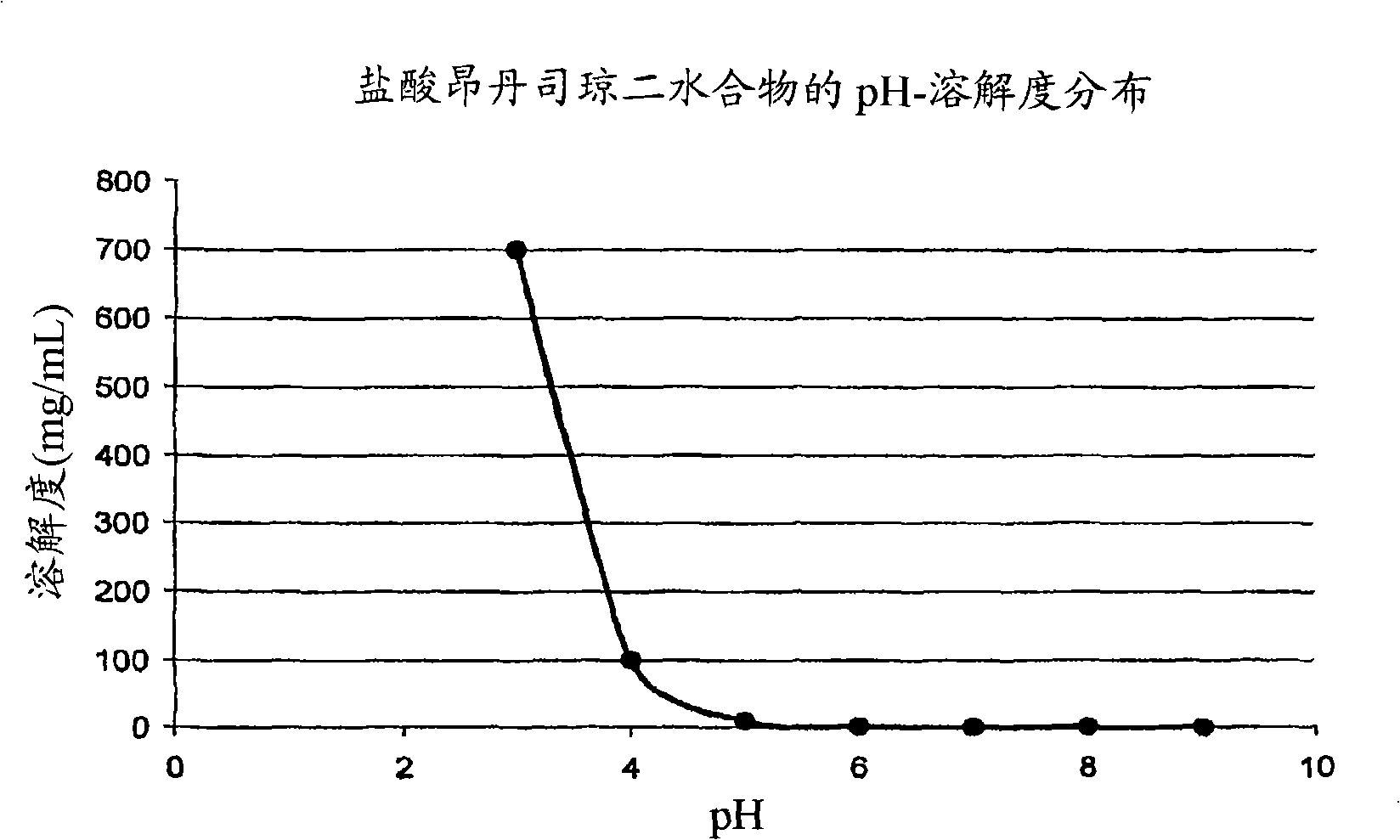
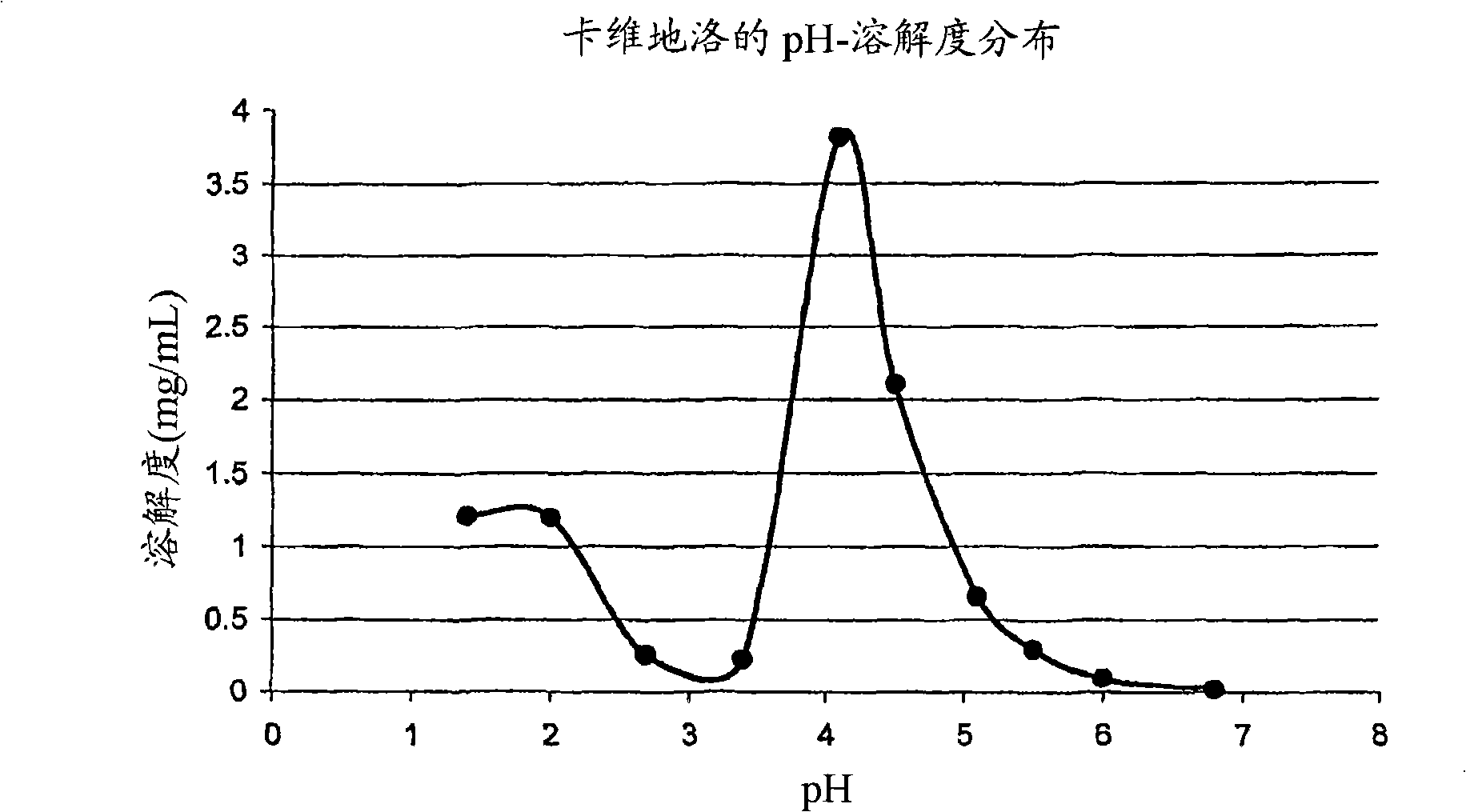
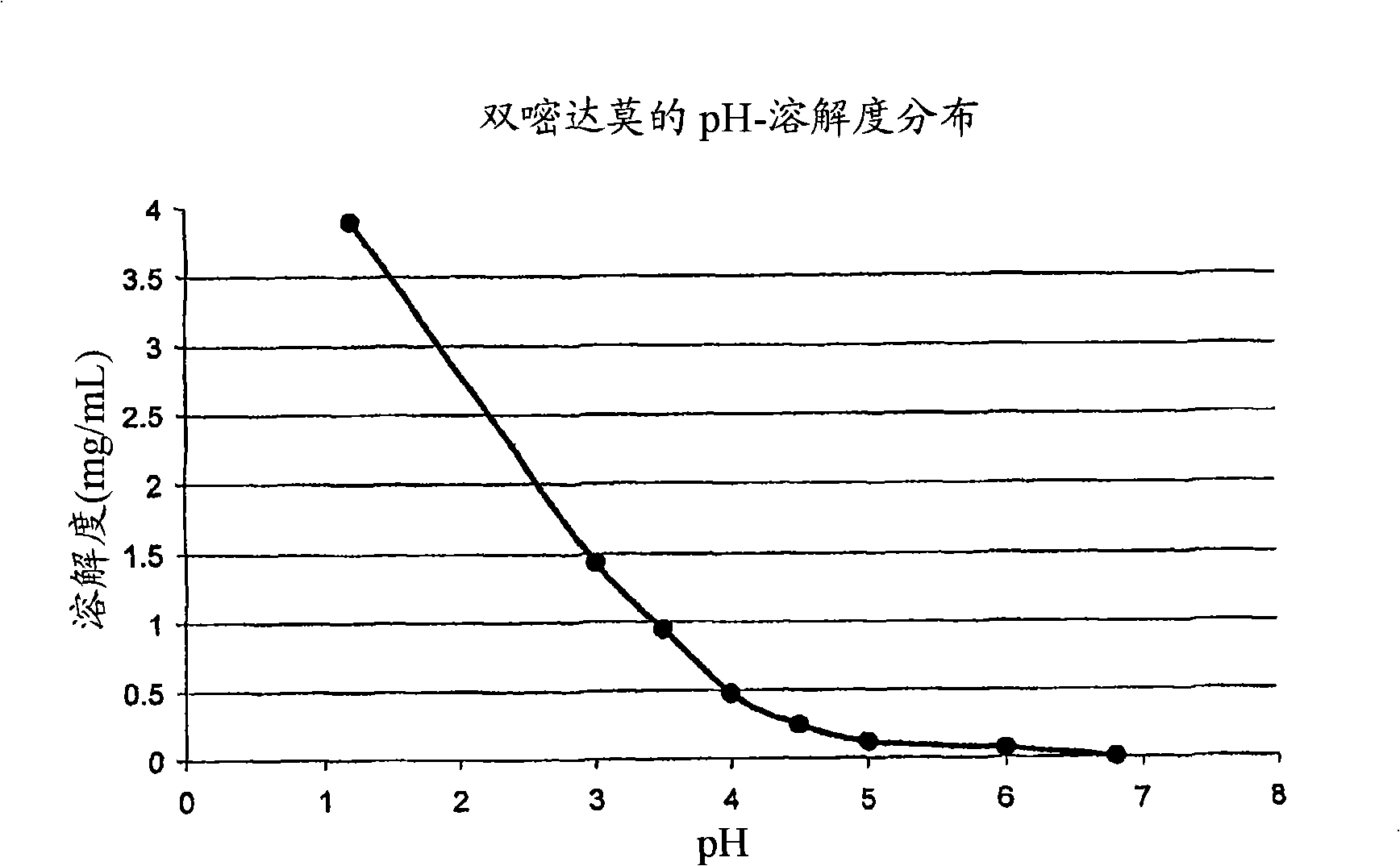
Drug delivery systems comprising weakly basic selective serotonin 5-HT3 blocking agent and organic acids
A 5-HT3, organic acid technology, applied in the direction of drug delivery, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of unable to maintain plasma distribution dosing regimen and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0079] According to one embodiment of the present invention, the method may include the following steps:
[0080] a. Provide organic acid containing core particles (e.g. organic acid crystals with desired size distribution or particles comprising inert particles (e.g. sugar spheres, cellulose spheres, silica spheres) layered with polymer organic acids in the binder solution);
[0081] b. Core particles containing organic acids are coated with an SR coating film consisting of a single water-insoluble polymer (e.g. EC-10 (ethylcellulose with an average viscosity of 10 cps)) or a combination of a water insoluble polymer with a water soluble polymer such as povidone or PEG 400 or an enteric polymer such as hydroxypropylmethylcellulose phthalate (eg HP-55);
[0082] c. Coat weakly basic drugs (such as ondansetron hydrochloride dihydrate) layer on the SR-coated core particles containing organic acids, and then further coat Pharmacoat 603 or Clear's protective seal coat to form IR...
Embodiment 1
[0108] A. SR-coated fumaric acid crystals
[0109] 40-80 mesh fumaric acid crystals (3750 g) were loaded into a fluid bed coater Glatt GPCG 5 equipped with a 9" bottom spray Wurster insert, column length 10" and tubing 16 mm . 250g of ethyl cellulose (Ethocel Premium, 10cps) and 166.7g of polyethylene glycol (PEG 400) (the ratio of the two is 60 / 40) were dissolved in 98 / 2 acetone / water (6528.3g), with the The resulting solution (6% solids concentration) was used to coat the above-mentioned acid crystals with a gain of up to 10% by weight. The treatment conditions are as follows: atomization air pressure is 2.0 bar; nozzle diameter is 1.00 mm; bottom distribution plate is B with No. 15 100 mesh screen; spray / shaking interval is 30s / 3s; product temperature is maintained at 35 ±1 °C; inlet air volume of 155-175 cubic feet per minute (cfm); and spray rate increased from about 8 g / min to 30 g / min.
[0110] In addition, fumaric acid crystals were coated with different ratios of e...
Embodiment 2
[0117] To evaluate the type of in vitro release profile required to achieve a once-daily plasma concentration profile, use Bozigian et al. in "Ondansetron Absorption in Adults: Effect of Dosage Form, Food, and Antacids", Journal of Pharmaceutical Sciences Vol. (1994) The reported pharmacokinetic parameters of ondansetron hydrochloride were used for modeling experiments. Using the software program WinNonlin TM Standard Version 2.1, assuming first-order elimination kinetics, will yield mean plasma concentrations in 24 healthy adult male volunteers who received a single 8 mg ondansetron hydrochloride IR tablet in the fasted state Substitute into the 1-compartment level model together with the lag time. get the following parameters:
[0118] Primary parameters: F=1.0 (assumed); V d =238.26;K a = 1.49 / hour; K e = 0.19 / hour (thus t 1 / 2 = 3.65 hours); T lag = 0.41 hours. Secondary parameters: AUC=0.17mg.hr / L; Cl=46.06L. / hour; T max = 1.98 hours; C m = 0.0248 mg / L. These p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com