Therapeutic agents comprising isolated recombinant oncolytic adenoviruses and immune cells and uses thereof
An oncolytic adenovirus and immune cell technology, applied in the direction of medical preparations containing active ingredients, drug combinations, anti-tumor drugs, etc., can solve problems affecting the effect of tumor treatment, reduce protein expression levels, and enhance anti-tumor immunity Killing effect, safety-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0257] MTT method used in Example 4: Add 10 μl of MTT solution (5 mg / ml) to each well of cells, incubate for 4 hours in a 37° C. The crystals were fully dissolved and mixed, and their absorbance at 490 nm (OD 490 ). Inhibition rate calculation formula: cell proliferation inhibition percentage (IR%)=1-(OD 490 Test article-OD 490 Blank) / (OD 490 negative control-OD 490 Blank) × 100%.
[0258] Counting by trypan blue staining: wash cells with PBS, digest with trypsin, suspend cells in PBS, add trypan blue staining solution with a final concentration of 0.04% (w / v), count under a microscope, dead cells will be Stain light blue, live cells are rejected. The number of living cells was taken as the final data.
[0259] Sources of culture plates: 6-well cell culture plates (the culture volume of each well is 2 ml), 12-well cell culture plates (1 ml of culture volume per well), 24-well cell culture plates (500 μl of culture volume per well) adopted in each example, All 96-well c...
Embodiment 11
[0265] The MTT method used in embodiment 11 illustrates:
[0266] Digest the cells in the logarithmic growth phase with trypsin, collect them by centrifugation after termination, blow them evenly, and prepare a single-cell suspension; ), inoculated in a 96-well cell culture plate with a culture system of 100 μl / well, placed at 37°C, 5% CO 2 Cultivate in an incubator overnight to make the cells completely adhere to the wall and reach 70-80% the next day; the counting method is counted by a counting plate, and the correctness of the counting is verified by a countstar counter. Take out the 96-well plate, add 100 μl pre-prepared T cell and TCR-T cell suspension, vortex slightly before adding the sample, add 100 μl serum-free medium for corresponding cell culture in the blank control well; place at 37°C, 5% CO 2 Culture in the incubator for 24 hours respectively; after 24 hours, take the cells, centrifuge at 400g, absorb 180 μl of medium after 10 minutes and put them into a new 9...
preparation example 1
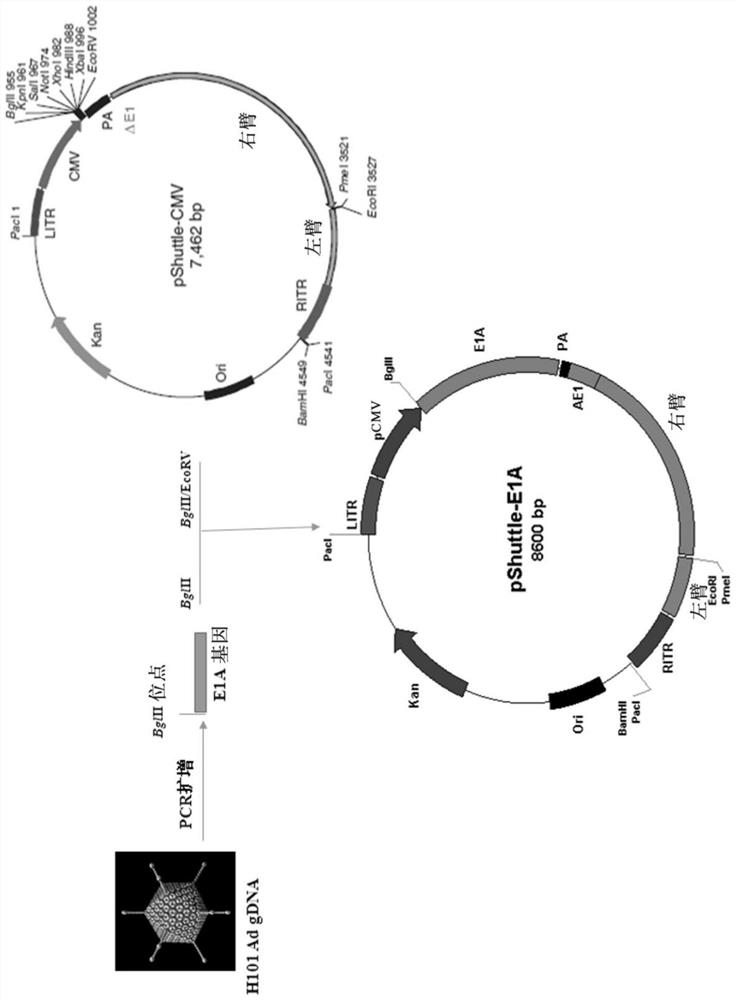
[0271] Preparation Example 1: Construction of E1A Gene Expression Vector
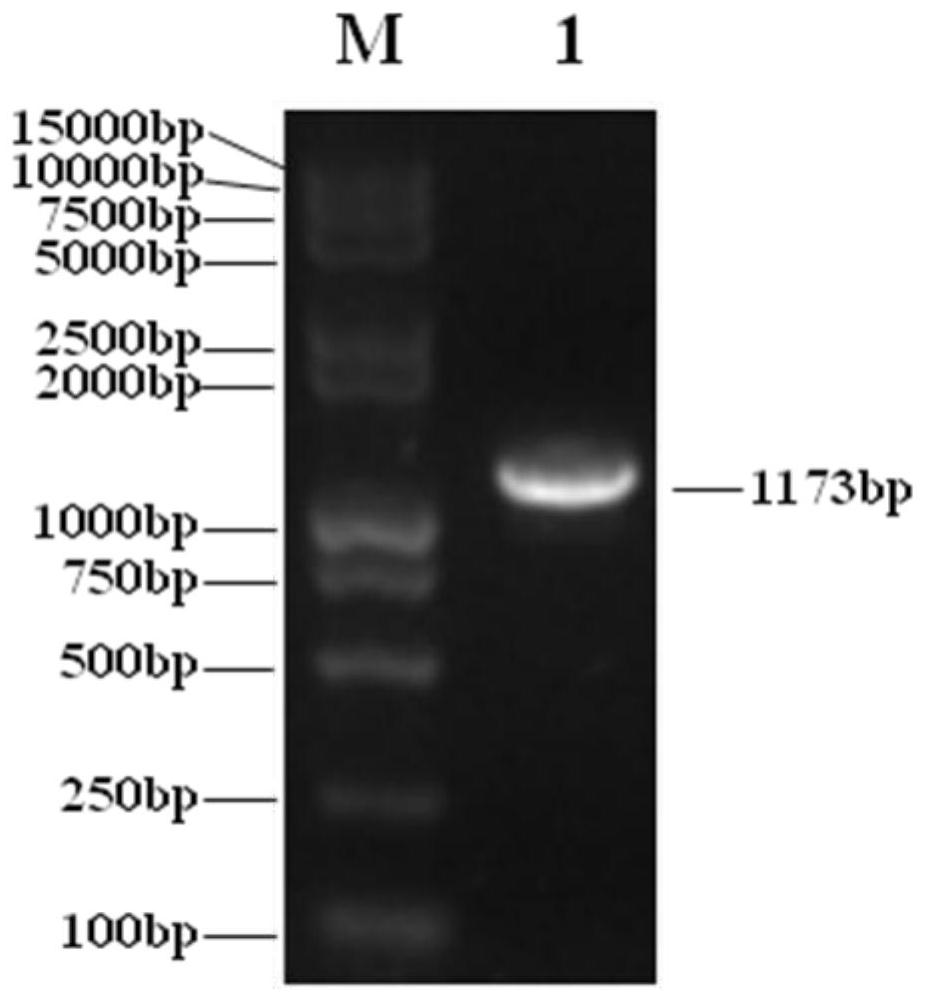
[0272] According to the human adenovirus type 5 (AD5) genome DNA sequence (ACCESSION: AC_000008) in the Genbank of NCBI (that is, the National Center for Biotechnology Information, website: https: / / www.ncbi.nlm.nih.gov) design two PCR primers (P1: GGA AGATCT GGACTGAAAATGAG (SEQ ID No.30), P2: TGAGGTCAGATGTAACCAAGATTA (SEQ ID No.31); note: the 5' end of the primer P1 has added a BglII restriction site, underlined); extract Shanghai Sanwei Biotechnology Co., Ltd. Oncolytic virus (H101) genomic DNA was used as a template for high-fidelity PCR amplification of the 1164bp sequence between 551-1714 on the AD5 genomic DNA, and the actual size was 1173bp (see figure 1 ), this sequence includes the coding region of the E1A gene (excluding the E1A promoter sequence) and part of the 3'UTR region. Utilize the PCR product that BglII digestion obtains, and it is cloned between the BglII and the EcoRV site in the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com