Pectin-5-efudix colon cancer double-target ahead body medicament and preparation method
A technology of fluorouracil and colon cancer, which is applied in the field of colon cancer targeted therapy drugs and its preparation, can solve the problems of low concentration in the colon, high toxicity and side effects, and difficult tolerance of patients, and achieves the reduction of immunosuppression, stable drug loading, and improved selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
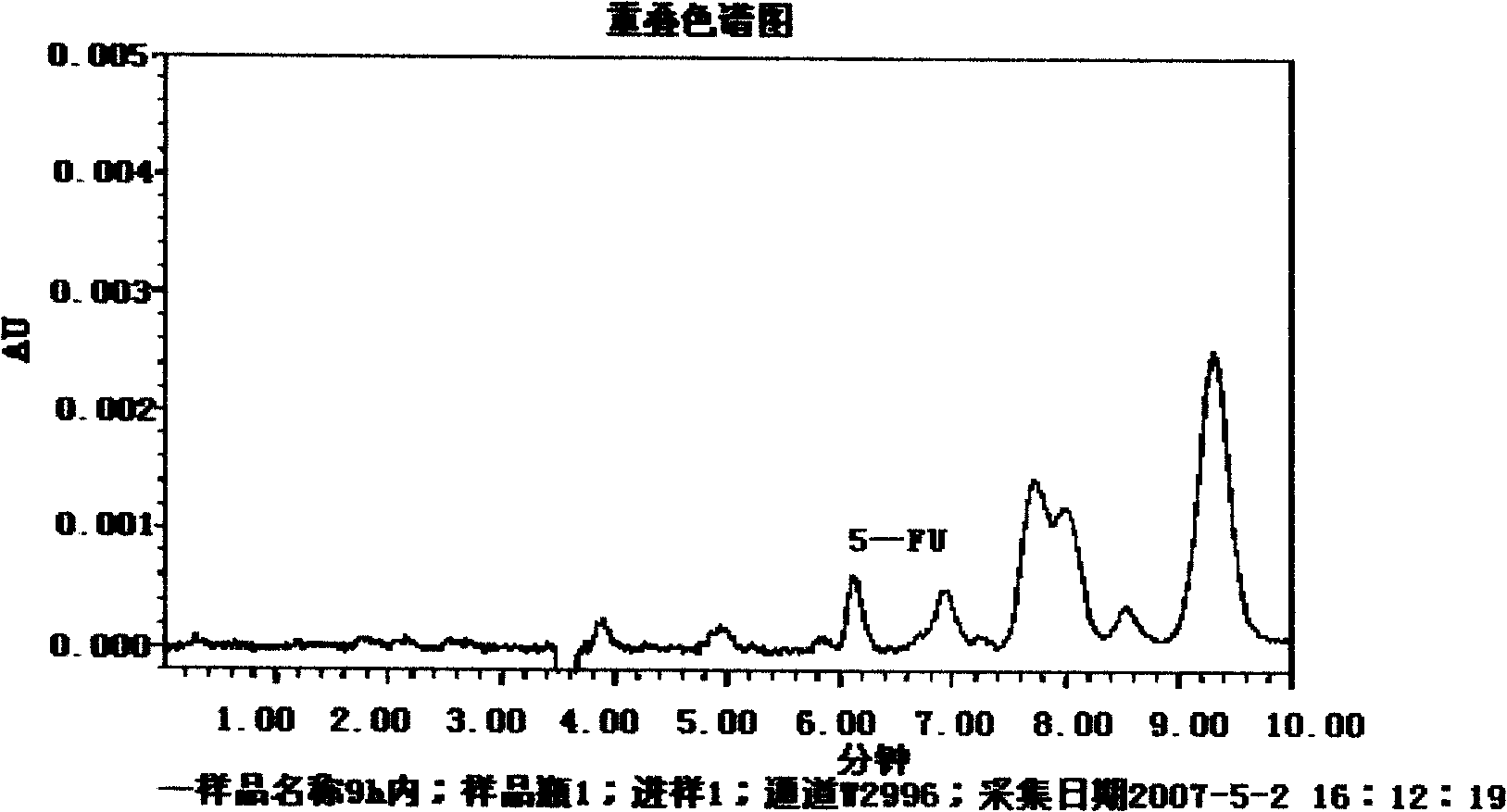
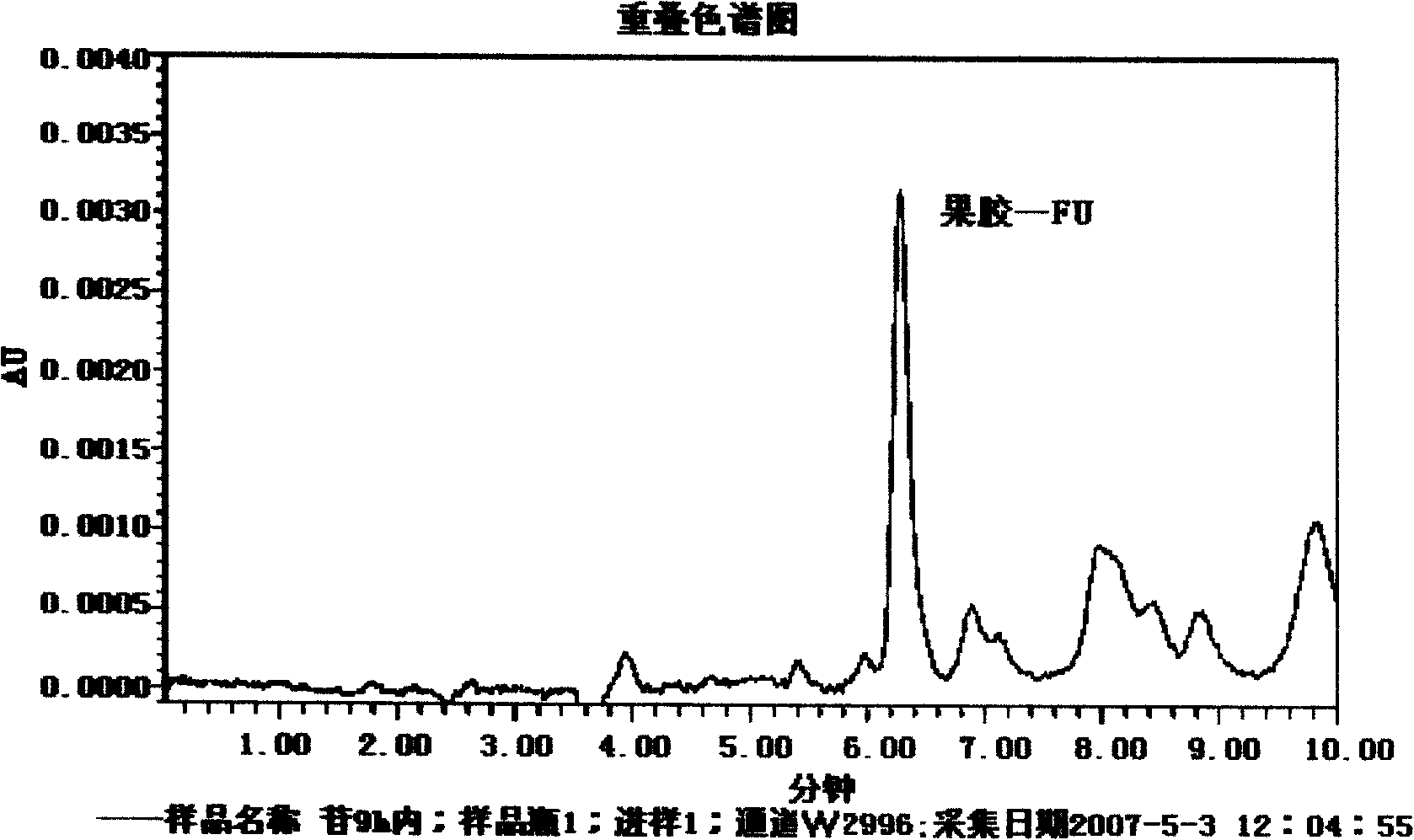
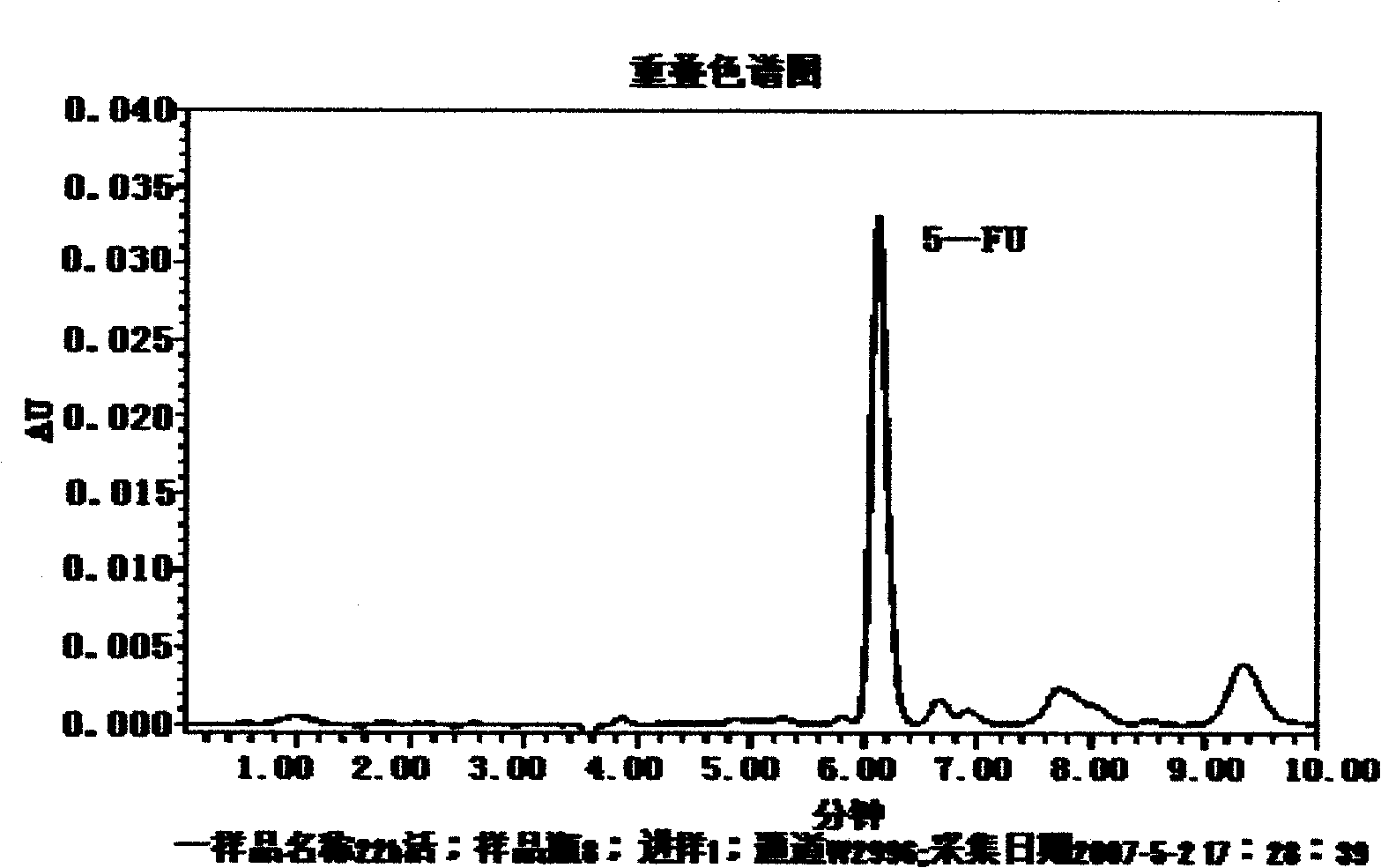
Image
Examples
Embodiment 1
[0043]Add 15g of pectin and 3N NaOH aqueous solution, stir overnight at 37°C, cool to room temperature, ethanol precipitate, add concentrated hydrochloric acid to adjust the pH value to 3, stir overnight at 37°C, adjust the pH value to neutral, evaporate the solvent under reduced pressure, and concentrate Dissolve the suspension in an appropriate amount of distilled water, move it to a dialysis bag (molecular weight cut-off 8000), use distilled water as the external fluid for dialysis, and freeze-dry for 24 hours after concentration until the color of the internal dialysis fluid no longer changes to obtain 13.6 g of light brown powder; HPLC Detect that the degree of esterification is less than 30%; weigh 0.5g of modified pectin and dissolve it in 20mL DMSO, add 0.25g of DCC and 15mg of DAMP, then add 0.5g of 5-FU, stir at 40°C for 24h, after the reaction is over, pour the reactants into In ethanol, the jelly was precipitated, filtered, rinsed with methanol, and dried in vacuum ...
Embodiment 2
[0046] Take 15g of pectin, add pectinase to citrate buffer (pH=4-5), stir at 50°C for 4-6h, cool to room temperature, distill off the solvent under reduced pressure, add appropriate amount of distilled water to the concentrated suspension until fully dissolved , moved to a dialysis bag (molecular weight cut-off 8000), and distilled water was used as the external fluid for dialysis. The dialysis was stopped until the color of the dialysis internal fluid no longer changed. After concentration, freeze-dry for 24 hours to obtain 13.6 g of light brown powder. The degree of esterification detected by HPLC is less than 30%. Weigh 0.5g of modified pectin and dissolve it in 20mL of DMSO, add 0.25g of DCC and 15mg of DAMP, then add 0.5g of 5-FU, and stir at 40°C for 24h. Filter, rinse with methanol, and dry in vacuo to obtain the product.
[0047]
Embodiment 3
[0049] Add 15g of pectin to methanol solution of 2mol / L NaOH, stir vigorously, heat and reflux at 60-80°C for 4-6h, cool to room temperature, add concentrated hydrochloric acid to adjust the pH value to 3, stir vigorously, heat and reflux at 50-70°C for 7- After 10 hours, evaporate the solvent under reduced pressure, add an appropriate amount of distilled water to the concentrated suspension until it is fully dissolved, transfer it to a dialysis bag (molecular weight cut-off 8000), use distilled water as the external fluid for dialysis, and stop dialysis until the color of the internal fluid no longer changes. Freeze-dried for 24 hours to obtain 13.6g of light brown powder. The degree of esterification detected by HPLC is less than 30%. Weigh 0.5g of modified pectin and dissolve it in 20mL of DMSO, add 0.25g of DCC and 15mg of DAMP, then add 0.5g of 5-FU, and stir at 40°C for 24h. Filter, rinse with methanol, and dry in vacuo to obtain the product.
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com