Enteric-coated gastro-retentive oral dosage form in tablet form, and application and pharmaceutical composition thereof
An enteric coating, gastric retention technology, used in the treatment of upper gastrointestinal and throat diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Embodiment 1: the production process of large-scale batch
[0165] 1. Intragranular mixing
[0166] The intragranular components (except magnesium stearate) were dispersed and passed through a 20 mesh screen. The components were added to the V-blender and mixed at 25 RPM for 10 minutes. Then, the intragranular magnesium stearate was dispersed and passed through a 20 mesh screen. Magnesium stearate was added to the V-blender and mixed at 25 RPM for 2 minutes. Remove the resulting intragranular blend from the V-blender.
[0167] 2. heavy pressure
[0168] A 24-station Fette tablet press was equipped with eight 0.6250" disc, flat B-shaped die elements. The intragranular blend obtained above was added to the feed hopper of the tablet press. Using Tablet press turret speed of 15-25RPM to compress the blend into a pre-compaction body with a target weight of 1200-1400mg and a hardness of 9-15kp. Appearance, weight, hardness and thickness are checked during re-pressing in...
Embodiment 2
[0185] Example 2: Large-Scale Batches Produced
[0186] Following the procedure described in Example 1 the following large scale batches were produced. Table 1 below summarizes the amounts of excipients used during the production of each of the 6 batches.
[0187] Table 1
[0188]
[0189] Microcrystalline cellulose (MCC) was used as a filler / compression aid in the formulation. Two types of MCC have been evaluated. One manufacturer has shown that Prosolv SMCC90, a siliconized microcrystalline cellulose, has better powder flow than non-siliconized MCC.
[0190] KG-100 is another MCC used as a substitute for SMCC90. According to one manufacturer: KG-1000 has the lowest bulk density among the MCC grades. Compared to other standard MCC grades, KG-100 exhibits superior firmness. KG-1000 particles have a very large L / D value. The particles are easily aligned upright against the force of the compacting body; thus, the contact area of the MCC particles is increased. Entan...
Embodiment 3A
[0196] Example 3A: In Vitro Dissolution / Drug Release
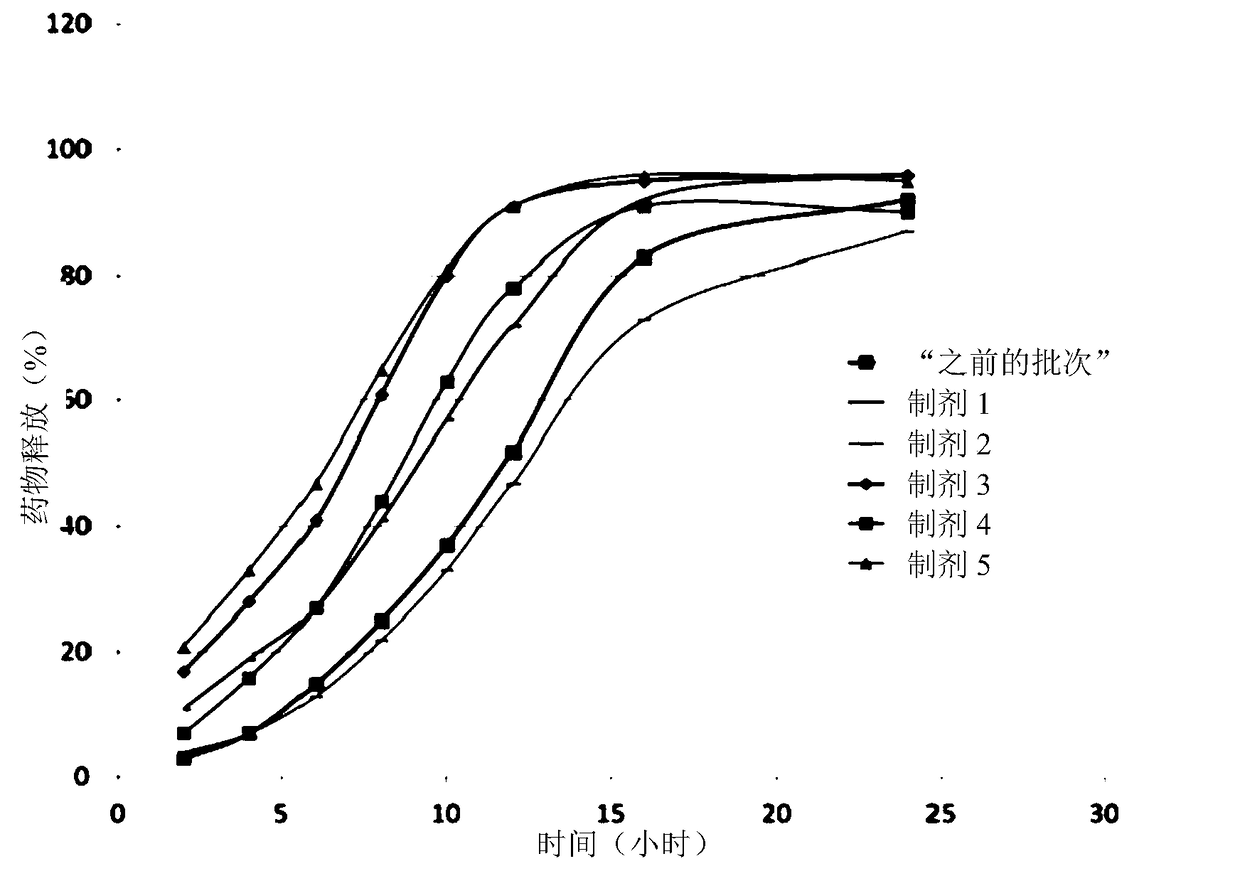
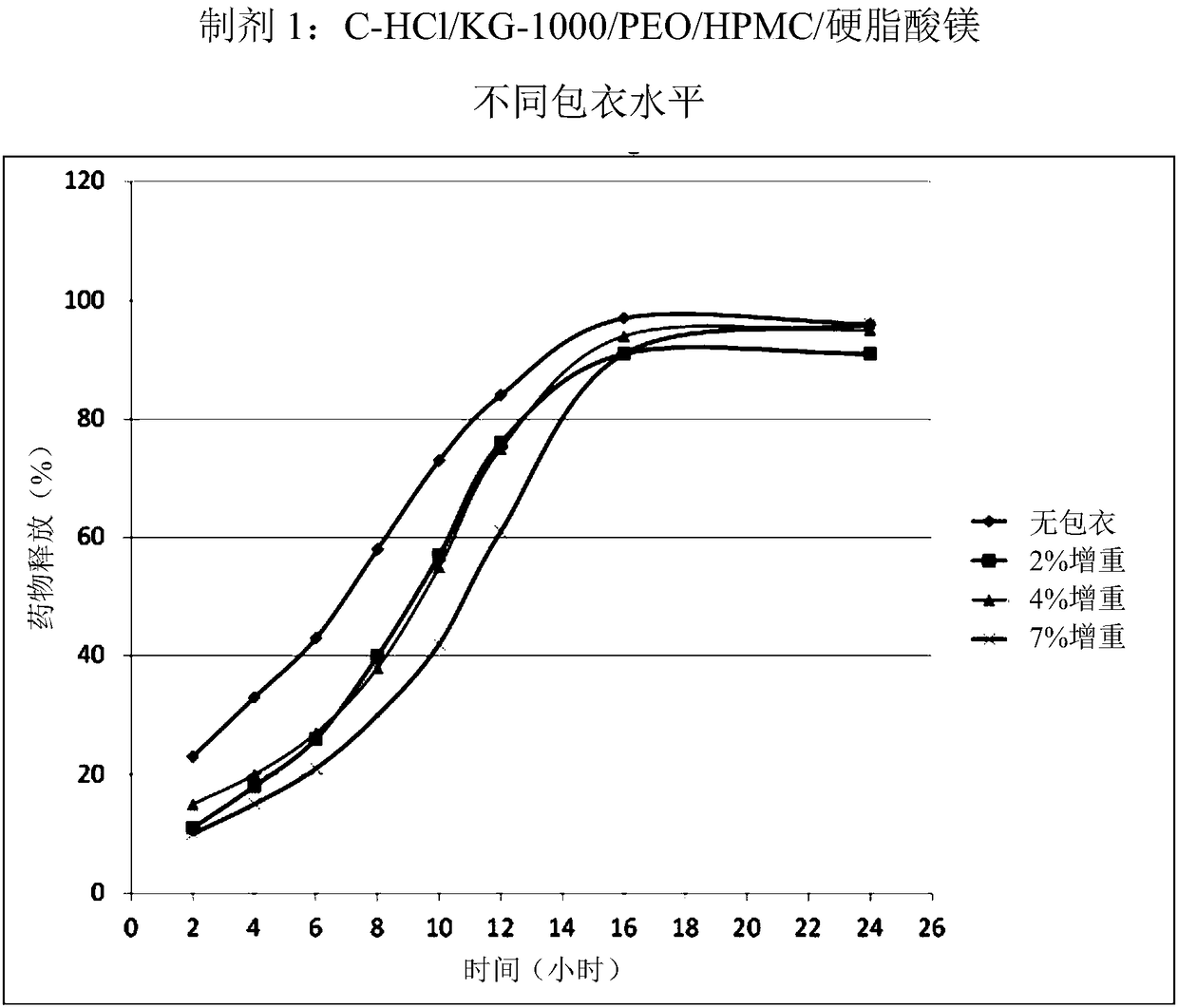
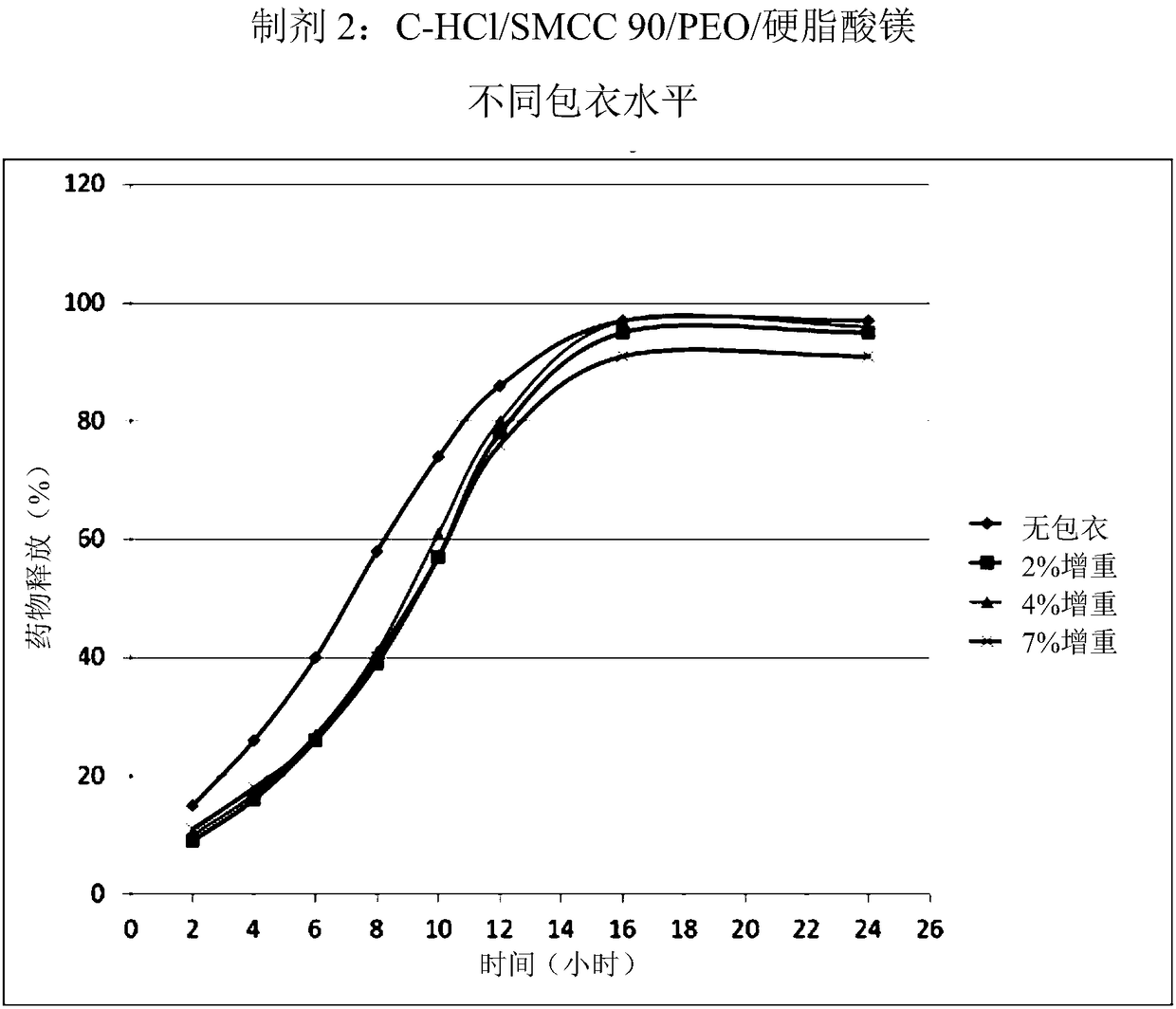
[0197] The drug release rates of six dosage forms produced on a large scale were determined using an indirect method, considering that colesevelam and colesevelam HCl are insoluble polymers and their concentrations could not be determined by standard direct HPLC methods for soluble drugs.
[0198] The drug release rate from the tablets was determined in vitro in acetate buffer at pH 4.5 (100 mM) containing a known bile acid (glycocholic acid) at a concentration of 2 mg / mL. The bile acid consumption of the tablets during swelling and erosion was determined and compared with values obtained from a series of standard solutions of known bile acid concentrations. The rate of bile acid consumption corresponds to the rate of drug release. These drug release rate results were obtained using a USPI Type II (paddle) apparatus with tablets placed in a settler. The results are summarized in figure 1 middle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com