Use of micro-ribonucleic acid (MIRNA) to diagnose transplant rejection and tolerance of immunosuppression therapy
a technology of immunosuppression therapy and microribonucleic acid, which is applied in the field of use of microribonucleic acid (mirna) to diagnose transplant rejection and tolerance of immunosuppression therapy, can solve the problems of graft loss, high invasiveness of allograft biopsies, and limited diagnostic tools in the ability to diagnose acute rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Serum miRNA Signatures for the Detection and Prediction of Acute Cellular Rejection (ACR)
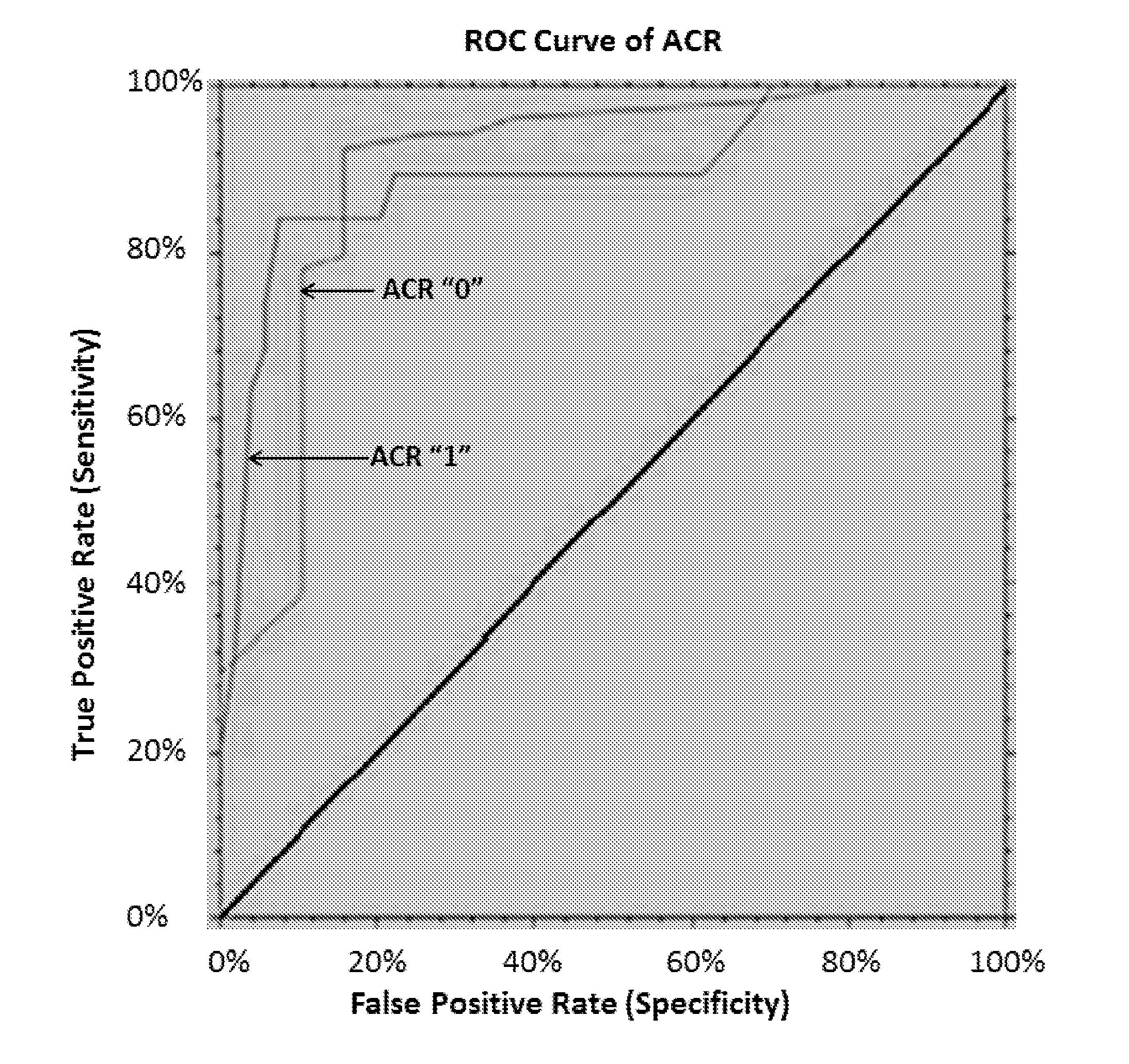
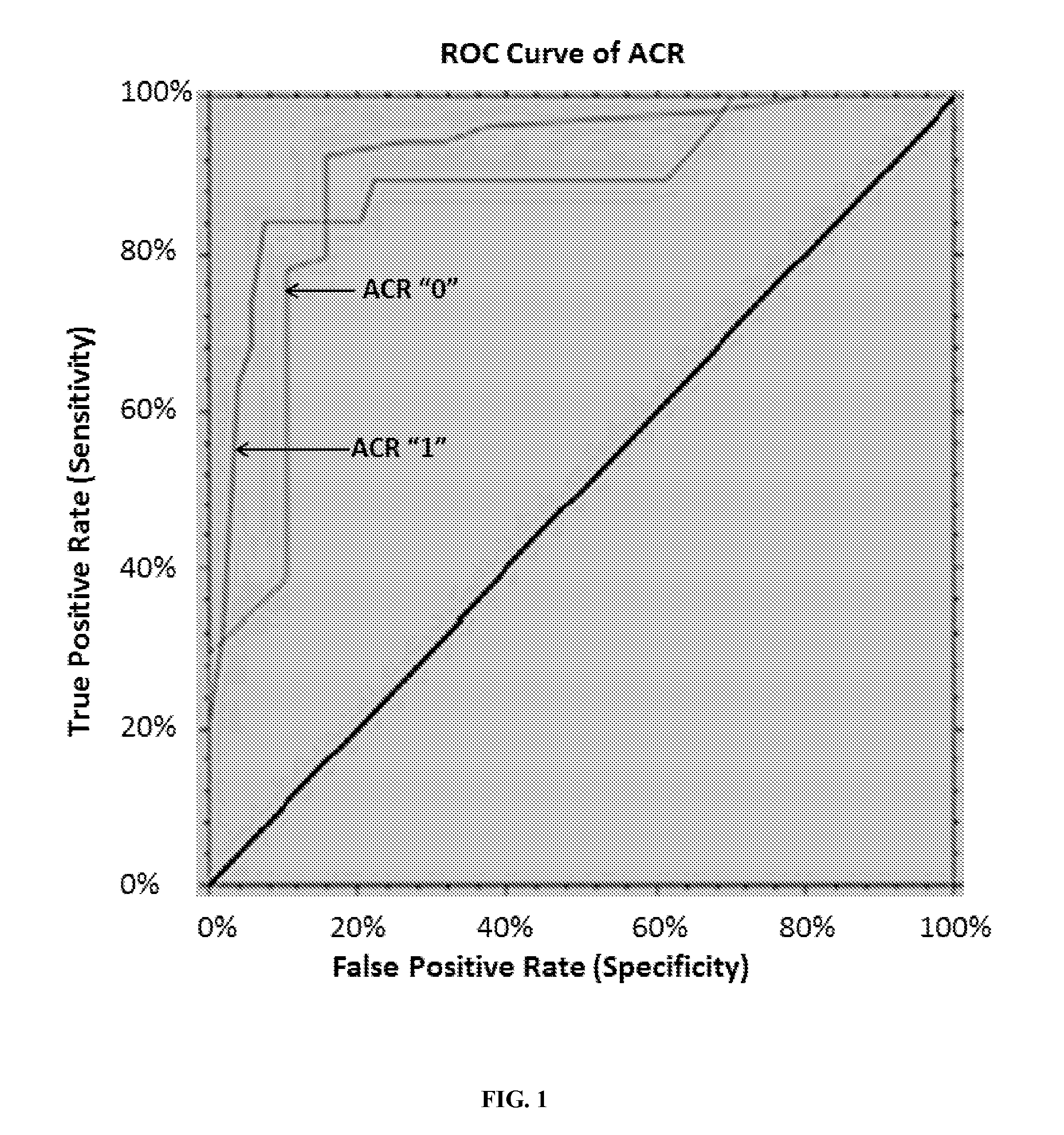
[0149]The results presented herein demonstrate that the miRNA profiles obtained at the time of clinically indicated biopsy are diagnostic of biopsy-confirmed acute rejection at any time after transplantation.
[0150]miRNA profiling was performed on 233 serum samples from 42 clinical trial participants from the National Institutes of Health Immune Tolerance in Transplantation-30 (ITN-30) study. This included 33 subjects randomized to immunosuppression withdrawal and 9 subjects randomized to maintenance, using the miRCURY LNA™ Universal RT microRNA PCR v3 panel (Exiqon, Denmark). The primary aims of this study were: 1) to identify serum miRNA signatures for diagnosis of acute cellular rejection (ACR) events; 2) to identify serum miRNA signatures for prediction of ACR events; 3) to identify serum miRNA signatures to differentiate subjects who fail immunosuppression withdrawal from s...
example 2
Identification of Serum miRNA Signatures for the Prediction of Immunosuppression Minimization Tolerance
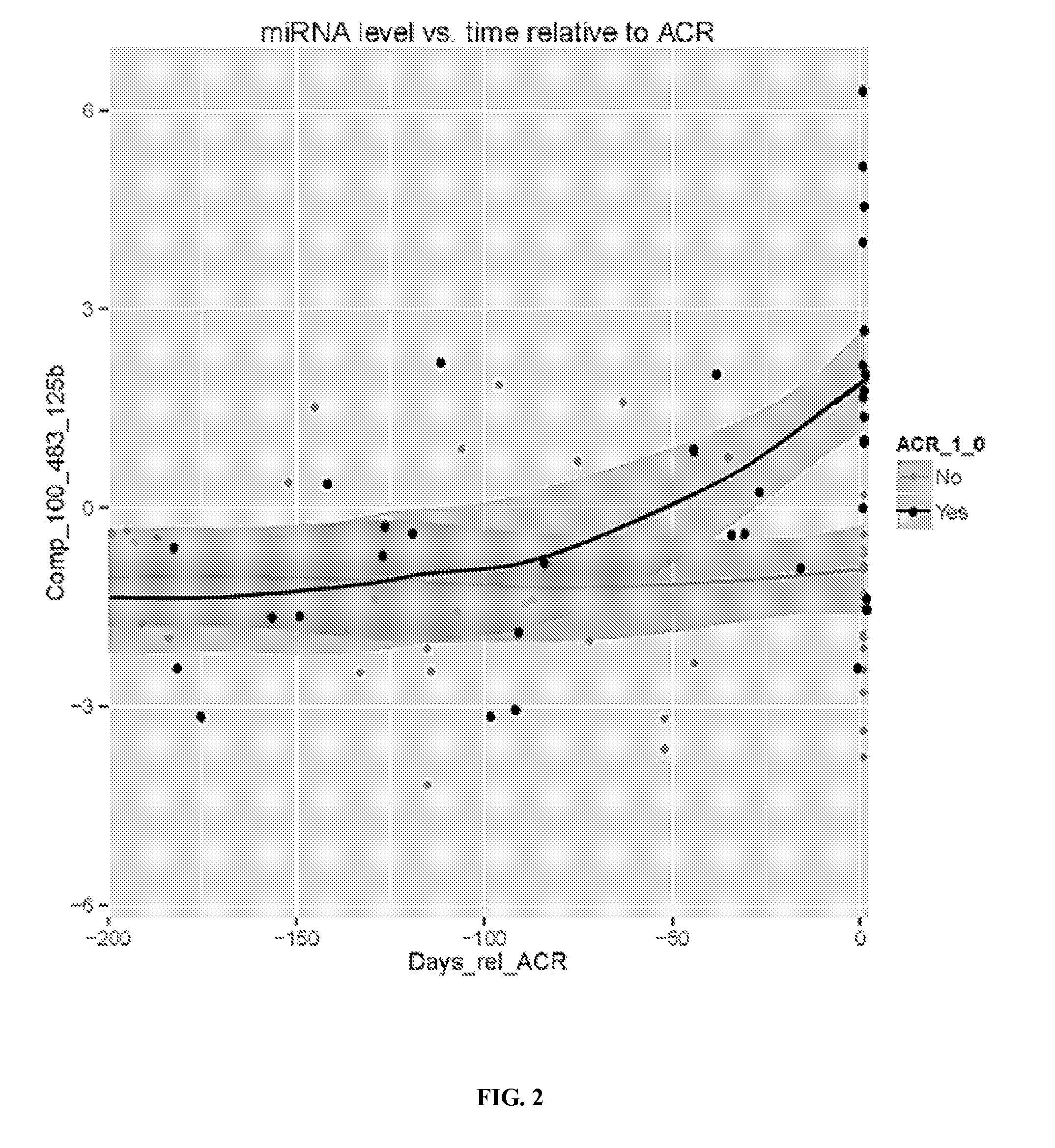
[0153]These experiments were designed to identify additional clinically relevant biomarkers for immunosuppression minimization that are predictive of which subjects will be able to tolerate lower doses of medication without inducing rejection. To identify each tolerance biomarkers, 10 serum samples from those subjects who were tolerant at the 25% immunosuppression dose (also meant by that a decrease of the initial IST dose by 75%) were compared with 11 serum samples from subjects who failed at the 25% IST dose (these serum samples were taken 58 days on average before rejection occurred). The 25% IST dose was used as the basis for this comparison because a large proportion of subjects (40% of all participants) failed at this stage, and this may represent where major changes occur in serum miRNAs that best differentiate patients who may tolerate or fail at lower IST doses As shown in...
example 3
Replication of miRNA Sera Signatures for Detection and Prediction of Acute Cellular Rejection (ACR)
[0156]To initially replicate the list of 23 ACR-associated miRNAs as well as the 3-miRNA signature identified previously for ACR diagnosis signature (Phase I study, presented previously in Example 1), miRNA profiling of serum samples from two independent studies were subsequently analyzed via the 752 miRCURY LNA™ Universal RT microRNA PCR v3 panels from Exiqon (Denmark). The first study comprised 15 ACR and 5 non-ACR serum samples from the ITN participants who were not randomized to IST withdrawal or maintenance. The second study comprised 4 ACR and 11 non-ACR samples from the NIH-CTOT03 prospective study. After excluding miRNAs that failed standard quality control (QC) measures, 20 of the 23 ACR-associated miRNAs were included in logistic regression modeling to test their association with ACR. As shown in Table 3, all 20 miRNAs were replicated in this follow-up study.
[0157]The perform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| double-stranded nucleic acid | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com