Autoimmune hepatitis model and medicine screened by using autoimmune hepatitis model
A technology for autoimmunity and hepatitis, applied in the field of non-human animal models, can solve problems such as inability to reflect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
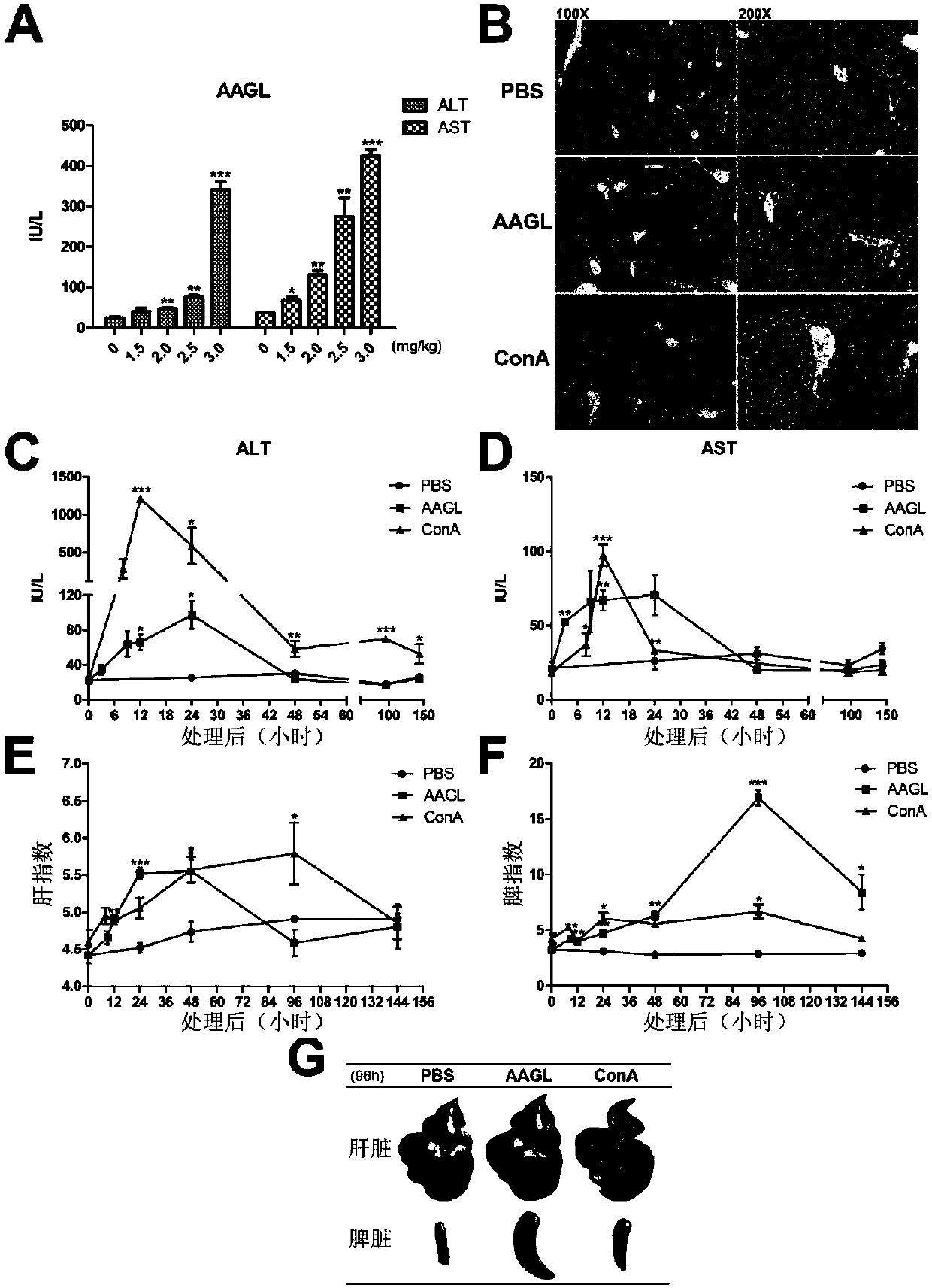
[0037] Tea Tree Mushroom was treated with ddH 2 O soaking, the soaking solution was dialyzed by ammonium sulfate precipitation method to obtain the total protein; then the target protein AAGL was obtained by using the affinity chromatography column of lactose-Sepharose 6B, AAGL 2 Dialyzed overnight in O, the dialysate was freeze-dried, and the SDS-PAGE electrophoresis results of the purified AAGL were as follows figure 1 shown. Dissolve the freeze-dried powder of AAGL in PBS, prepare the concentration of 0.15 mg / ml, 0.2 mg / ml, 0.25 mg / ml, and 0.3 mg / ml, filter it with a 0.22um filter, and inject it into the tail vein according to the weight of the mouse In C57BL / 6 mice, the final injections were 1.5 mg / kg, 2.0 mg / kg, 2.5 mg / kg, 3.0 mg / kg, respectively. Five mice in each group were sacrificed 9 hours later to collect blood, and the serum was separated to measure the contents of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum;
[0038] Depend on ...
Embodiment 2
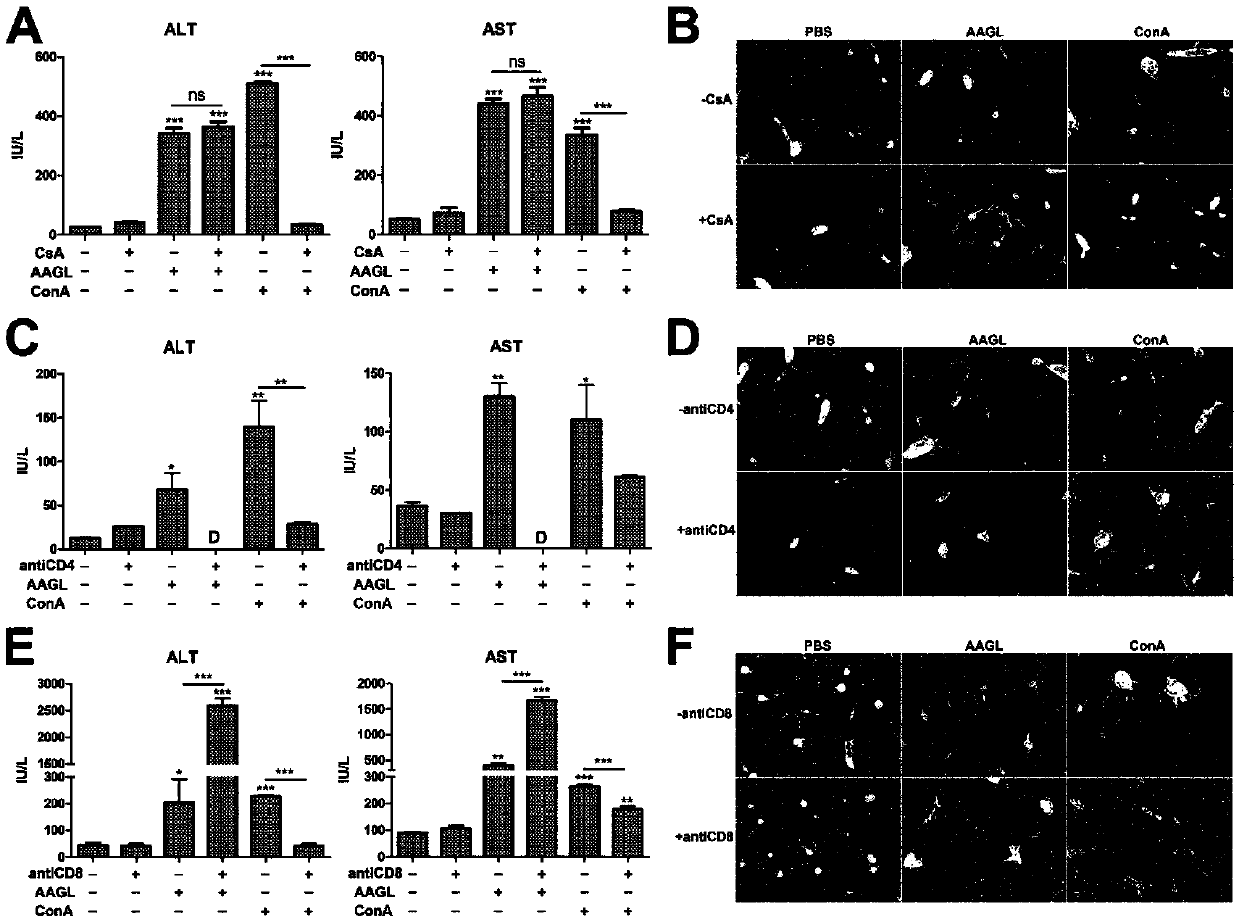
[0044] Dissolve the immunosuppressant cyclosporine CsA in olive oil, inject mice intraperitoneally at 50 mg / kg for 24 hours, and then inject mice with 2.5 mg / kg AAGL through the tail vein, 5 mice in each group, and kill the mice 9 hours later. Blood and liver tissue were collected from the rats to detect the changes in ALT and AST levels in the serum; the liver tissue was embedded in paraffin and stained with H&E; at the same time, the Con A model was used as a positive control to detect the efficacy of CsA in inhibiting T cell activation. The specific operation was as follows: After the mice were pretreated with CsA, they were injected with 15 mg / kg Con A through the tail vein, and the mice were sacrificed 8 hours later to collect blood and liver tissue. After the serum was separated, the changes of ALT and AST in the serum were detected; the liver tissue was stained with H&E.
[0045] Depend on image 3 The results of A and B show that the immunosuppressant cyclosporine CsA,...
Embodiment 3
[0049] Download the glycan data of AAGL and Con A version 5.0 from the CFG database, the concentration of the glycan data of AAGL is 200, 100 and 20 μg / ml, the concentration of the data of Con A is 100, 10 and 1 μg / ml; The negative RFU value in the sugar spectrum data is unified as 1, and then the log is calculated 10 (RFU), and finally conduct sugar map heat map analysis according to different sugar chain ends.
[0050] Depend on Figure 4 The results of A show that AAGL can specifically bind sugar chains terminated with galactose, sialylated galactose and sulfonated galactose, while Con A can bind with higher affinity to sugar chains terminated with mannose .
[0051] AAGL and Con A were biotin-labeled with a commercial biotin labeling kit, and BCA was quantified after labeling; mouse spleen lymphocytes were isolated, and the mouse spleen was first separated and ground with rough-edged glass in PBS, and the cell suspension was passed through 200-mesh filter, centrifuge at 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com