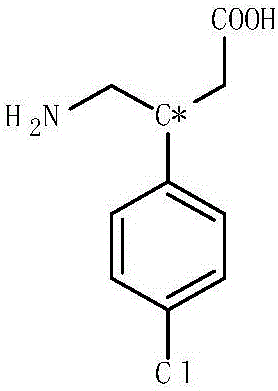
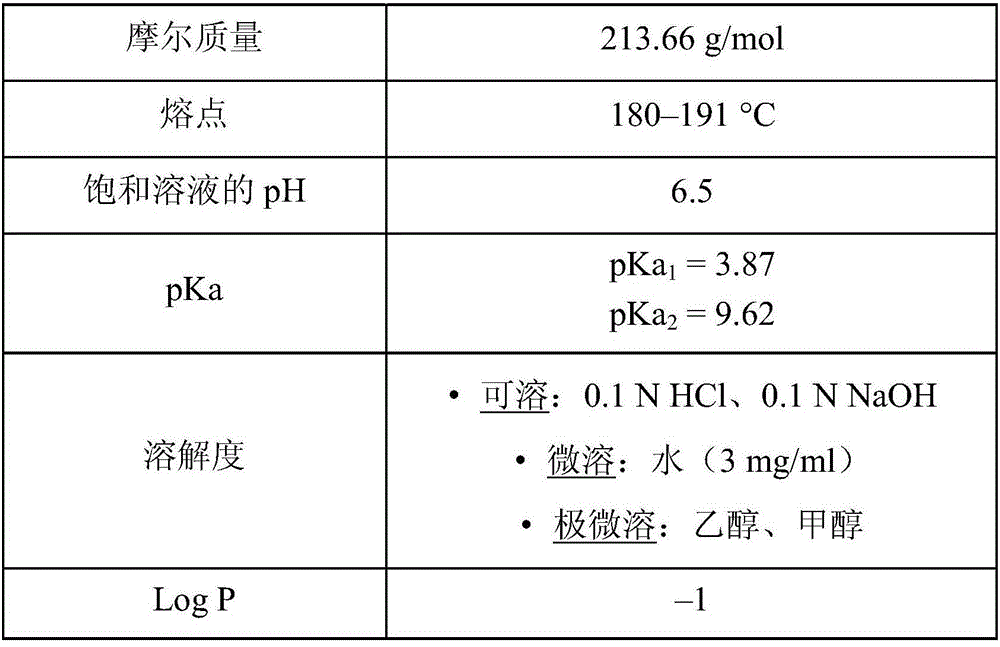
Gastro-retentive oral pharmaceutical compositions
A drug and tablet technology, which is applied in the field of oral galenic preparations of three types of BCS molecules such as baclofen, can solve the problems of long time and achieve the effect of improving floating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
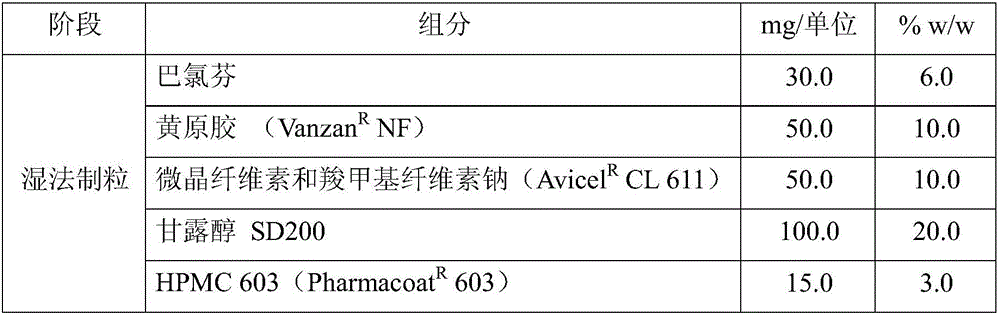
[0062] 1. Example of composition according to patent EP1745775 (comparative example)
[0063] In this example, the composition of the tablet follows the quantities described in patent EP1745775.
[0064] Table 1: Similar percentage composition as described in patent EP1745775
[0065]
[0066]
[0067] Baclofen was mixed with xanthan gum, Avicel CL611, mannitol SD200, and grain. After drying and sizing on a 425 μm mesh vibrating sieve, the granulated active ingredient was mixed with the excipient mixture in a multidirectional mixer (Turbula 2L) for 10 minutes, then lubricated with magnesium stearate for an additional 1 minute. The final blend was then compressed on a rotary tablet press (SVIACPR12) equipped with 12 mm diameter round punches and a pressure feed system.
[0068] Tablets were produced using a compression force of 18kN and had a hardness of 105N.
[0069] Table 2 shows the dissolution results in 500 ml of pH 4.5 media according to USP test II (100 rpm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com