Pharmaceutical compositions containing diacerein
A technology of diacerein and formulations, applied in the field of pharmaceutical compositions containing diacerein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of solid dispersions
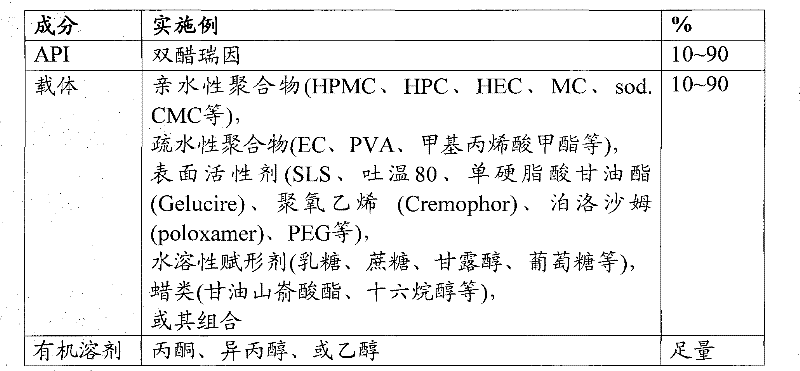
[0064] Typical acceptable ranges for solid dispersion ingredients are shown in Table 3.
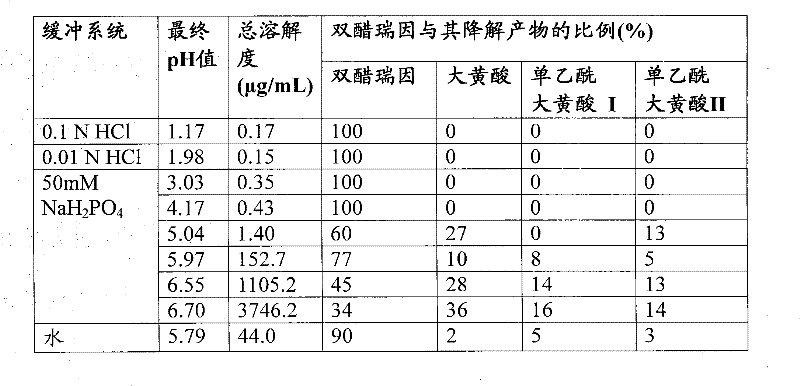
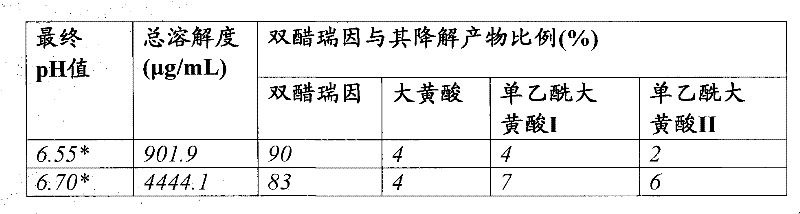
[0065] table 3
[0066]
[0067] Process:
[0068] Diacerein is dissolved in a suitable organic solvent to form a drug solution. Carriers such as hydrophilic polymers, hydrophobic polymers, surfactants, water-soluble excipients or waxes, or a combination of the above carriers are then dissolved or dispersed in the drug solution. Solid dispersions can be obtained by spray drying the above solutions, or by coating the solutions on a suitable vehicle (a water soluble material used as a secondary carrier) using a fluidized bed.
Embodiment 2
[0070] complexed with cyclodextrin
[0071] Acceptable ranges for the composition of typical complexes complexed with cyclodextrins are shown in Table 4.
[0072] Table 4
[0073]
[0074] Process:
[0075] Aqueous solutions of cyclodextrins can be prepared in different percentages. Diacerein was added to the above solution to make a saturated solution. The solution was stirred for at least 72 hours and then allowed to stand until precipitation of the insoluble material was complete. The supernatant is filtered and dried via oven, spray drying or freeze drying, or coated on a suitable vehicle (as a diluent) using a fluidized bed.
Embodiment 3
[0077] Matrix System (Tablet)
[0078] The acceptable ranges for the ingredients of a typical tablet matrix system are shown in Table 5.
[0079] table 5
[0080]
[0081] Process:
[0082] API fractions were prepared as described in the above examples. The Diacerein API portion is physically mixed or granulated with the controlled release material and the mixture is then compressed to obtain matrix tablets. Optionally, acidulants or buffers are added to the tablet formulation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com