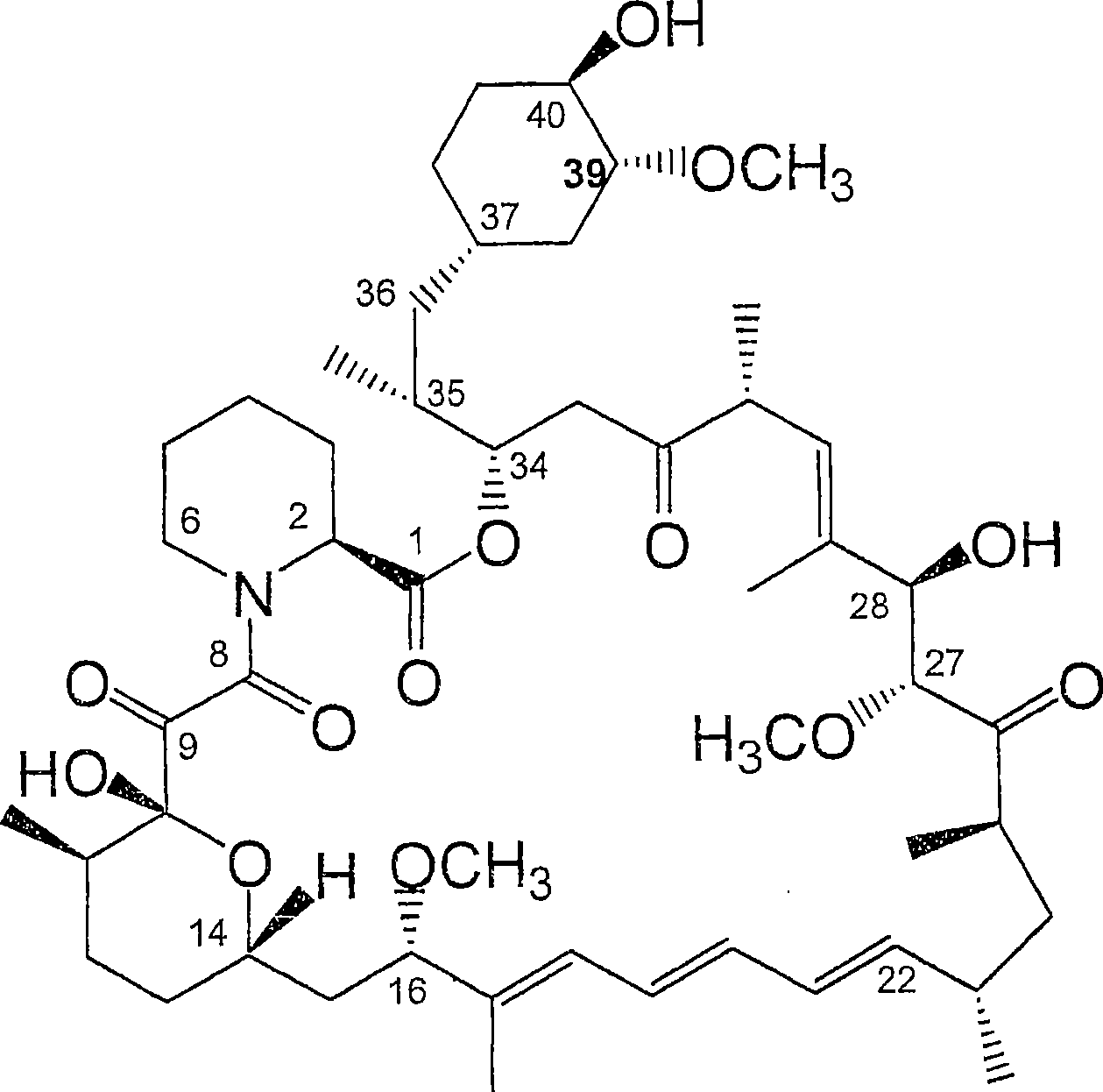
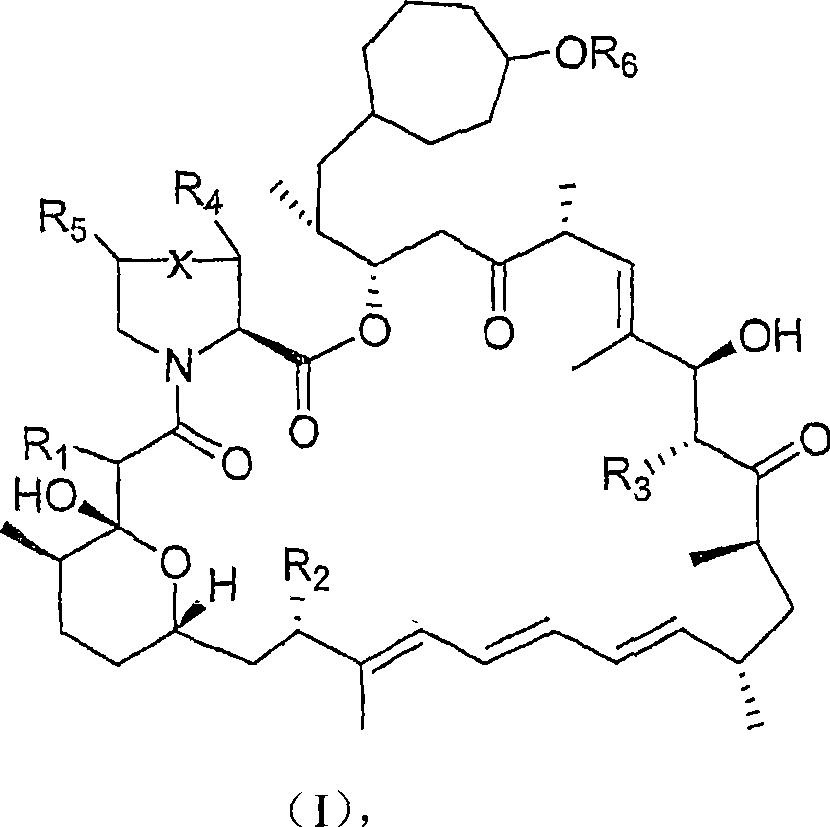
36 -des (3 -methoxy-4 -hydroxycyclohexyl) 36 - (3 -hydroxycycloheptyl) derivatives of rapamycin for the treatment of cancer and other disorders
A hydroxycyclohexyl, hydroxycycloheptyl technology, applied in the field of novel 36-de-36-rapamycin derivatives, can solve the problems of immunosuppression loss and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0183] General Methods and Materials
[0184] Material
[0185] All reagents were obtained from commercial sources and used without further purification unless otherwise stated.
[0186] to cultivate
[0187] S. hygroscopicus MG2-10 [JMNOQLhis] was maintained on Medium 1 agar plates (see below) at 28°C. Spore stocks were prepared after growth on Medium 1, kept in 20% w / v glycerol:10% w / v lactose in distilled water and stored at −80 °C. Vegetative cultures were prepared by inoculating 0.1 mL of frozen stock into 50 mL of Medium 2 (see below) in 250 mL flasks. The cultures were grown at 28°C for 36 to 48 hours at 300 rpm.
[0188] Preparation:
[0189] Vegetative cultures were inoculated into Medium 3 at 2.5-5% v / v. The culture was carried out at 26°C and 300 rpm for 6-7 days.
[0190] Feeding program:
[0191] Feeds / additions of selected carboxylic acids were performed 24-48 hours after inoculation and were fed at 1-2 mM unless otherwise stated.
[0192] Medium 1:
[...
example 1
[0245] Example 1: Fermentation and isolation of 36-des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin
[0246] 36-Des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin was prepared according to the method described in WO 04 / 007709. Briefly, cultures of S. hygroscopicus MG2-10 were transformed with appropriate expression vectors carrying the rapamycin genes rapJ, rapM, rapN, rapO, rapQ, and rapL to generate strain S. hygroscopicus MG2-10[rapJMNOQLhis]. Cultures of S. hygroscopicus MG2-10 [rapJMNOQLhis] were grown and fed cycloheptanecarboxylic acid using the method described in WO 04 / 007709. LCMS and LCMS of culture extracts n Analysis revealed that the m / z ratio of the as-prepared rapamycin analog was 16 atomic mass units less than that of rapamycin and was substituted with the 3-hydroxycycloheptyl moiety at C-36. The methoxy-4-hydroxycyclohexyl moiety is consistent.
example 2
[0247] Example 2: Synthesis of 36-des(3-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin by lipase-catalyzed esterification Methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycyclohexyl)-40-O-[2,2-bis(hydroxymethyl)propionyl]rapamycin
[0248] Under argon atmosphere, 36-des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin (10 mg, 0.011 mmol), 2,2,5- Trimethyl[1.3-dioxane]-5-vinylcarboxylate (100mg, 0.5mmol), lipase PS-C "Amano" II (100mg) and 0.5nm molecular sieves (50mg) in anhydrous tert-butyl methyl The mixture in ether (2 mL) was heated to 43°C. After 72h, LC / MS monitoring showed complete conversion of starting material. THF (10 mL) was added and the mixture was filtered through a pad of celite. The enzyme was washed with THF (2 x 10 mL) and the combined organic extracts were concentrated under reduced pressure. The residue was dissolved in THF (7.5 mL), and H was added 2 SO 4 (2.5 mL, 0.5N). The solution was allowed to stand at room temperature for 5 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com