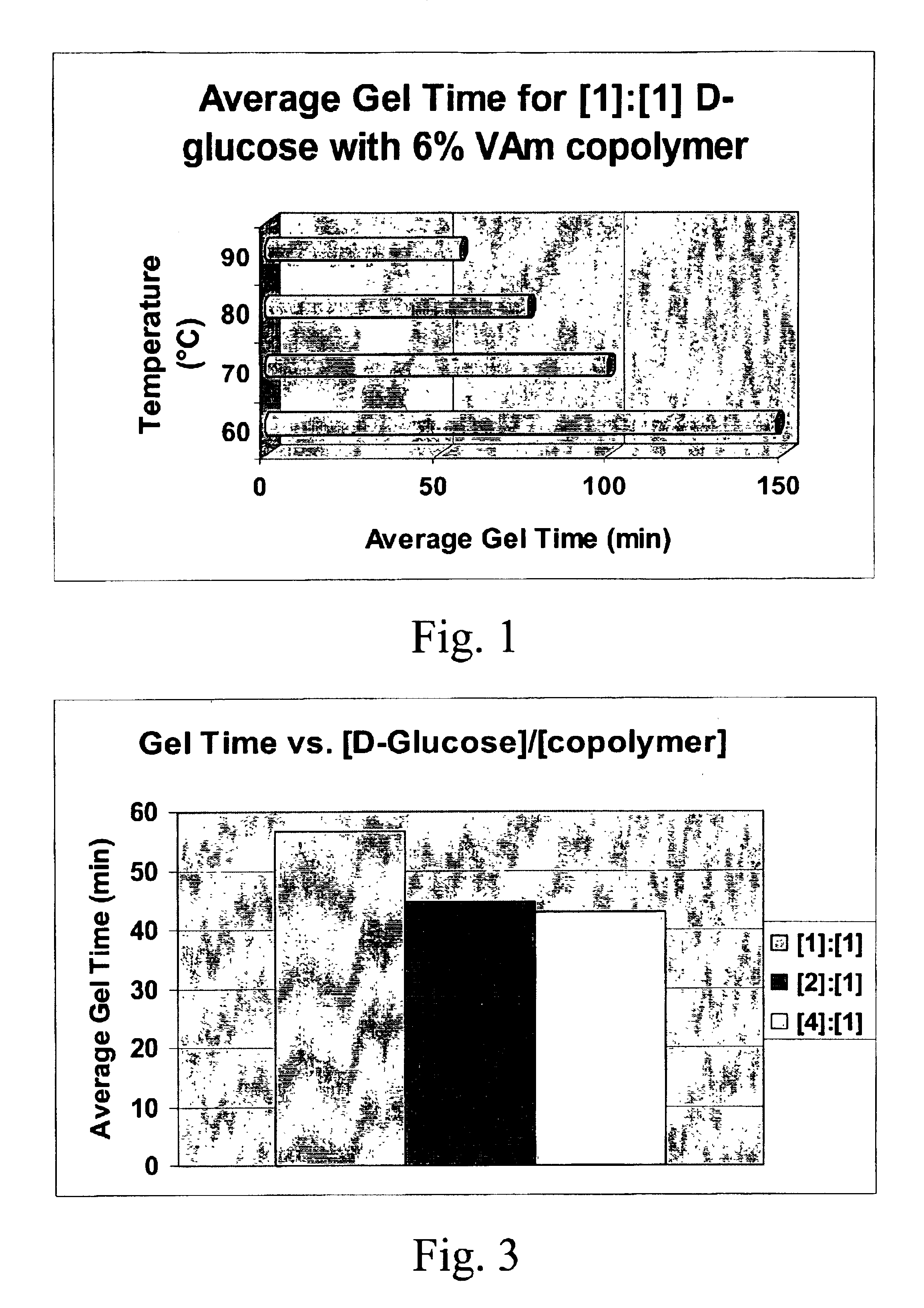
A composition includes at least one hydrophilic polymer containing primary (—NH2) and / or secondary (—NHR) amine groups and at least one saccharide containing a reducible function. A method of increasing the strength of paper includes the step of contacting the paper with a composition comprising (i) at least one hydrophilic polymer containing at least two groups which are independently the same or different a primary amine group or a secondary amine group and at least one saccharide containing a reducible function. A hydrogel composition is formed from a mixture of at least one hydrophilic polymer containing at least two groups which are independently the same or different a primary amine group or a secondary amine group and at least one saccharide containing a reducible function.
Owner:UNIVERSITY OF PITTSBURGH +1
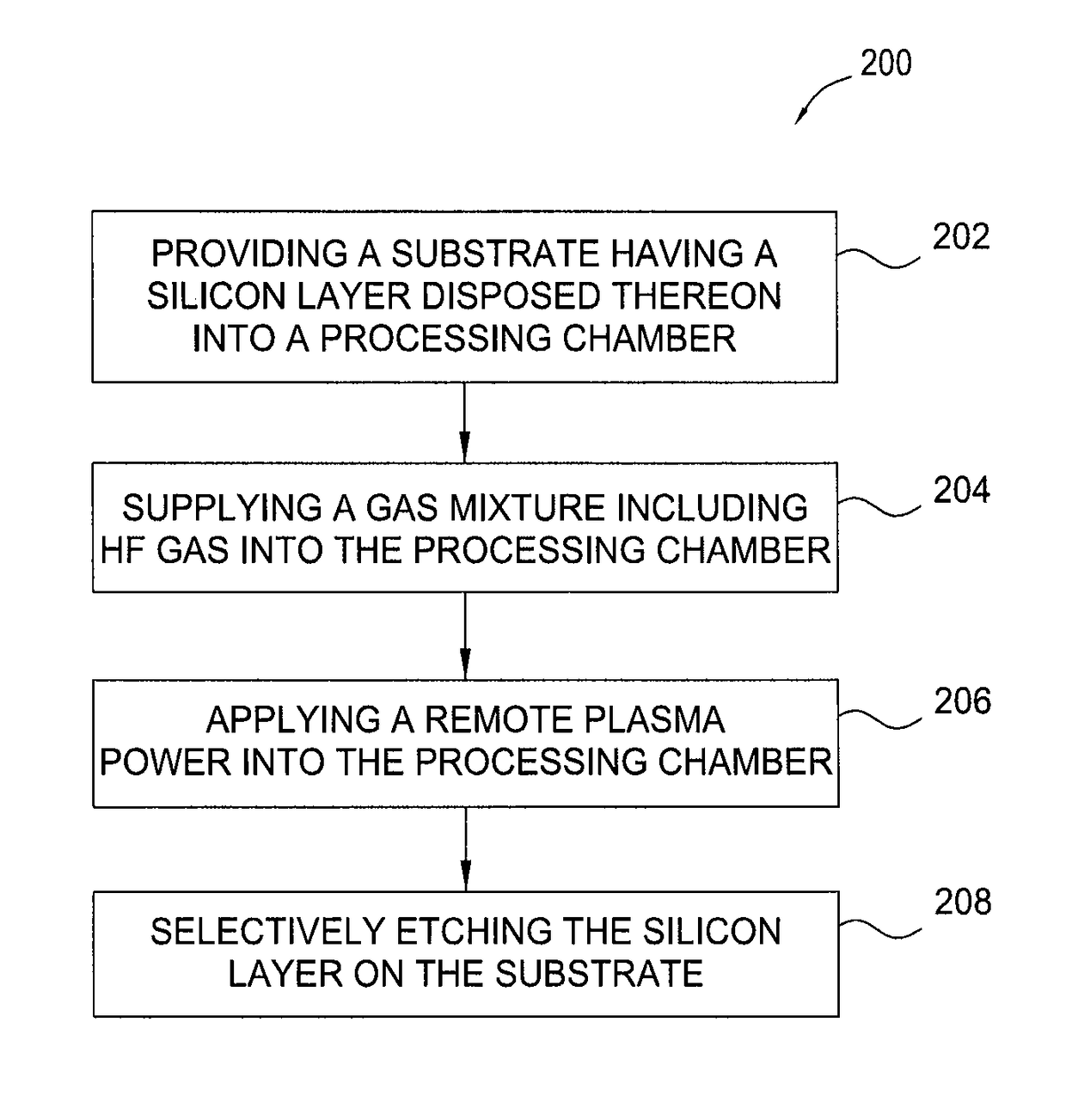
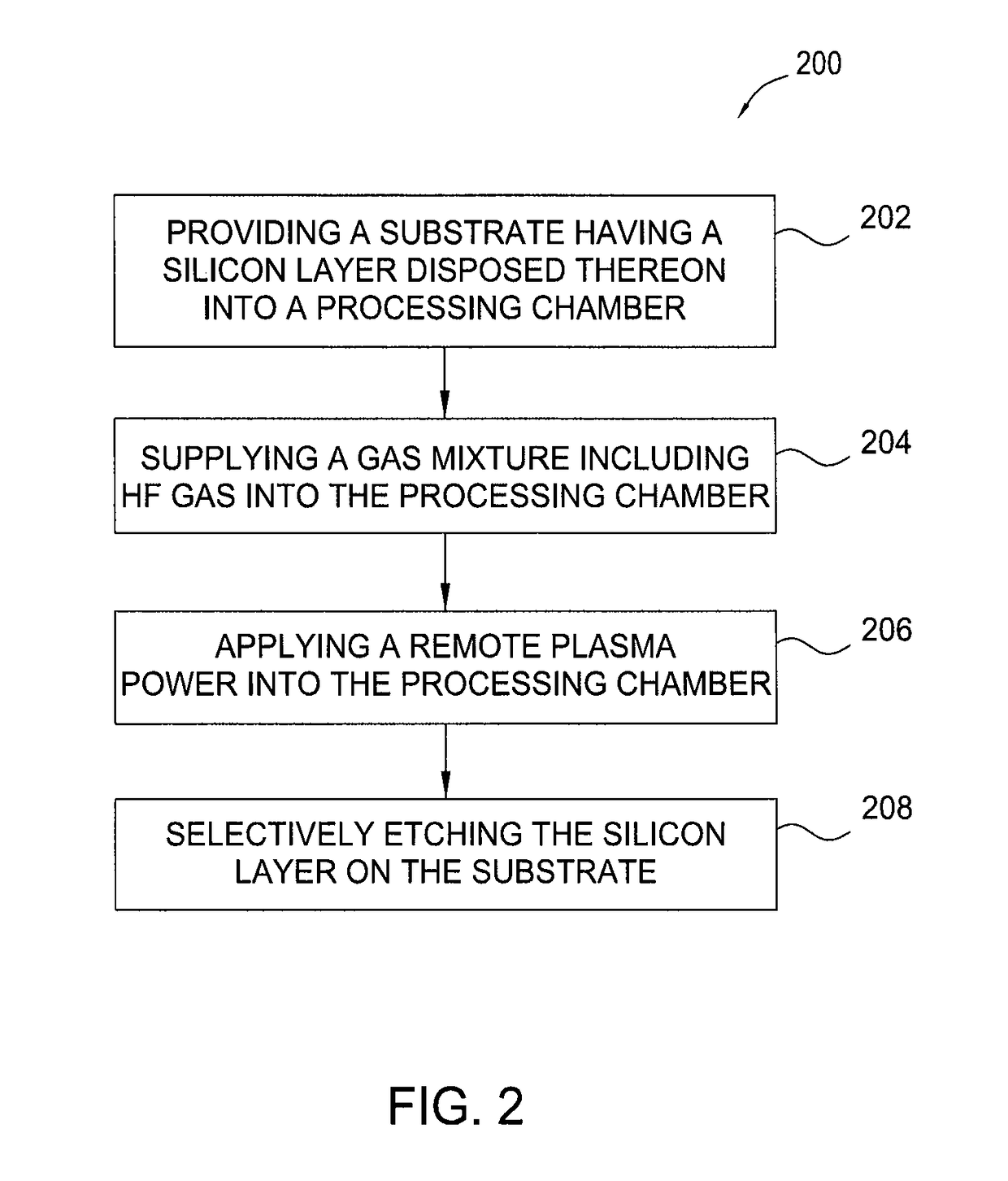
Methods for selective etching of a silicon material using HF gas without nitrogen etchants
InactiveUS9831097B2Electric discharge tubesSemiconductor/solid-state device manufacturingRemote plasmaEtching
The present disclosure provides methods for etching a silicon material in a device structure in semiconductor applications. In one example, a method for etching features in a silicon material includes performing a remote plasma process formed from an etching gas mixture including HF gas without nitrogen etchants to remove a silicon material disposed on a substrate.
Owner:APPLIED MATERIALS INC
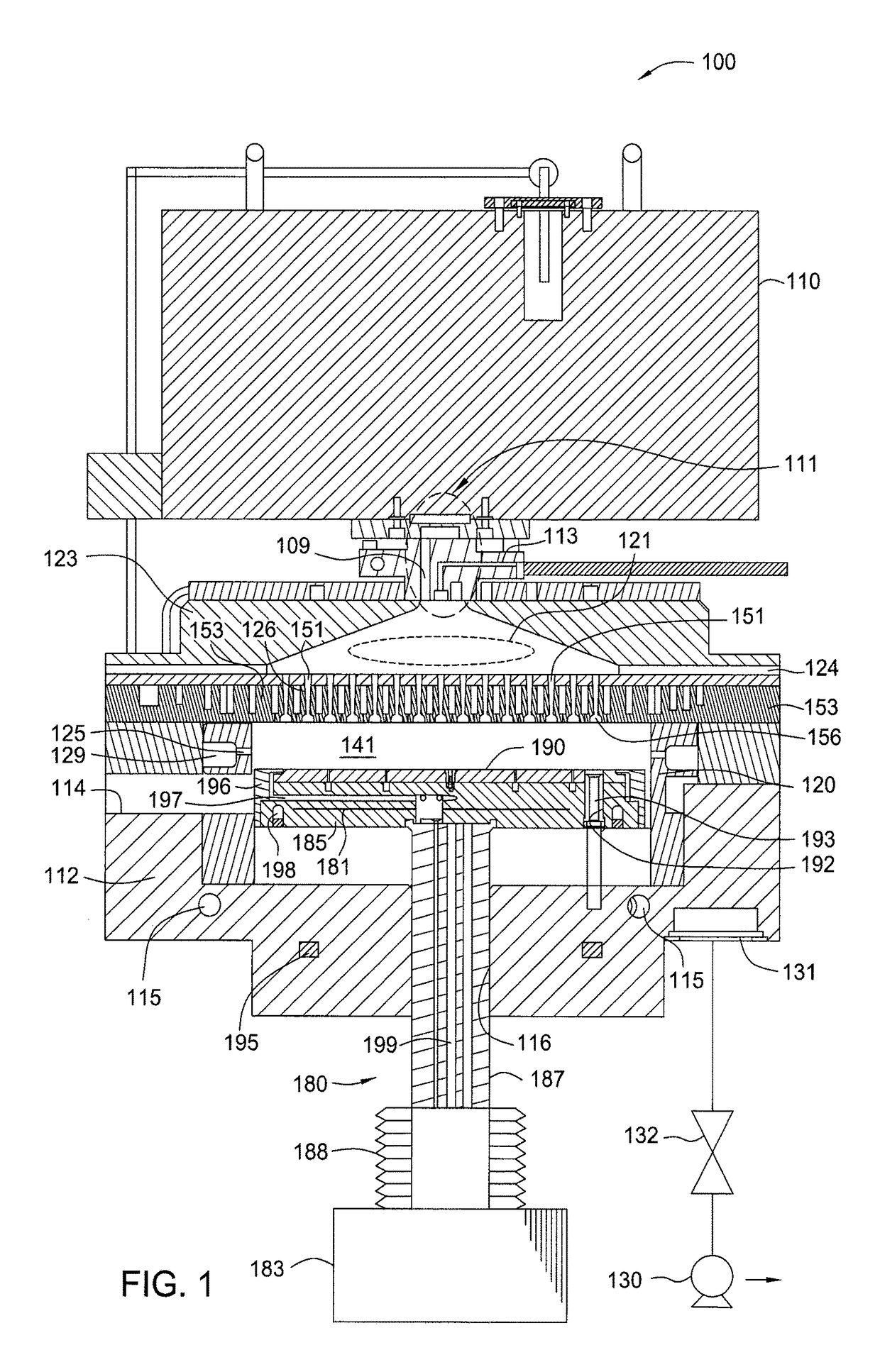
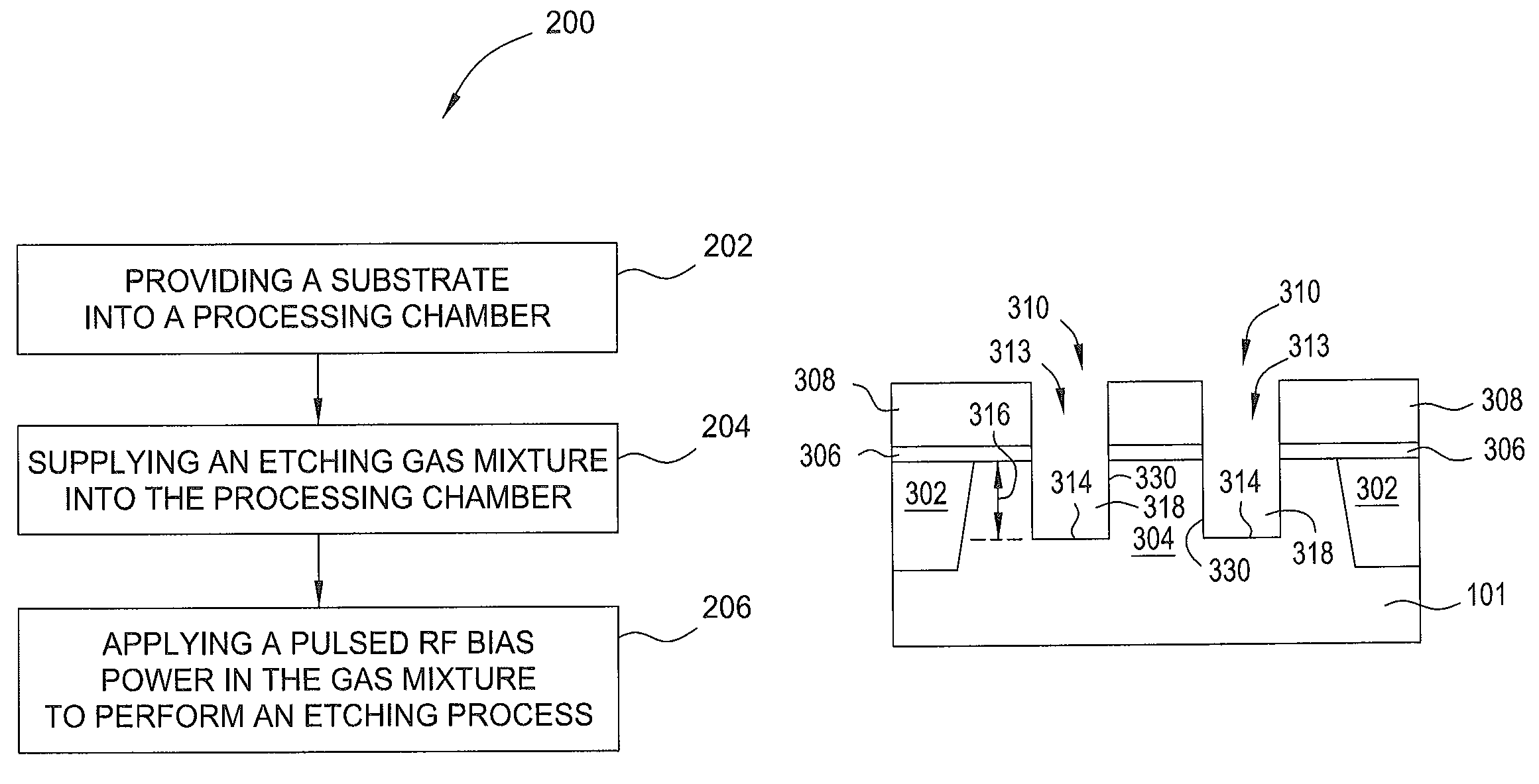
Methods for forming a round bottom silicon trench recess for semiconductor applications
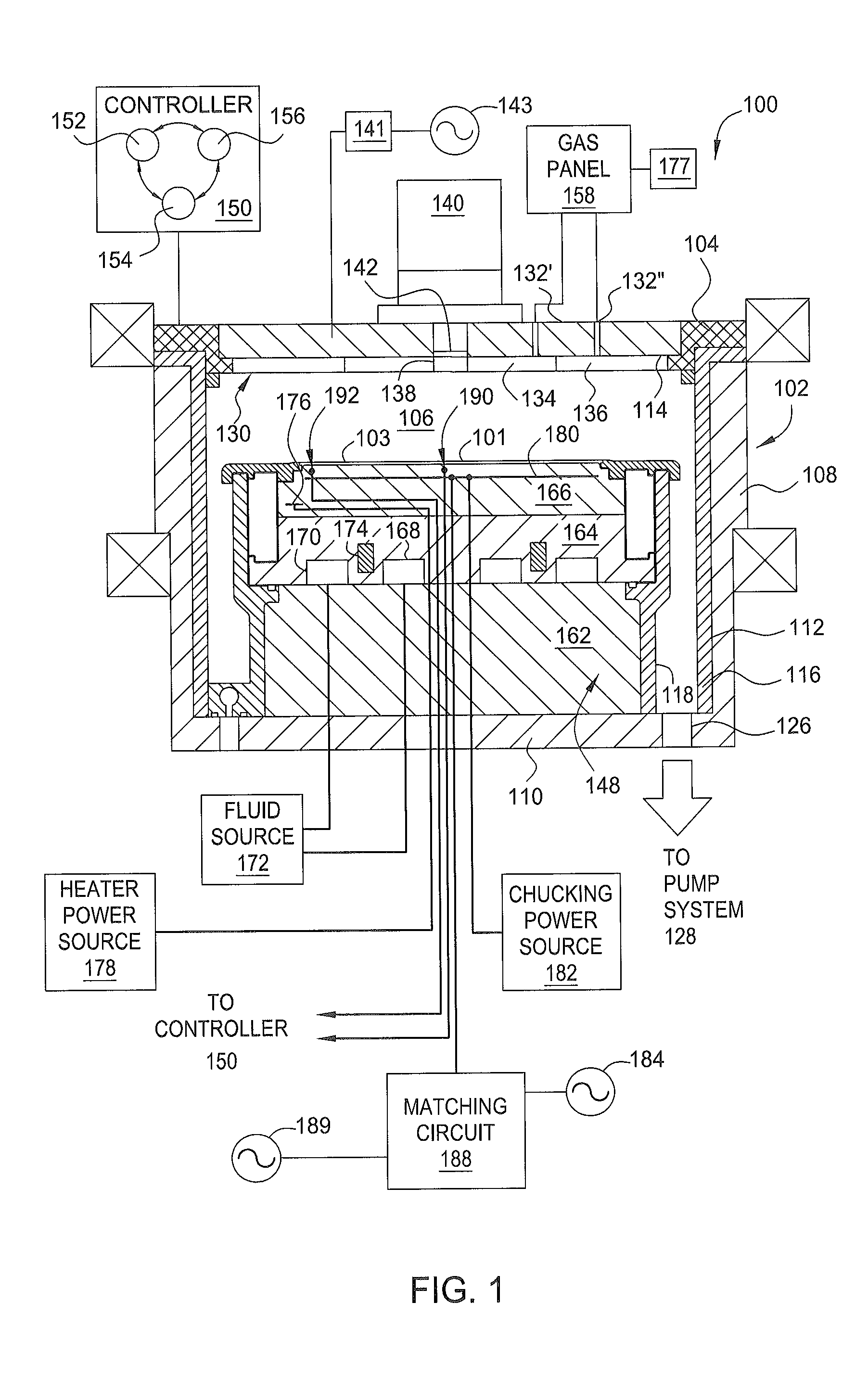
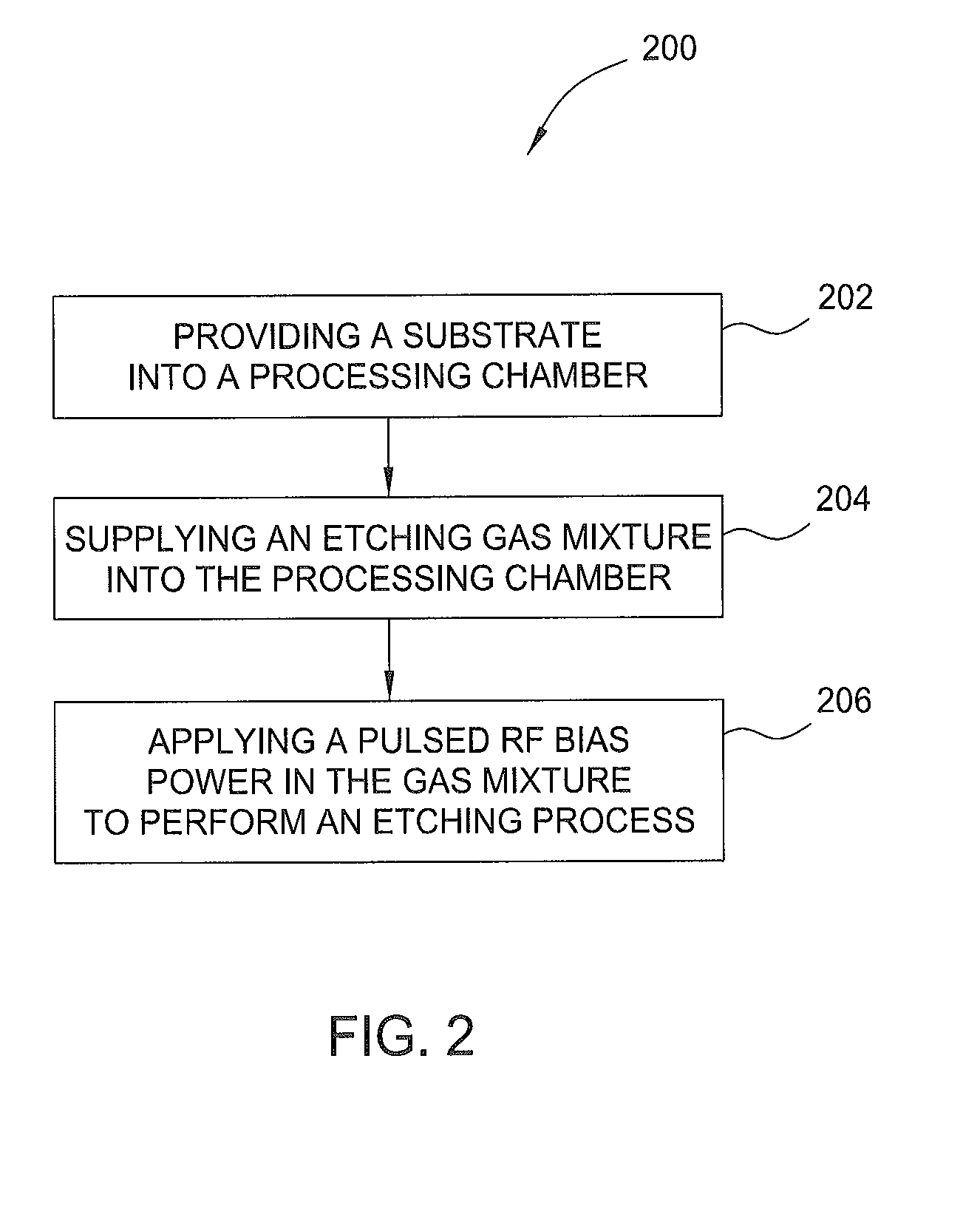
ActiveUS8932947B1Electric discharge tubesSemiconductor/solid-state device manufacturingPhotoresistSemiconductor
Embodiments of the present invention provide methods to etching a recess channel in a semiconductor substrate, for example, a silicon containing material. In one embodiment, a method of forming a recess structure in a semiconductor substrate includes transferring a silicon substrate into a processing chamber having a patterned photoresist layer disposed thereon exposing a portion of the substrate, providing an etching gas mixture including a halogen containing gas and a Cl2 gas into the processing chamber, supplying a RF source power to form a plasma from the etching gas mixture, supplying a pulsed RF bias power in the etching gas mixture, and etching the portion of the silicon substrate exposed through the patterned photoresist layer in the presence of the plasma.
Owner:APPLIED MATERIALS INC
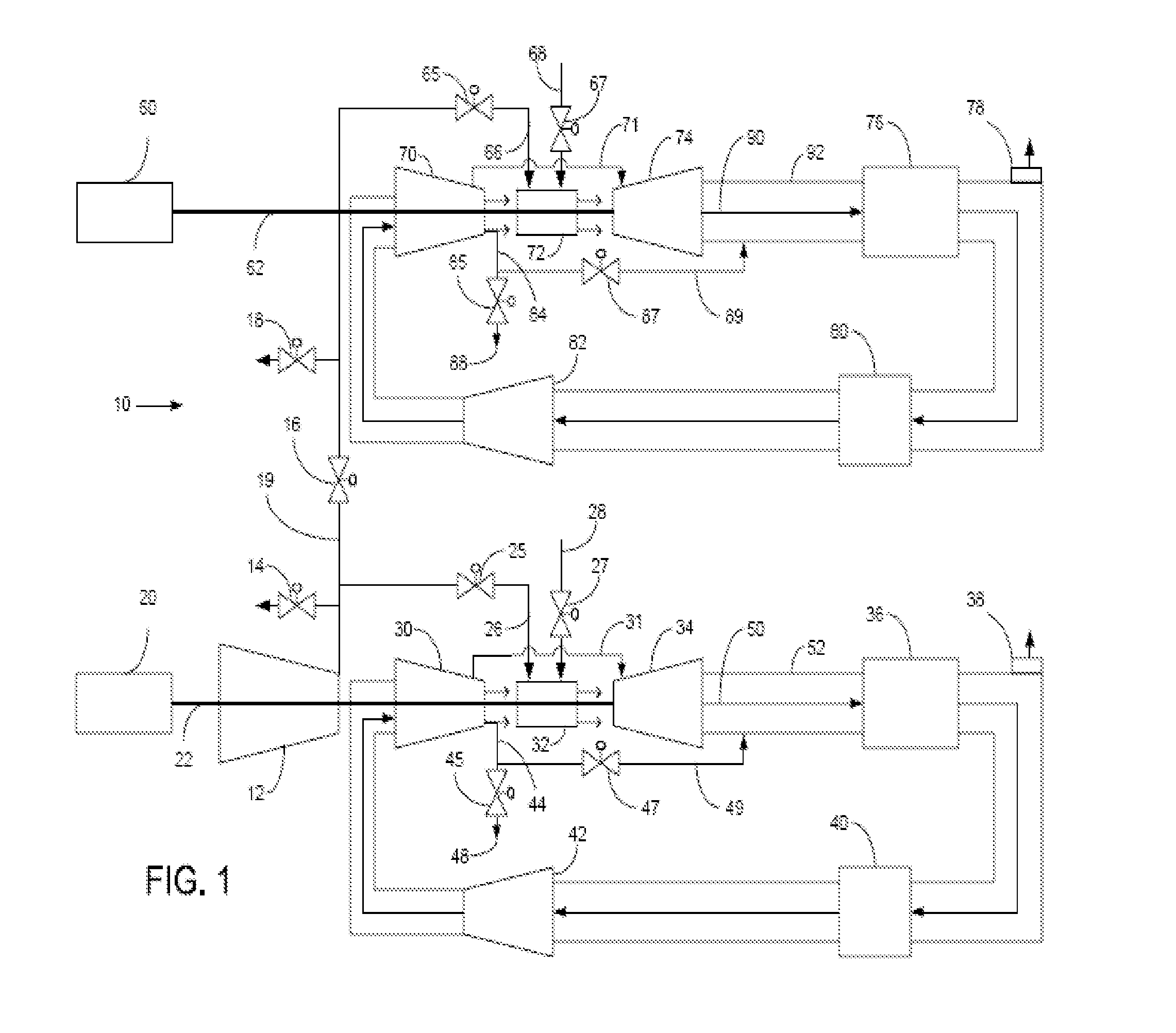
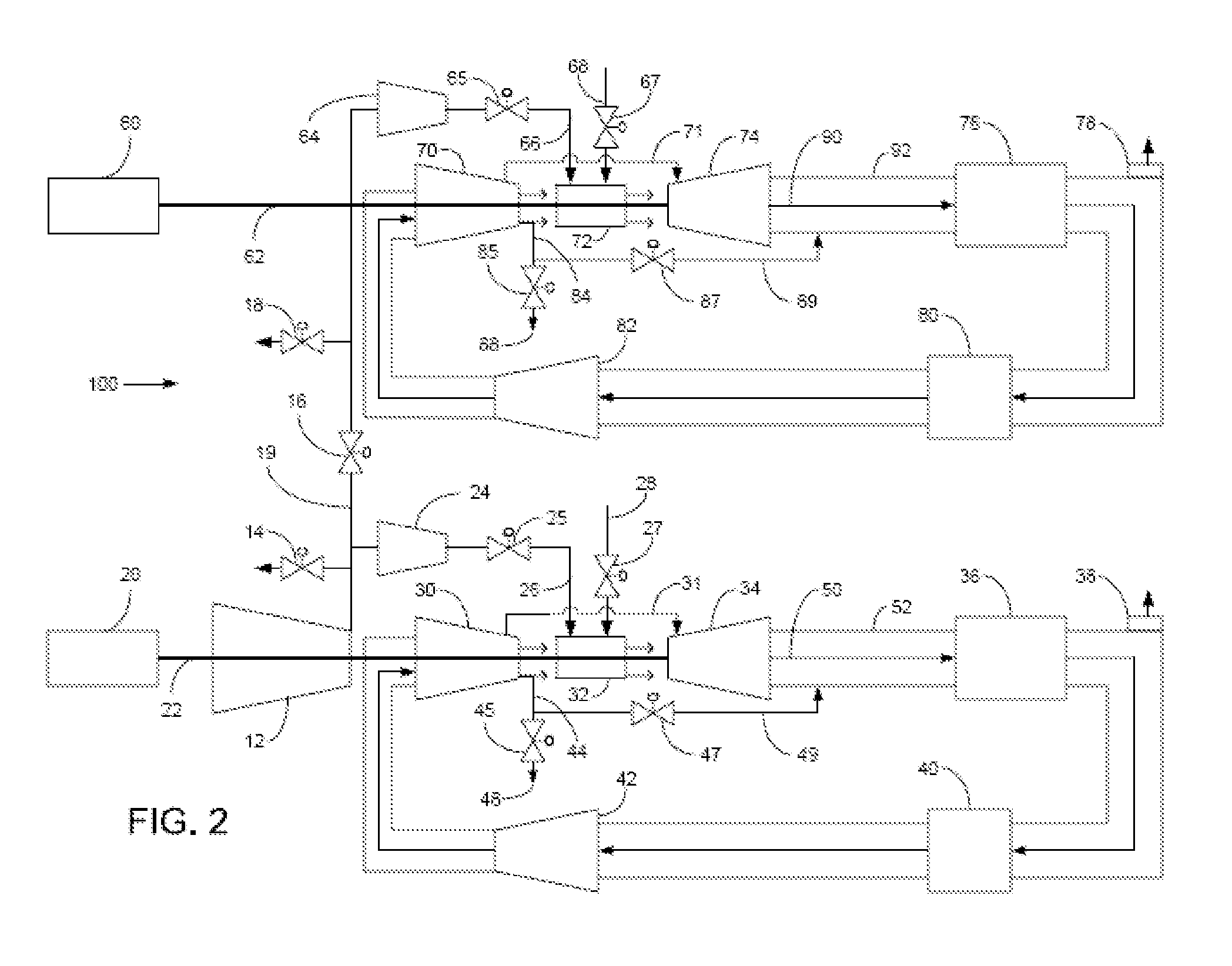
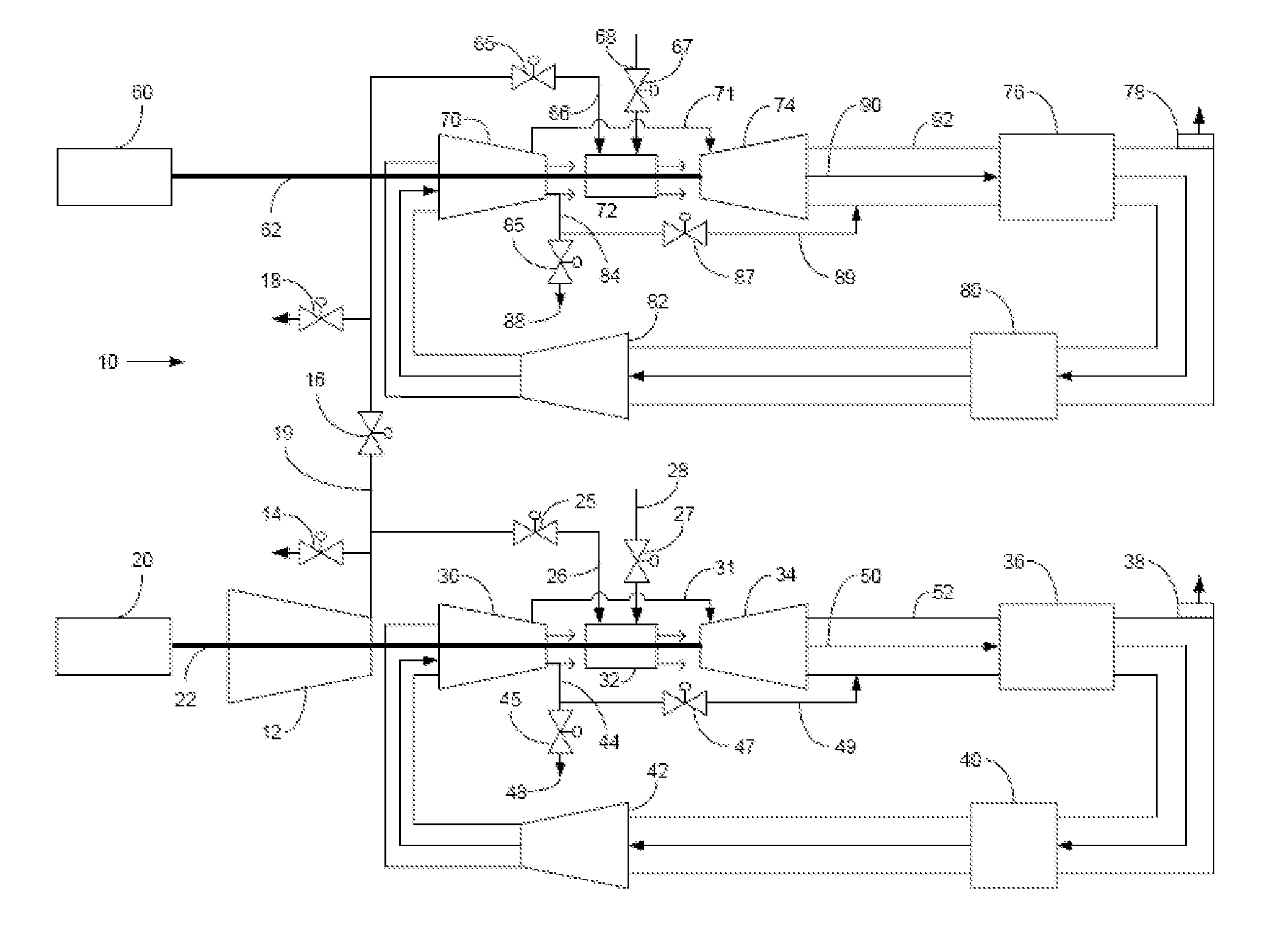
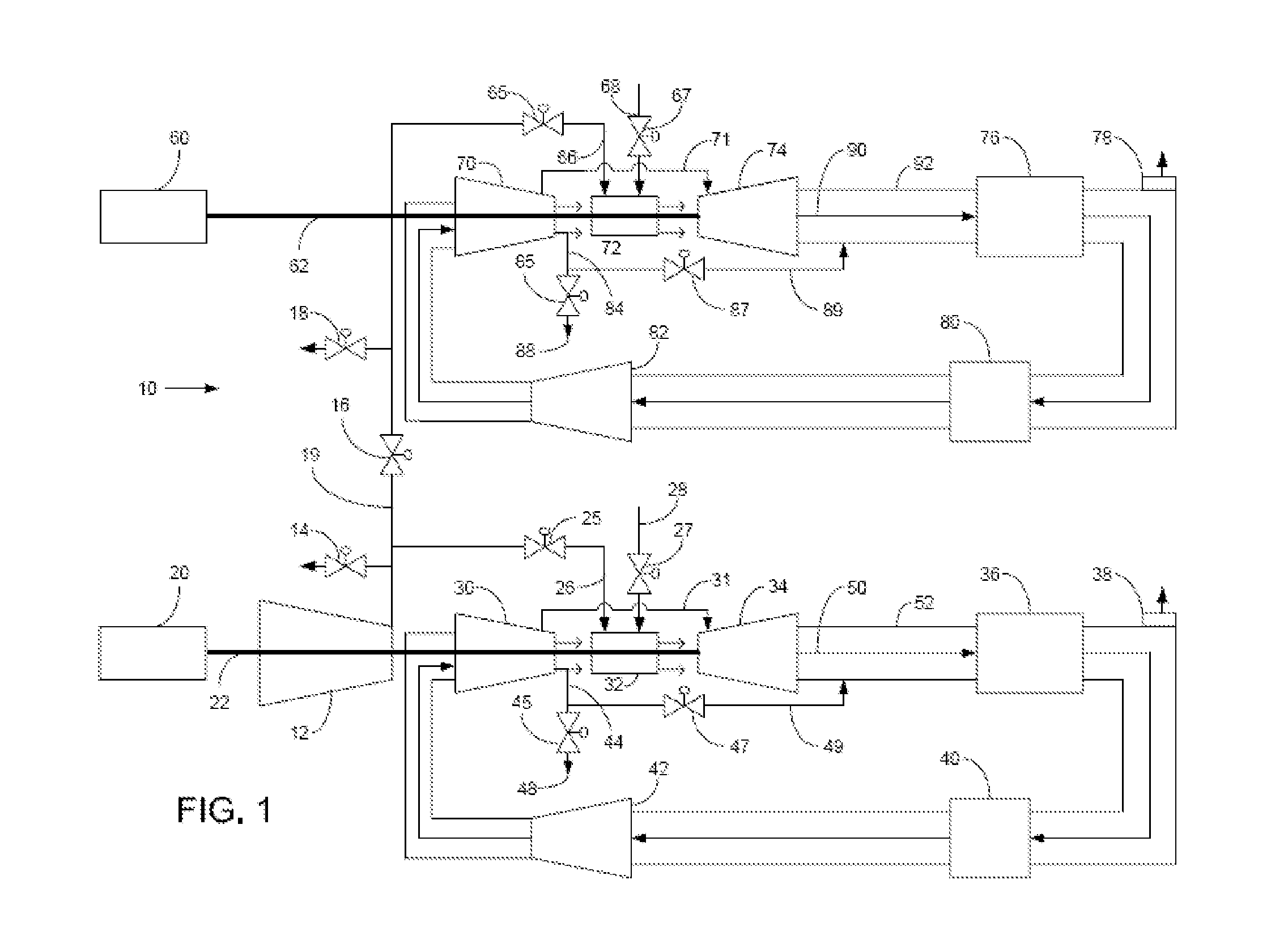
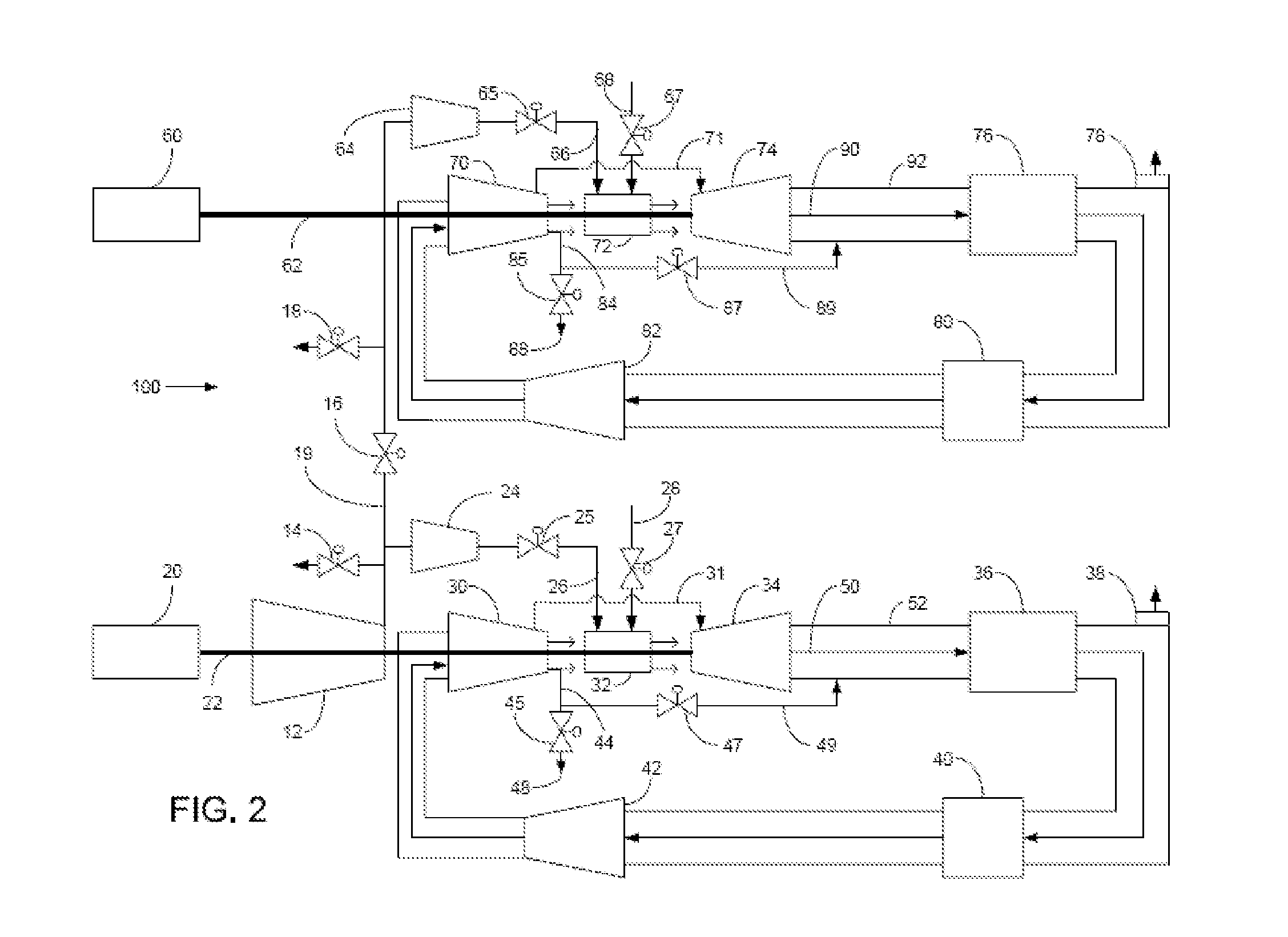
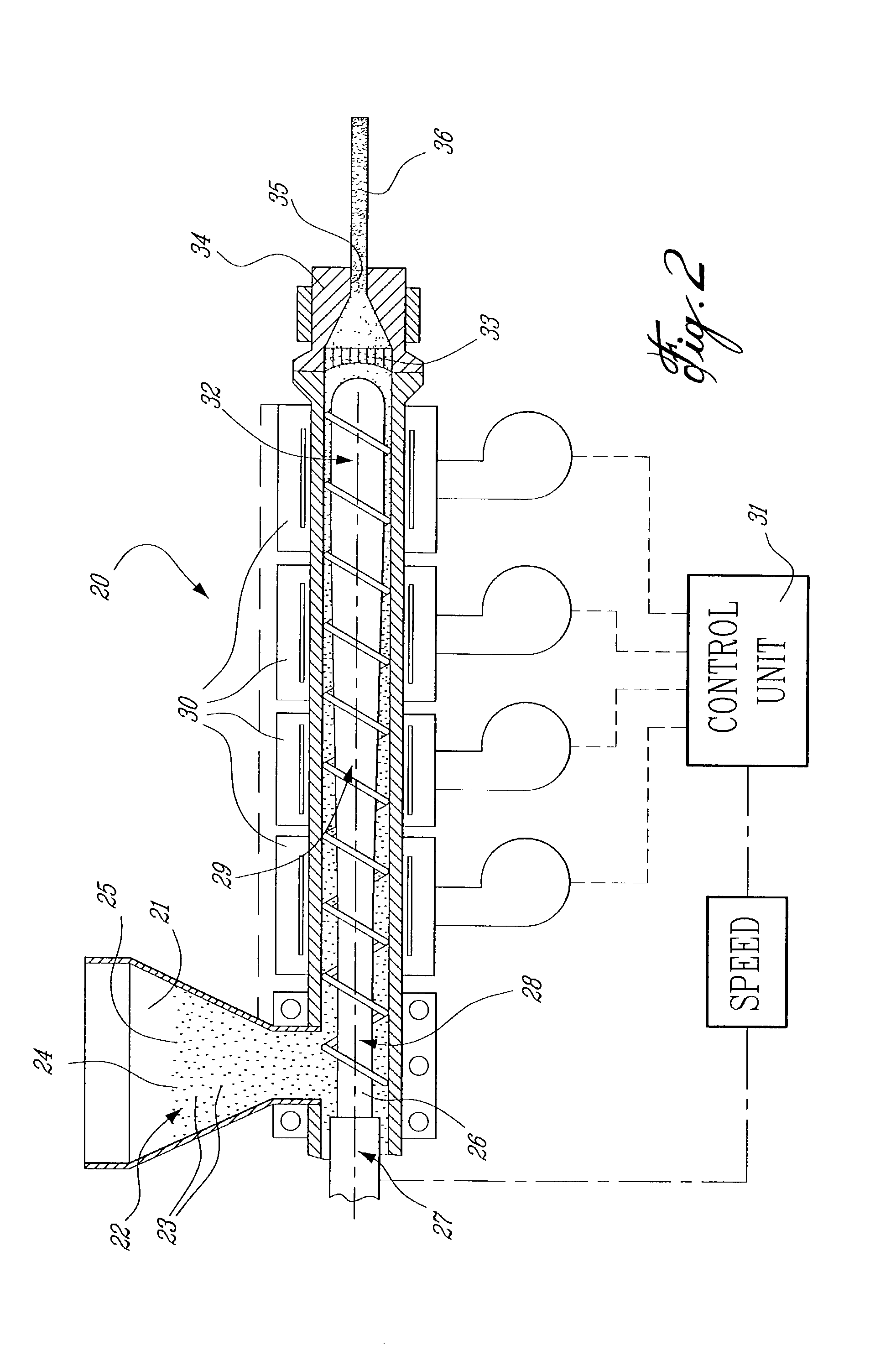
Method of operating a stoichiometric exhaust gas recirculation power plant
At least one main air compressor makes a compressed ambient gas flow. The compressed ambient gas flow is delivered to a turbine combustor at a pressure that is greater than or substantially equal to an output pressure delivered to the turbine combustor from a turbine compressor as at least a first portion of a recirculated gas flow. A fuel stream is delivered to the turbine combustor, and a combustible mixture is formed and burned, forming the recirculated gas flow. A turbine power is produced that is substantially equal to at least a power required to rotate the turbine compressor. At least a portion of the recirculated gas flow is recirculated through a recirculation loop. An excess portion of the recirculated gas flow is vented or a portion of the recirculated gas flow bypasses the turbine combustor or both.
Owner:GENERAL ELECTRIC CO
Water filter materials and water filters containing a mixture of microporous and mesoporous carbon particles
InactiveUS20050279696A1Reduce bacteriaReduce virusMembrane filtersLoose filtering material filtersWater filterActivated carbon filtration
A filter and filter material for providing or treating potable water is provided. The filter includes a housing having an inlet and an outlet, a filter material disposed within the housing, the filter material formed at least in part from a mixture of a plurality of mesoporous and microporous activated carbon particles. Preferably, at least some of the mesoporous activated carbon filter particles are coated with a cationic polymer, and even more preferably, at least some of the particles are coated with a cationic polymer and silver or a silver containing material. Kits comprising filters and information relating to the reduction, killing or removal of bacteria, viruses, microbials, and TTHM are also provided.
Owner:PUR WATER PURIFICATION PRODUCTS INC +2
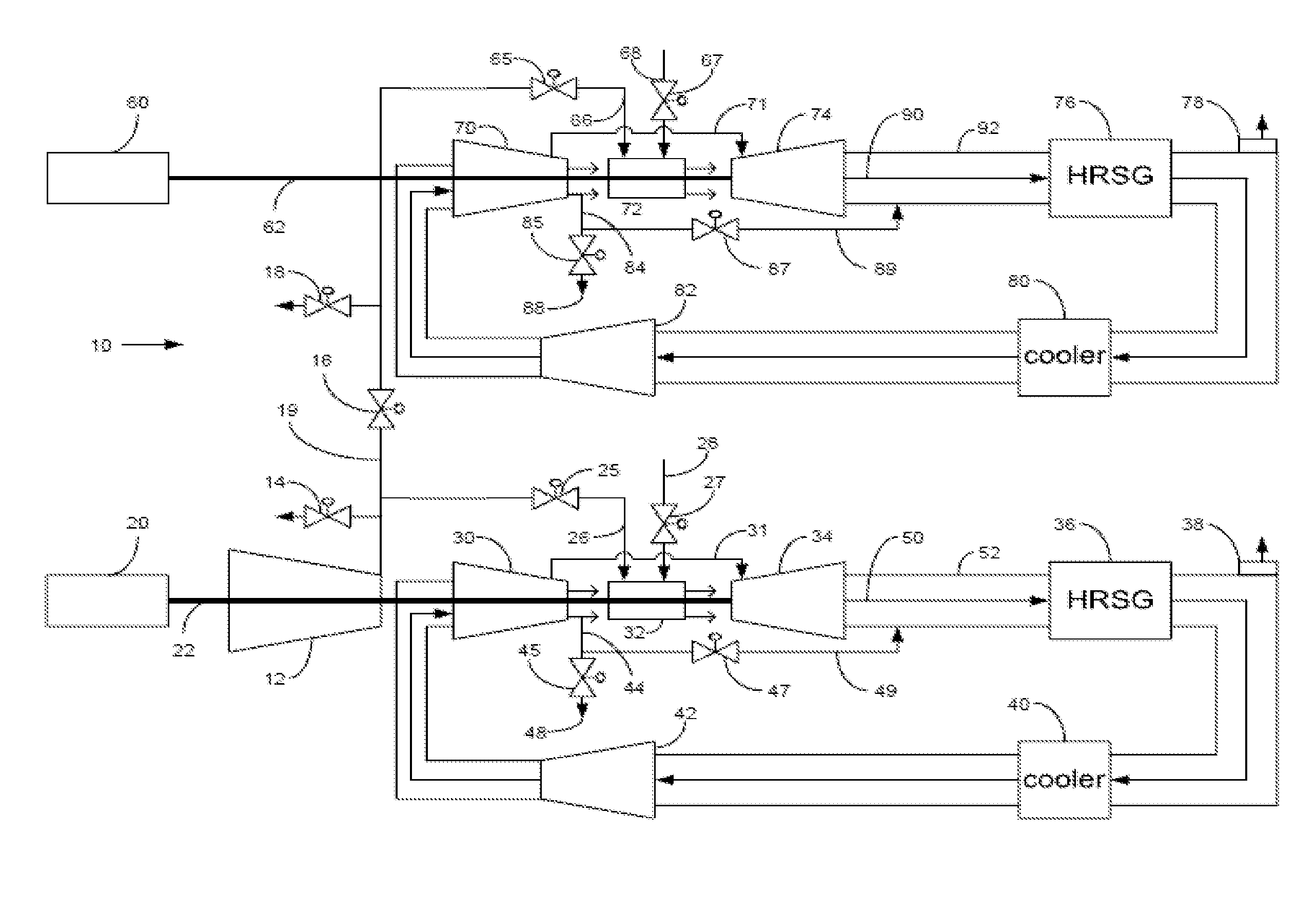
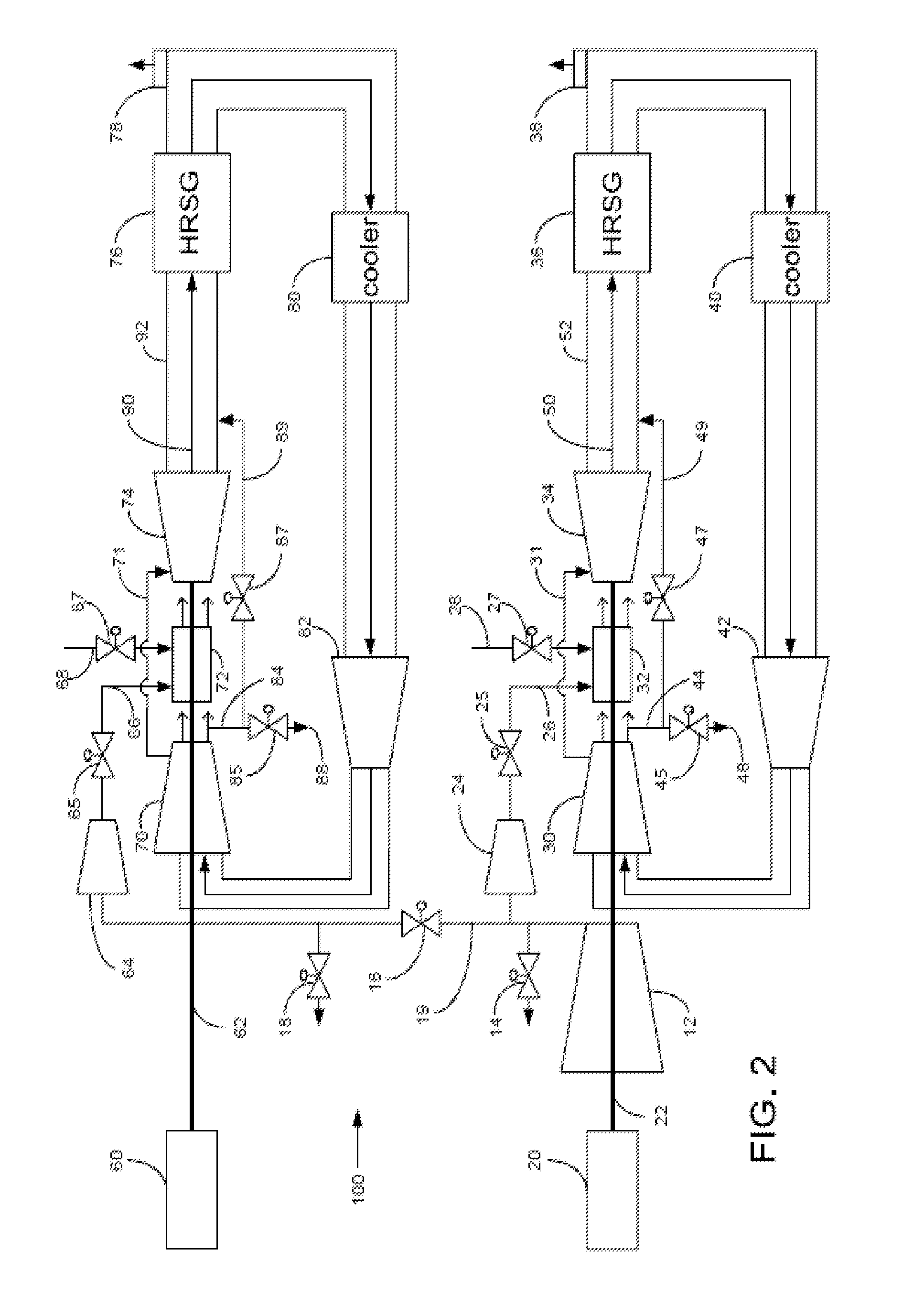
Power plant and method of operation
At least one main air compressor makes a compressed ambient gas flow. The compressed ambient gas flow is delivered to both master and slave turbine combustors at a pressure that is greater than or substantially equal to an output pressure delivered to each turbine combustor from each turbine compressor as at least a first portion of a recirculated gas flow. A fuel stream is delivered to each turbine combustor, and combustible mixtures are formed and burned, forming the recirculated gas flows. A master and slave turbine power are produced, and each is substantially equal to at least a power required to rotate each turbine compressor. At least a portion of the recirculated gas flow is recirculated through recirculation loops. At least a second portion of the recirculated gas flow bypasses the combustors or an excess portion of each recirculated gas flow is vented or both.
Owner:GENERAL ELECTRIC CO
Extruded wood imitation component and process
An extruded wood imitation component and process is described. The component has a solid core containing coloured polymer material formed from a mix of coloured thermoplastic polymer with veins of contrasting coloured polymer throughout the core and on outer surfaces of the component simulating natural wood. In the extrusion process the mixture includes coloured polymer pellets of different colours and sizes which are mixed and melted in an extruder under controlled conditions to provide contrasting streaks of molten polymer throughout a molten extruded core and on outer surfaces of the core of the extrudate which exits the land of the die.
Owner:STAR PLAST HLDG
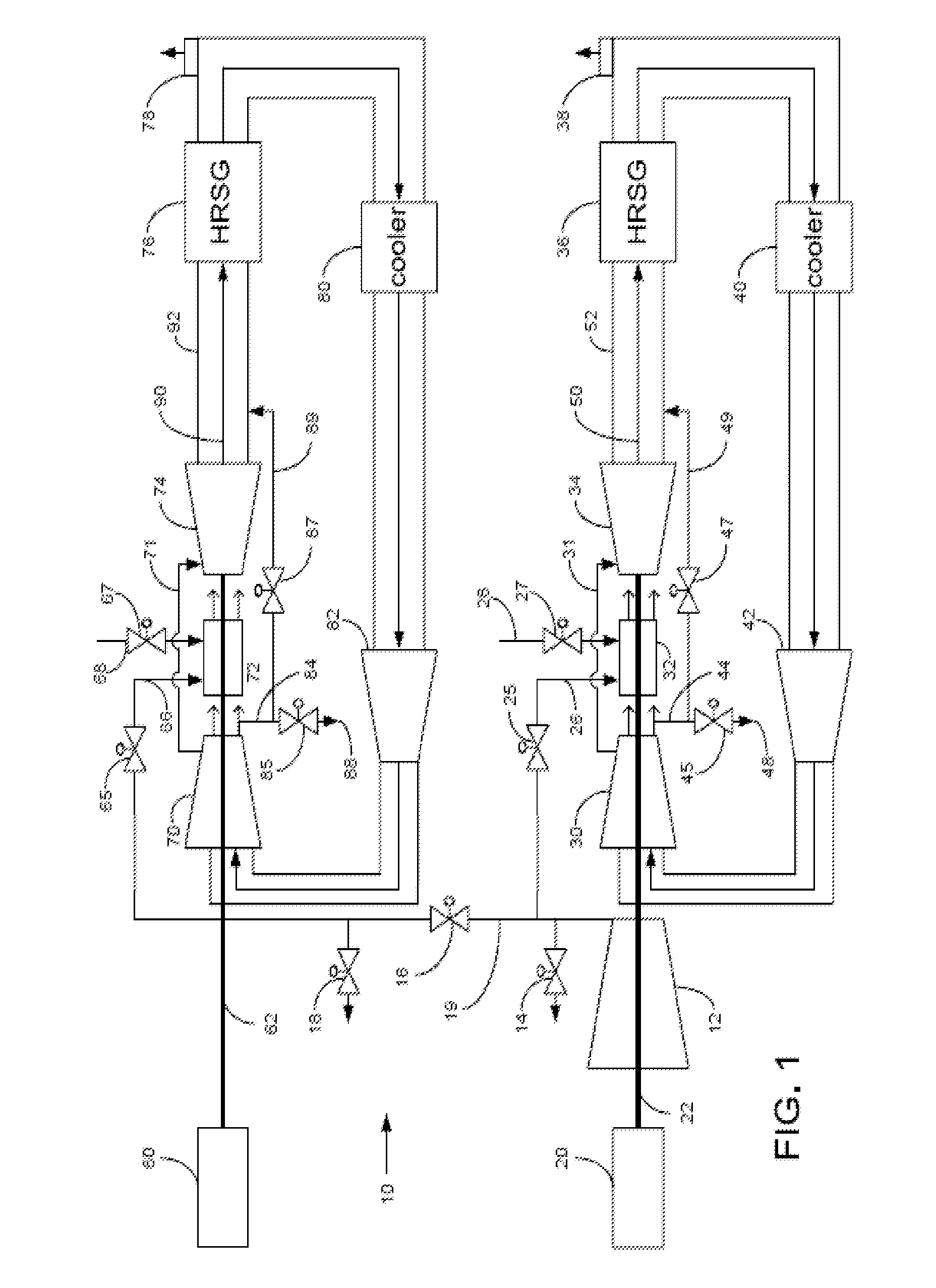
Power plant and method of operation
At least one main air compressor makes a compressed ambient gas flow. The compressed ambient gas flow is delivered to both master and slave turbine combustors at a pressure that is greater than or substantially equal to an output pressure delivered to each turbine combustor from each turbine compressor as at least a first portion of a recirculated gas flow. A fuel stream is delivered to each turbine combustor, and combustible mixtures are formed and burned, forming the recirculated gas flows. A master and slave turbine power are produced, and each is substantially equal to at least a power required to rotate each turbine compressor. At least a portion of the recirculated gas flow is recirculated through recirculation loops. At least a second portion of the recirculated gas flow bypasses the combustors or an excess portion of each recirculated gas flow is vented or both.
Owner:GENERAL ELECTRIC CO
Controlled porosity article
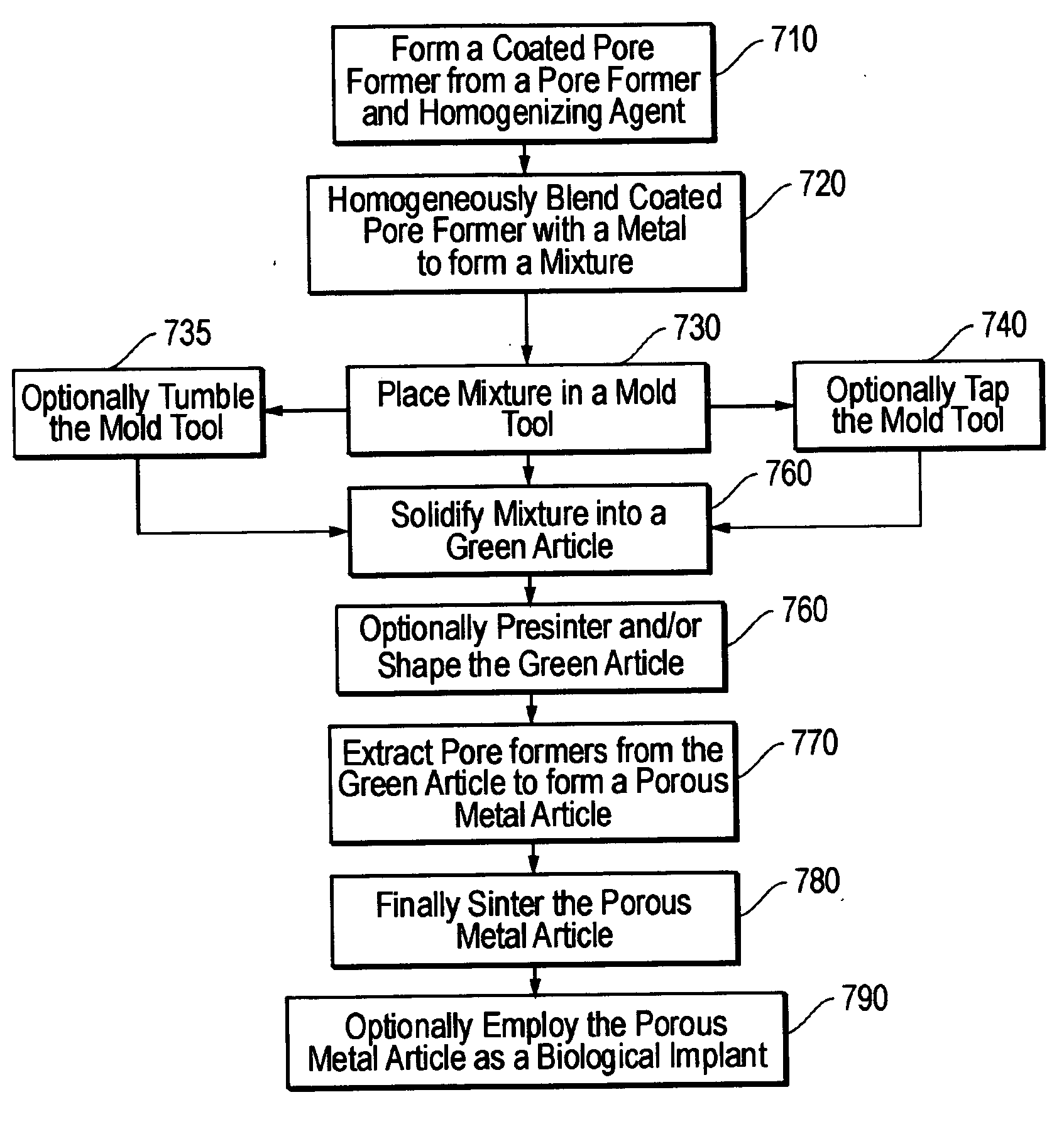
An article of controlled porosity. The porosity of the article may be controlled by display of a particular pore character with respect to pore size, morphology and distribution through a metal, including a uniform distribution. The uniform distribution of porosity within the metal may be provided by a way of a coated pore former including a homogenizing agent thereat to maintain a uniform distribution of pore former throughout a mixture of the coated pore former and a powdered metal. A metal article may be formed of varying layers of porous metals each formed from an independent mixture of coated pore former and metal as indicated.
Owner:PRAXIS POWDER TECH
Method of etching high aspect ratio openings
InactiveUS20020179570A1High aspect ratioIncrease the wall areaDecorative surface effectsSemiconductor/solid-state device manufacturingSilanesThinning
A method of etching a deep, high aspect ratio opening in a silicon substrate includes etching the substrate with a first plasma formed using a first gaseous mixture including a bromine containing gas, an oxygen containing gas and a first fluorine containing gas. The etching process with the first gaseous mixture produces a sidewall passivating deposit, which builds up near the opening entrance. To reduce this buildup, and to increase the average etching rate, the sidewall passivating deposit is periodically thinned by forming a second plasma using a mixture containing silane and a second fluorine containing gas. The substrate remains in the same plasma reactor chamber during the entire process and the plasma is continuously maintained during the thinning step. Holes of a depth greater than 40 times the width may be produced using repeated cycles of etching and thinning.
Owner:INFINEON TECH AG +1
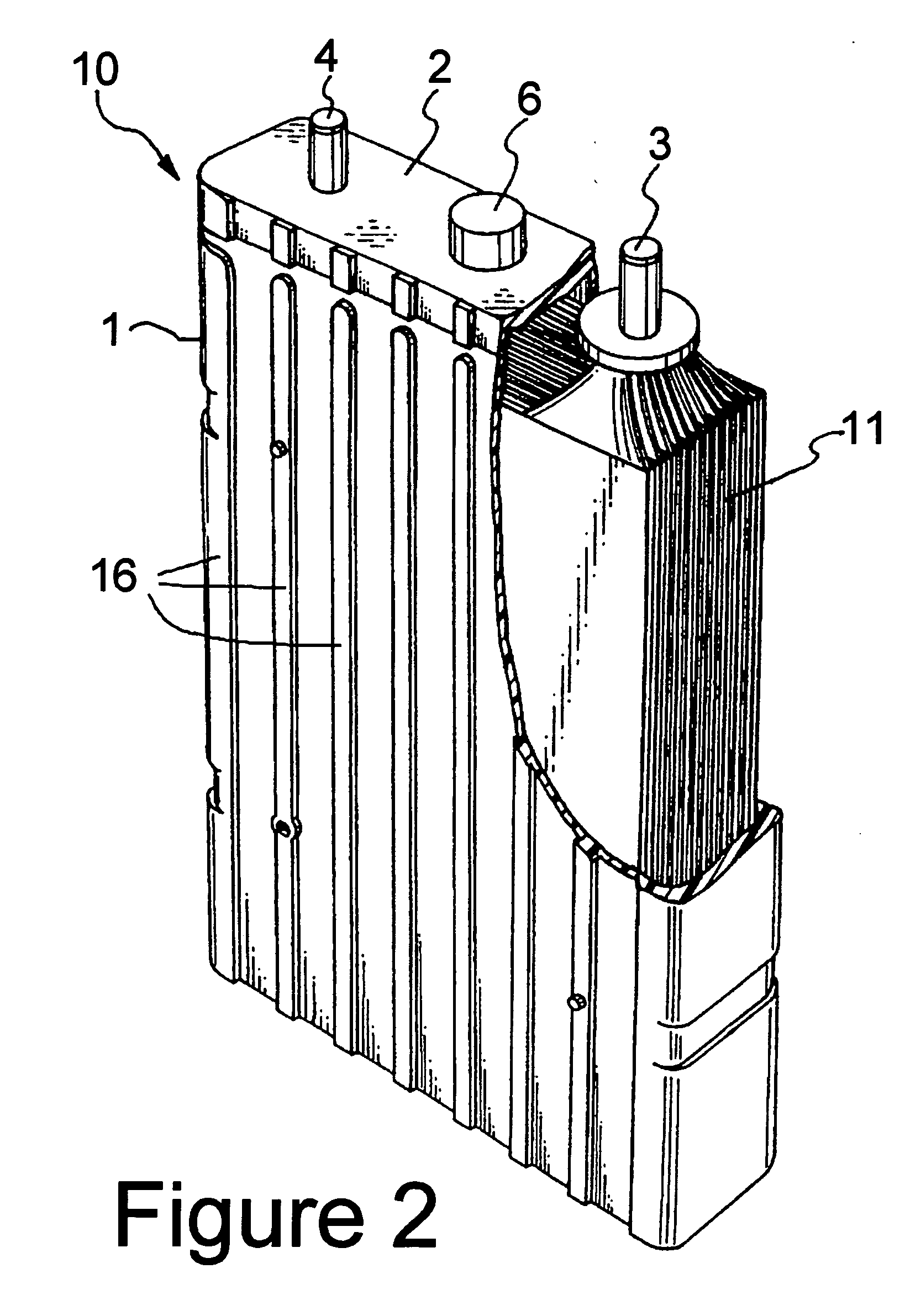
Battery employing thermally conductive polymer case
InactiveUS20050233206A1Improve thermal conductivitySmall-sized cells cases/jacketsLarge-sized cells cases/jacketsElectrical batteryConductive polymer
A battery having at least one group of electric power-generating elements each comprising at least a positive electrode, a negative electrode and a separator; and a battery case containing the group of electric power-generating elements. The battery case is formed from a mixture which includes a matrix material selected from the group consisting of plastics, polymers, resins or combinations thereof. The mixture further includes a thermally conductive, electrically insulating material distributed throughout the matrix material. The thermally conductive material has a thermal conductivity at least one order of magnitude higher than the thermal conductivity of the matrix material. The present invention also includes battery cases (lids and containers) used in making the batteries and formed of the mixture.
Owner:CHEVRON TECH VENTURES
Method for producing highly conductive sheet molding compound, fuel cell flow field plate, and bipolar plate
ActiveUS20070125493A1Improves Structural IntegrityAdhesive processesMechanical working/deformationFuel cellsSheet moulding compound
This invention provides a method of producing a highly electrically conductive sheet molding compound (SMC) composition and a fuel cell flow field plate or bipolar plate made from such a composition. The plate exhibits a conductivity typically greater than 100 S / cm and more typically greater than 200 S / cm. In one preferred embodiment, the method comprises: (a) providing a continuous sheet of a substrate material (bottom sheet) and a continuous sheet of flexible graphite (top sheet) from respective rollers; (b) feeding a resin mixture (comprising a thermoset resin and a conductive filler) to a space between the top sheet and the bottom sheet in such a way that the resin mixture forms a uniform core layer sandwiched between the two sheets to obtain a laminated structure; (c) compressing the laminated structure to obtain a SMC composition having two opposite outer surfaces; and (e) impressing a fluid flow channel to either or both of the outer surfaces (e.g., via embossing or matched-die molding) and curing the thermoset resin to obtain the plate.
Owner:NANOTEK INSTR GRP LLC
Tissue products having reduced lint and slough
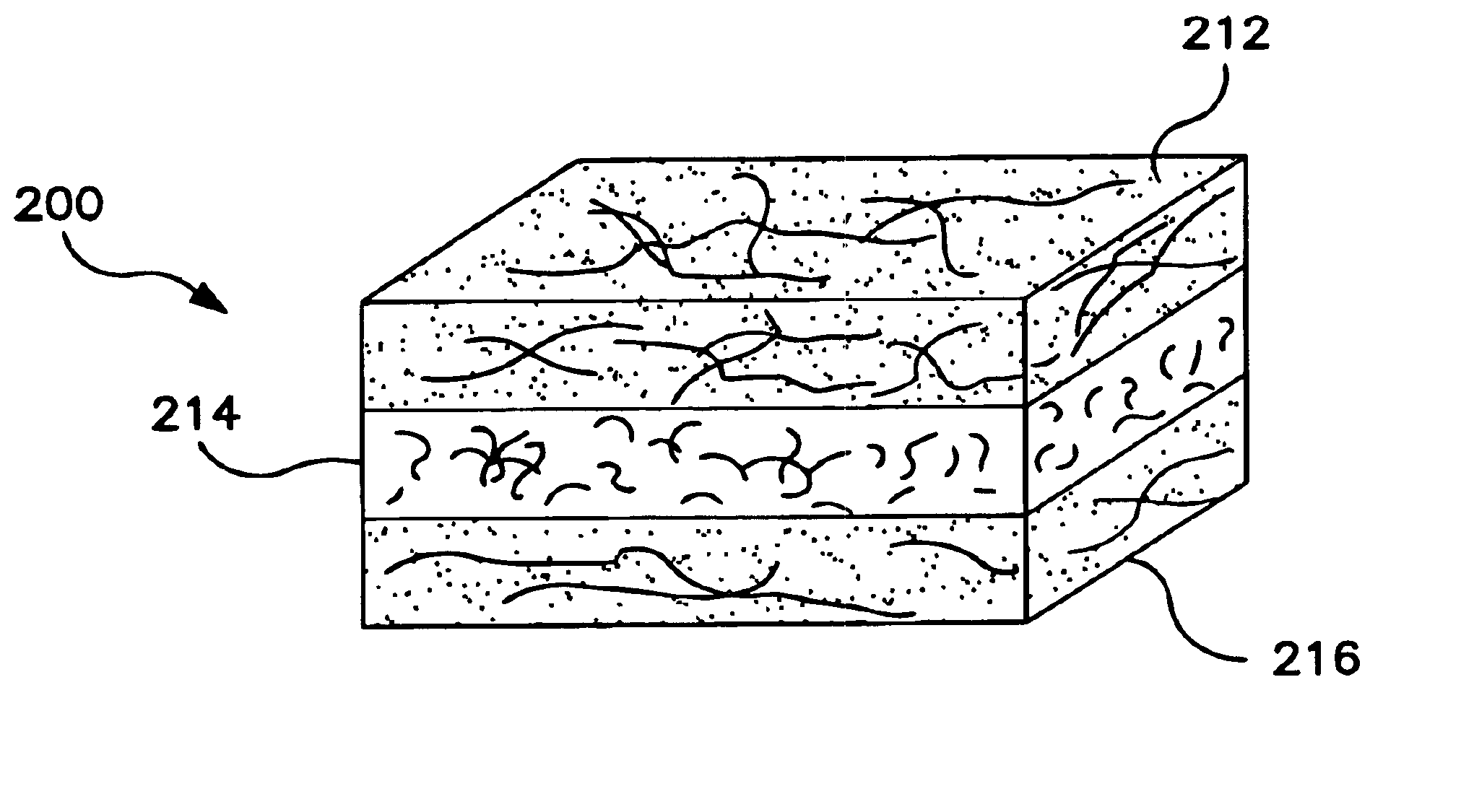
InactiveUS6861380B2Long fiber lengthAvoid short lengthSpecial paperPaper/cardboardPulp fibreBiomedical engineering
A tissue product containing a multi-layered paper web that has at least one layer formed from a blend of pulp fibers and synthetic fibers is provided. By containing at least one layer of synthetic and pulp fibers, it has been discovered that lint and slough of a tissue product formed according to the present invention can be substantially reduced. In addition, by limiting the amount and layers to which the synthetic fibers are applied, the increase in hydrophobicity and cost of the tissue product may be minimized, while still achieving the desired reduction in lint and slough. In some embodiments, the tendency of the synthetic fibers to sink or float in the fibrous furnish may be minimized to enhance processability by selecting certain types of synthetic fibers, e.g., those with a certain density imbalance.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com