Mouse HCV (hepatitis C virus) polypeptide epitope combined with MHC-I molecule and application thereof
A hepatitis virus and binding site technology, applied in the direction of viral peptides, medical preparations containing active ingredients, applications, etc., can solve problems such as research that has not been reported in the literature, and achieve convenient clinical application, large commercial industrialization value, The effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Screening method for polypeptide epitopes
[0034] The polypeptide sequence obtained by the present invention is directly fetched from the peptide library by the method of combining supermotif, quantitative motif and artificial neural network scheme, and the binding property with MHC-I class molecules is maintained.
[0035] 1. Obtaining the amino acid sequence of S protein of mouse hepatitis III virus
[0036] The full-length amino acid sequence (330 amino acids in total) of the natural murine hepatitis III virus S protein was found from the international open shared gene bank NCBI GeneBank, and is represented as: SEQ NO.1.
[0037] 2. Acquisition of supermotifs for polypeptide epitope prediction
[0038] Supermotifs are based on peptide motifs composed of the same and similar anchor residues for antigenic peptides received in the same leukocyte antigen (HLA) family, or even HLA allotypes of different families. Easy with H-2K K In the combined 8-peptide se...
Embodiment 2
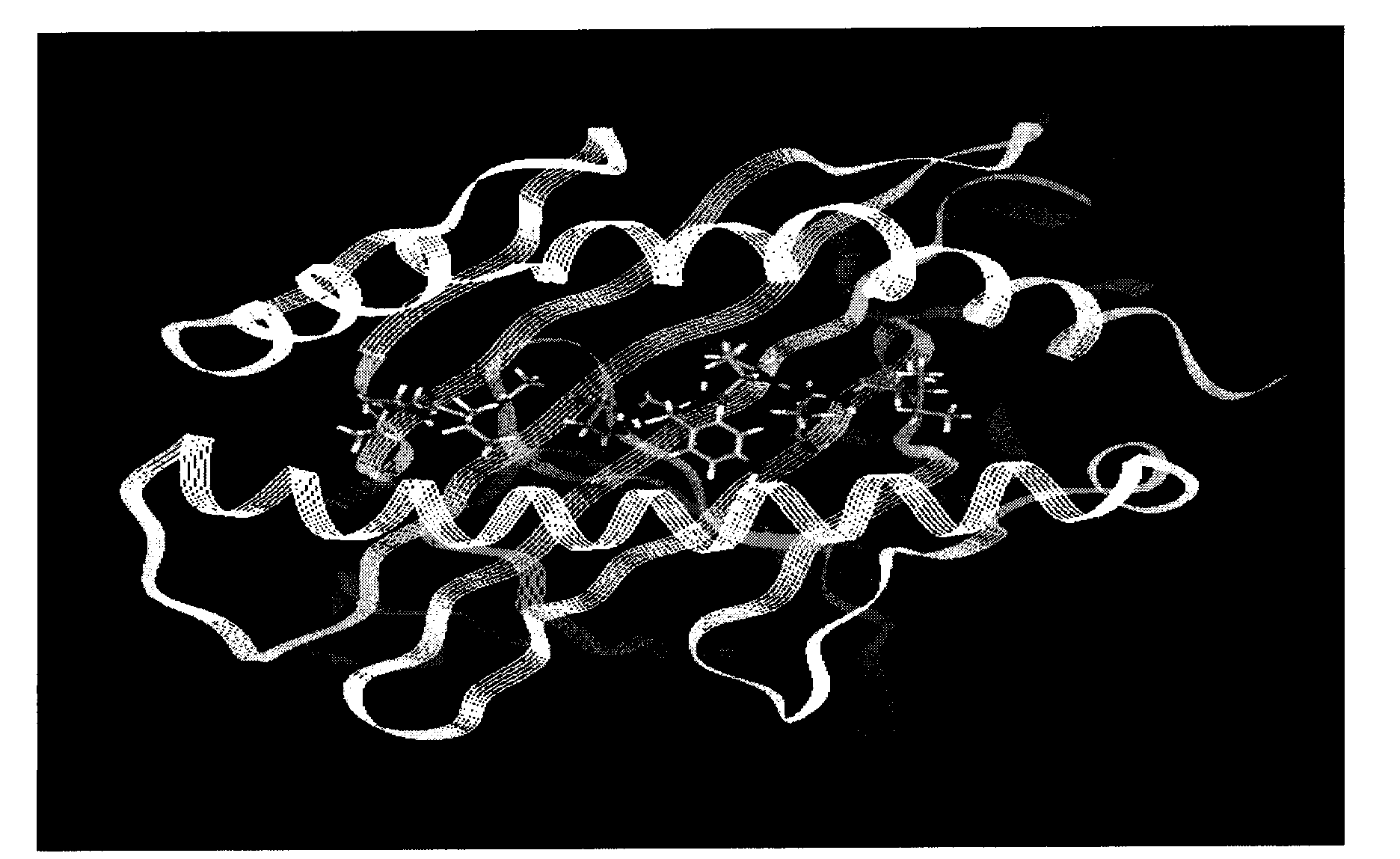
[0047] Example 2 Three-dimensional structural analysis and molecular dynamics simulation analysis of the binding of polypeptide epitopes to MHC-I
[0048] Using Silicon graphics workstation and Insight II software to establish the predicted polypeptide and H-2K epitope for the above-obtained polypeptide K The combined three-dimensional structure and molecular dynamics simulation, including the following methods: ① Molecular model construction, using the Discover3.0 module in the InsightII software package (using CVFF force field), the octapeptide from the mouse hepatitis III virus S protein and H-2K K Molecular dynamics simulations of molecular complexes. H-2K K The initial coordinates of are from the Protein Structure Database (PDB No. 1ZT1), and each octapeptide is obtained by performing amino acid substitutions on the polypeptide molecules in 1ZT1 using the Bioploymer module. The simulation process is as follows: First, the H-2K K Fixed with β2m, using the steepest desc...
Embodiment 3
[0051] Example 3 Acquisition of the polypeptide epitope of the present invention
[0052] (1) Synthesis of polypeptides
[0053] The standard Fmoc scheme was used for the polypeptide, and the arginine was coupled twice, and the peptide chain was extended from the carboxyl terminus to the amino terminus according to the sequence of the polypeptide. After the polypeptide is synthesized, the corresponding cleavage enzyme is selected for cleavage, and the protecting group of the polypeptide is removed to obtain the crude polypeptide.
[0054] (2) Purification and molecular weight analysis of peptide epitopes
[0055] The above-obtained polypeptide epitopes were purified and analyzed by RP-HPLC, and the purity of the four synthesized polypeptides determined by RP-HPLC was all above 95%. The results of mass spectrometry showed that the relative molecular mass of each peptide was basically consistent with the theoretical value, which could be used for subsequent experimental resear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com