Medicament for preventing and controlling alzheimer's disease
A technology for Alzheimer's disease and drugs, applied in the field of biomedicine, can solve the problems of bleeding, inflammatory response microvessels, etc., achieve broad clinical application prospects, avoid repeated drug administration, and reduce treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation method of the present invention is as follows: firstly, the scFv gene is cloned from the pGEX-6P-scFv plasmid by using the polymerase chain reaction (PCR) method, and the secretory signal peptide and the enzyme cutting site are introduced, and then enzyme cutting is connected into the AAV plasmid pSNAV1 , constituting the pSNAV1-scFv plasmid. The signal peptide sequence is: atggactgga cctggaggat cctcttcttg gtggcagcag ccacaggagc ccactcc. The resulting plasmid was transformed and amplified. Plasmids were extracted and identified by one-way DNA sequencing. The pSNAV1-scFv plasmid was introduced into BHK-21 cells, and G418 selected pSNAV1-scFv plasmid carrier cells. The helper virus rHSV1 / repcap containing AAV rep and cap genes is added to infect the pSNAV1-scFv vector cells for virus replication and packaging. Collect the culture supernatant and centrifuge. Add polyethylene glycol and NaCl, incubate, centrifuge, and collect the rAAV virus particle preci...
experiment example
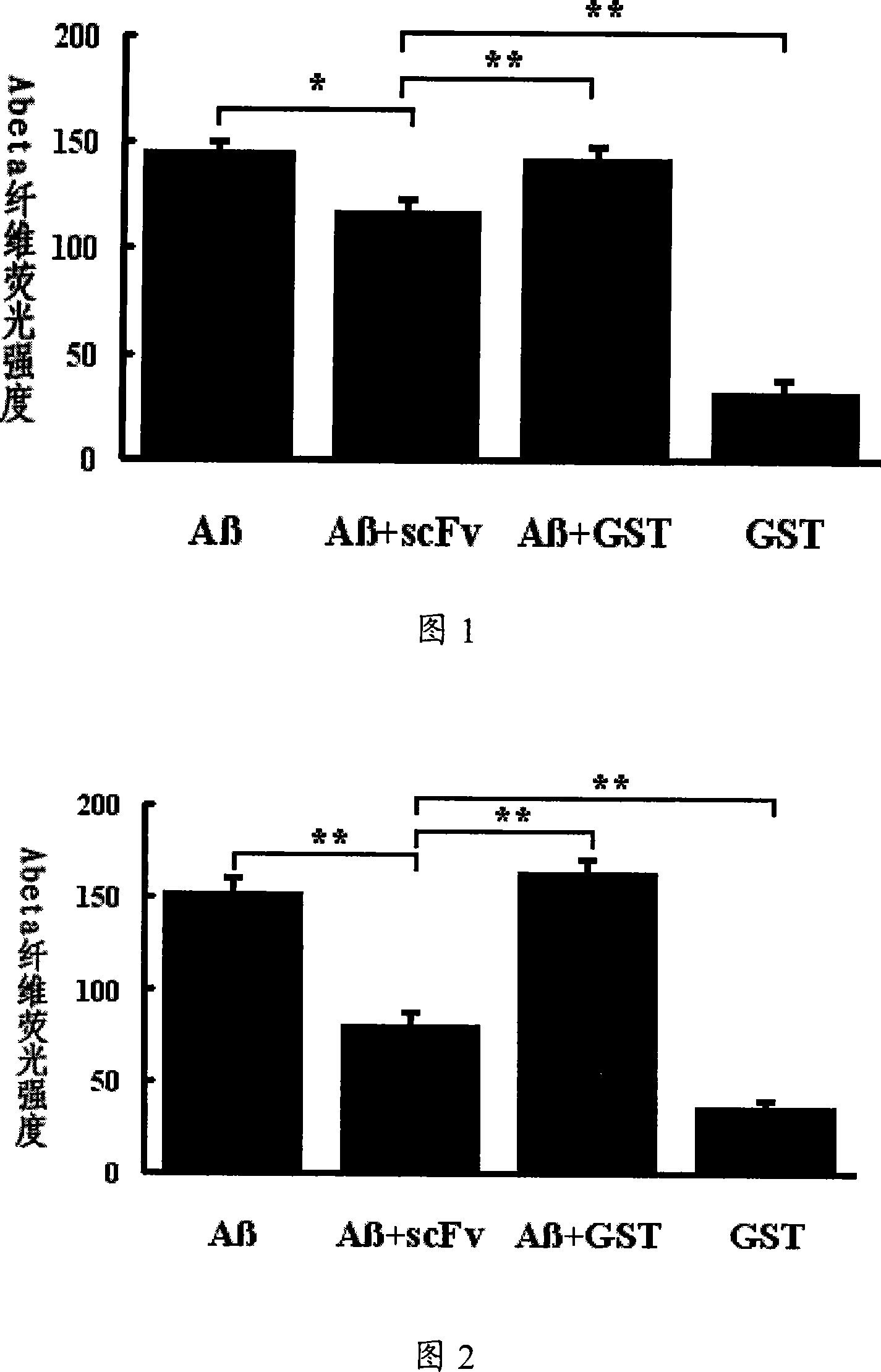
[0064] 1. Inhibitory effect of scFv on Aβ42 monomer polymerization
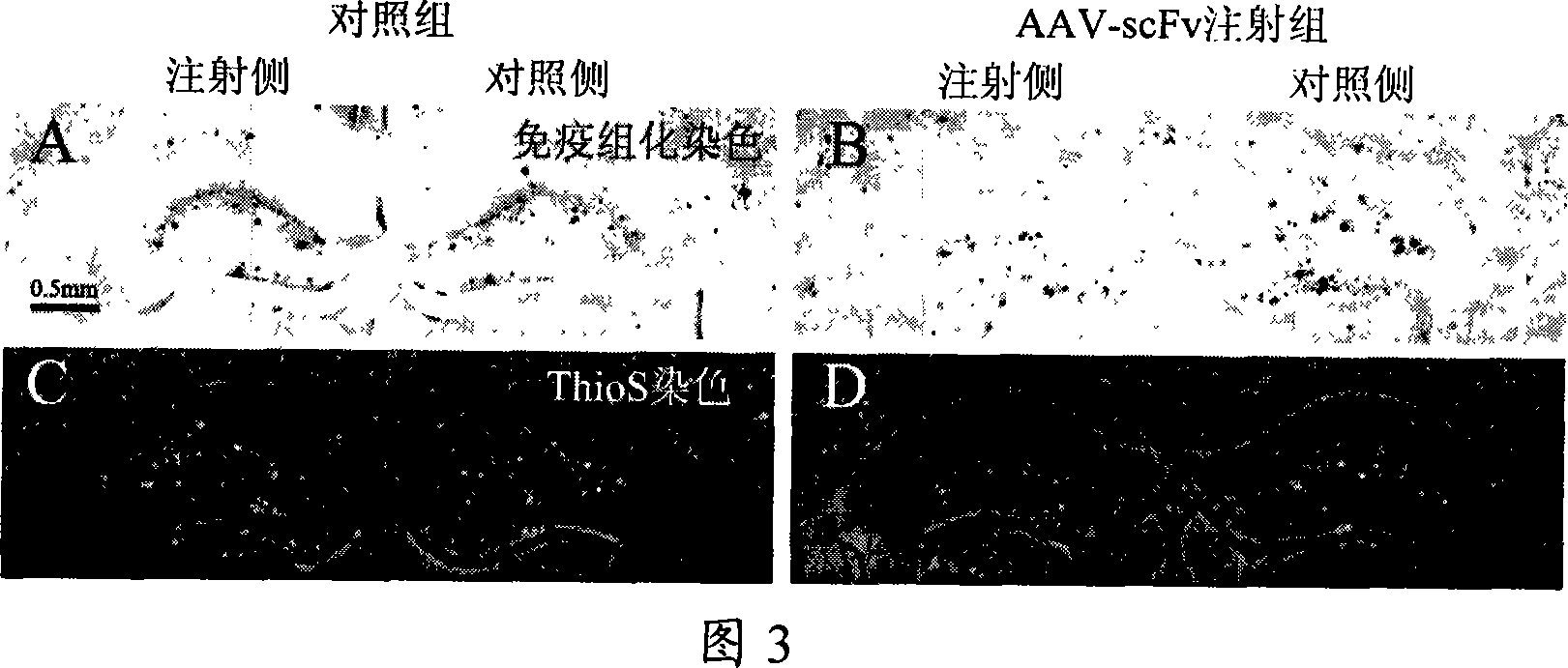
[0065] To test the effect of scFv on Aβ aggregation, 30 μg Aβ42 and equimolar scFv were mixed to a final concentration of 25 μM. Incubate at 37°C for 7 days. The results showed that after Aβ42 and scFv-GST were mixed and incubated at equimolar concentrations, the fluorescence intensity of Thioflavine T was significantly lower than that of the Aβ42 alone incubation group and the Aβ42 and GST protein co-incubation group, as shown in Figure 1, in Figure 1: Aβ refers to the group incubated with Aβ42 monomer alone, Aβ+scFv refers to the group incubated with Aβ42 monomer and scFv-GST protein, Aβ+GST refers to the group incubated with Aβ42 monomer and GST protein, and GST refers to the group incubated with GST protein alone . It can be seen from Figure 1 that there is no significant difference in the fluorescence intensity of the positive control Aβ42 incubation group alone and the Aβ42 and GST protein co-incubati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com