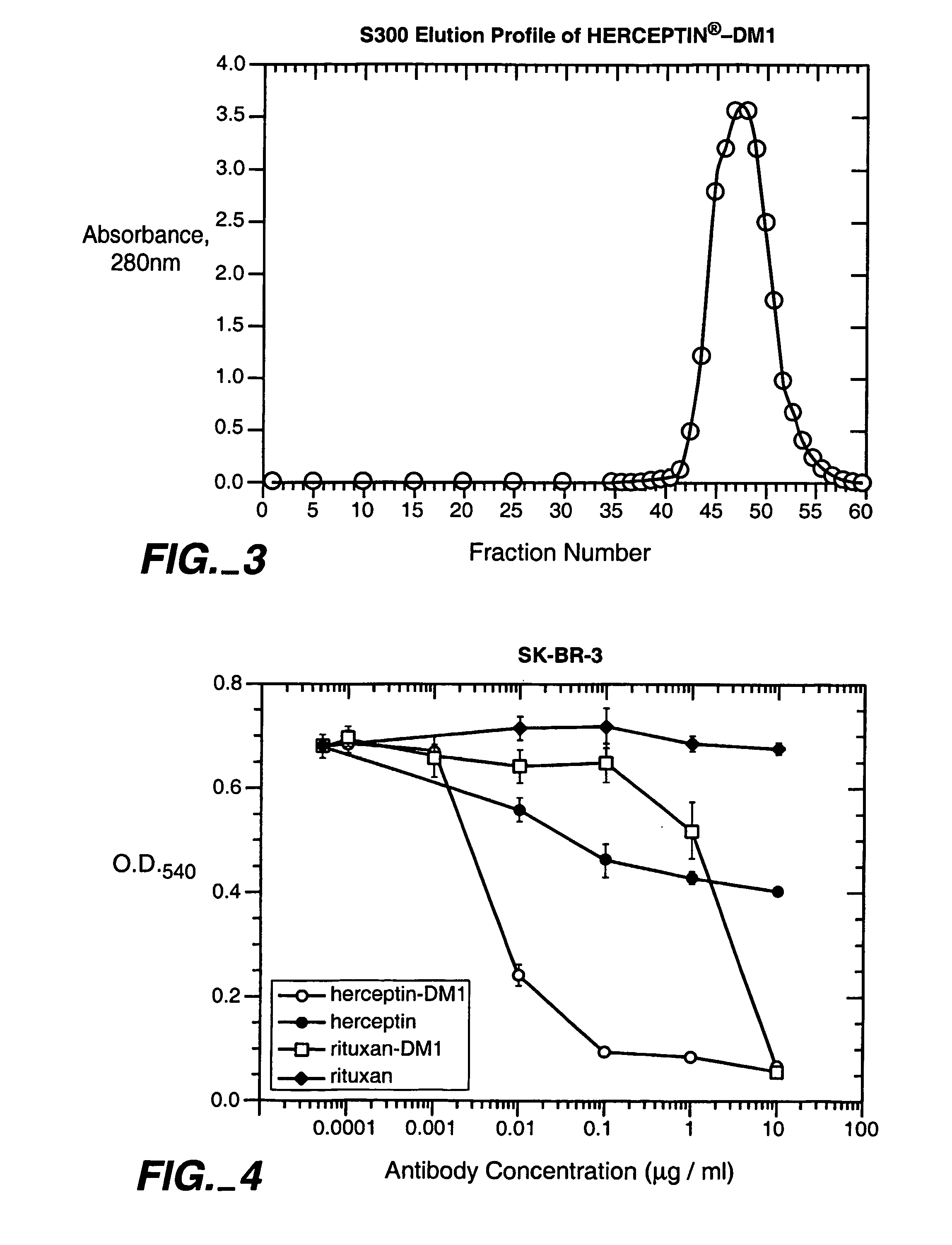
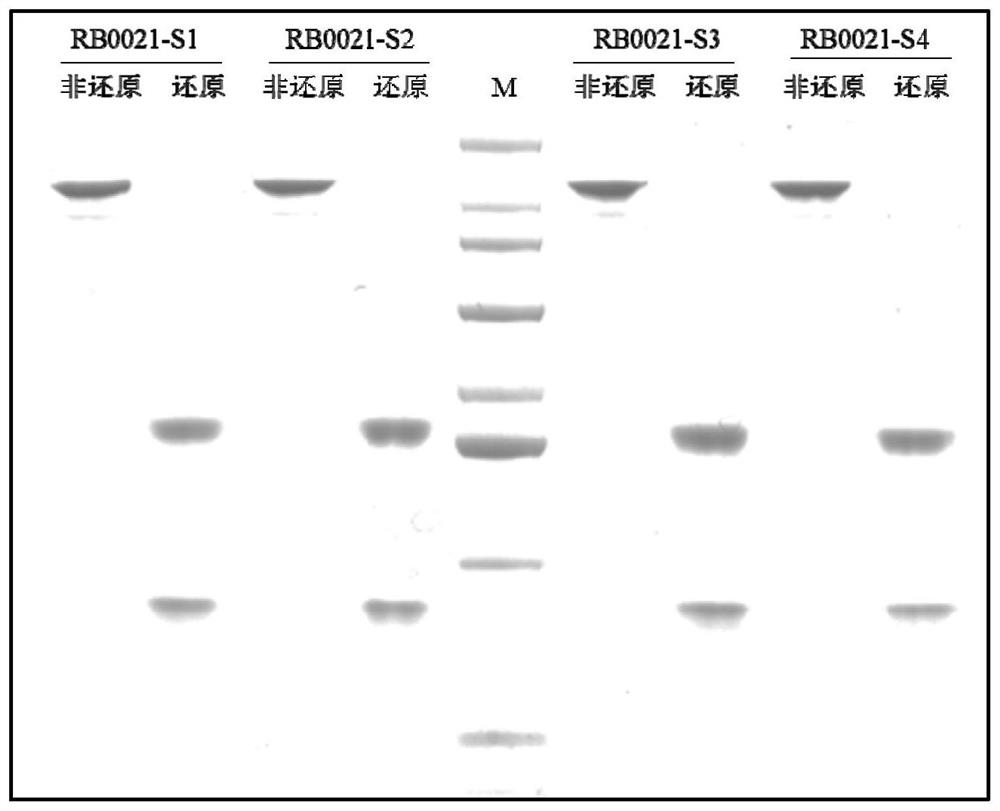
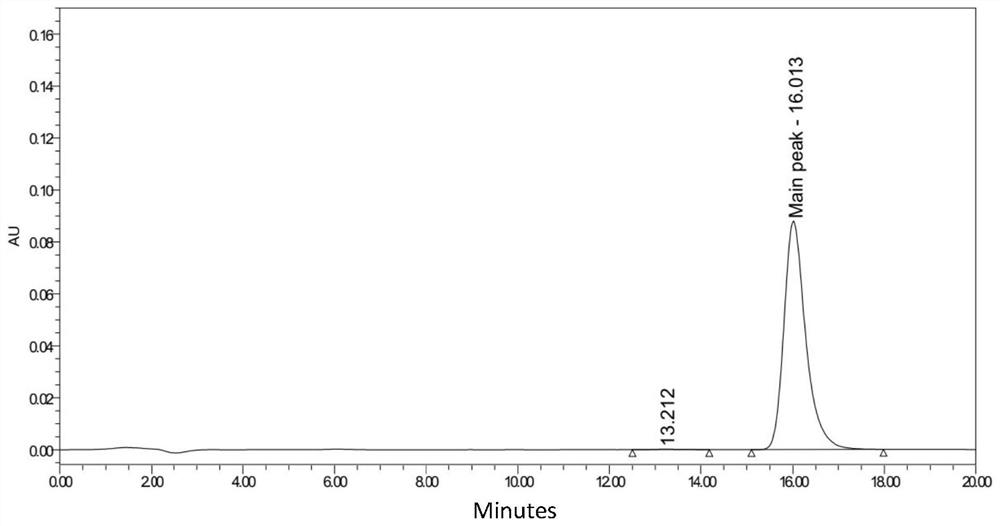
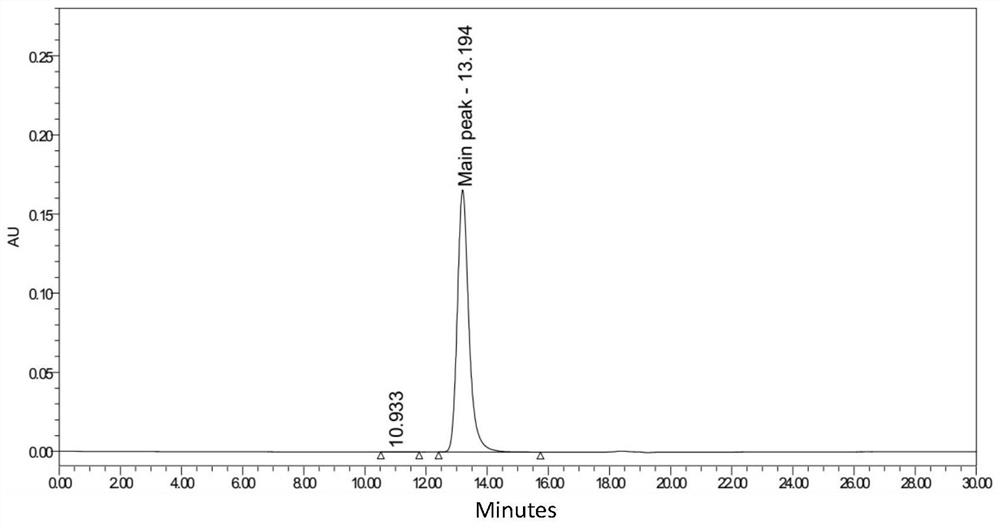
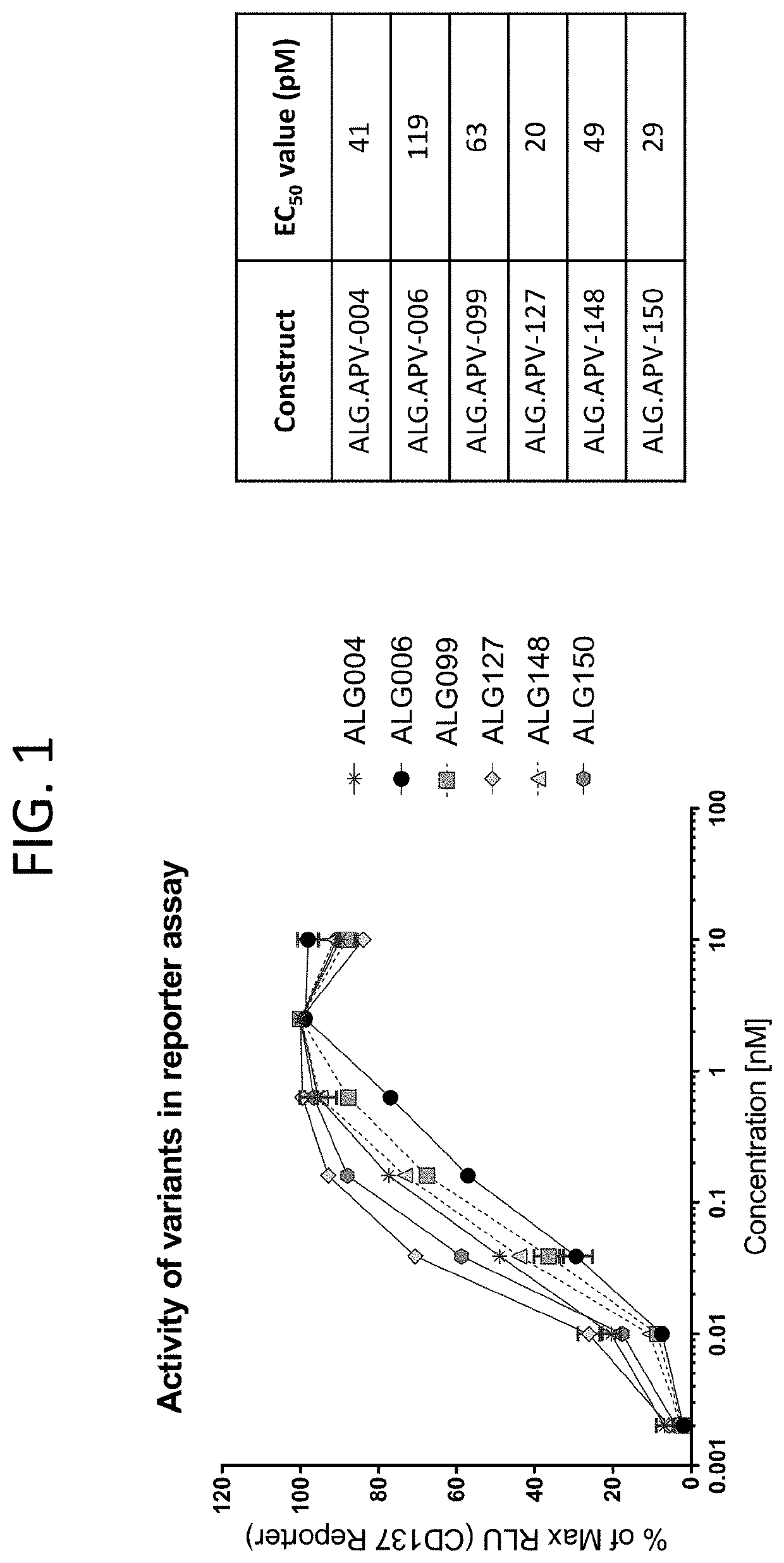
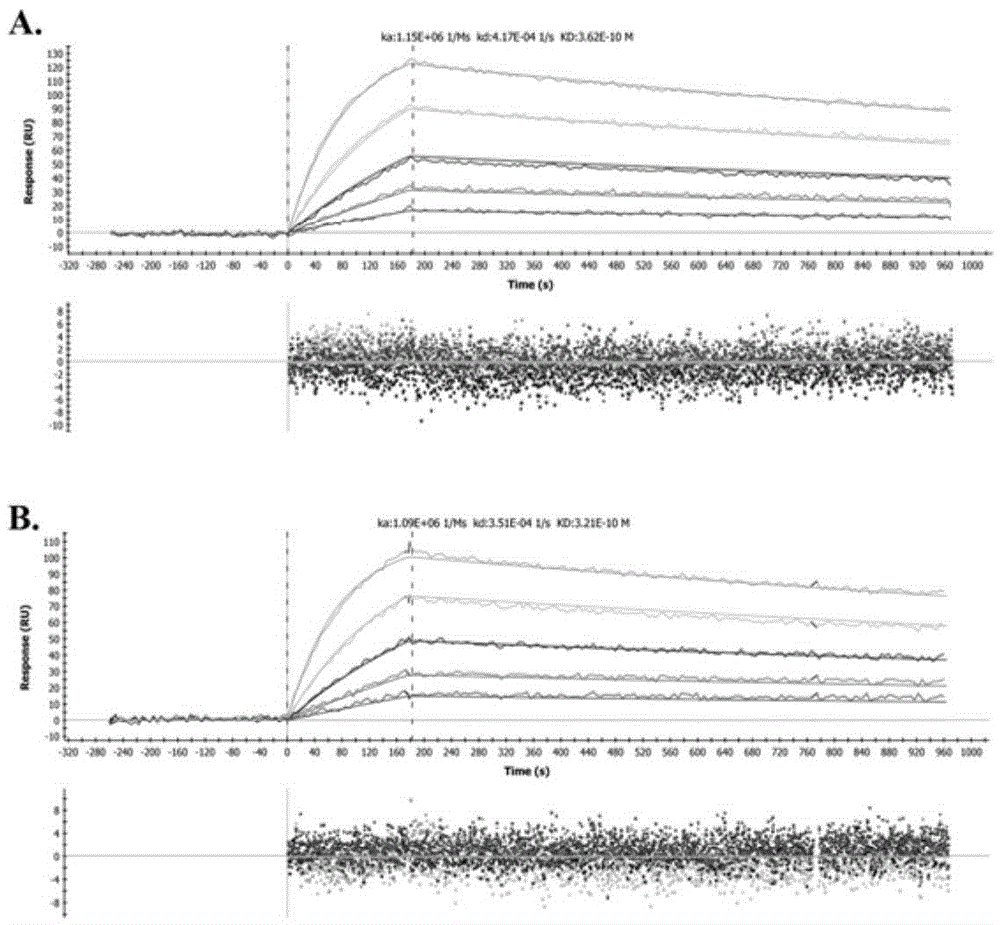
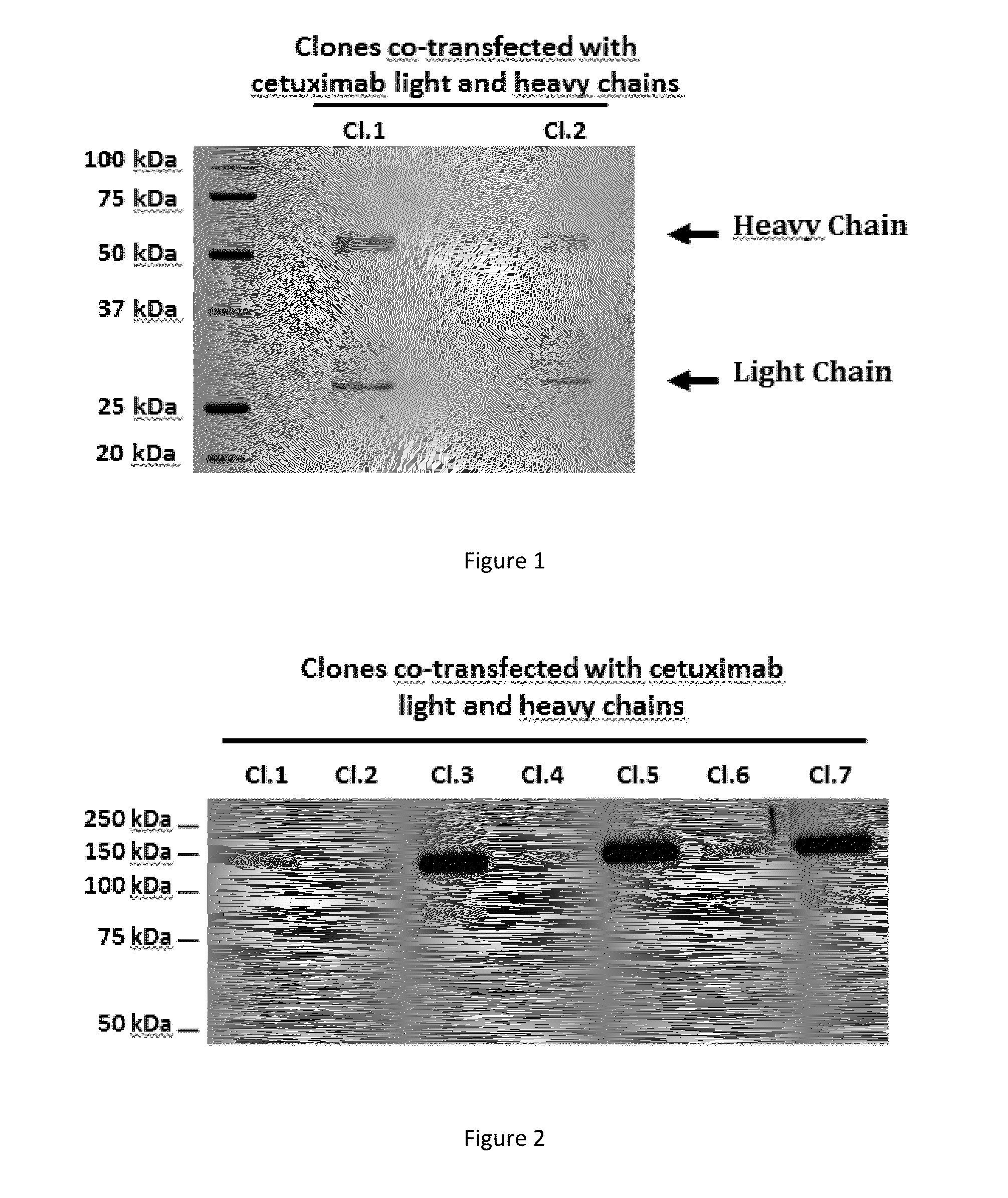
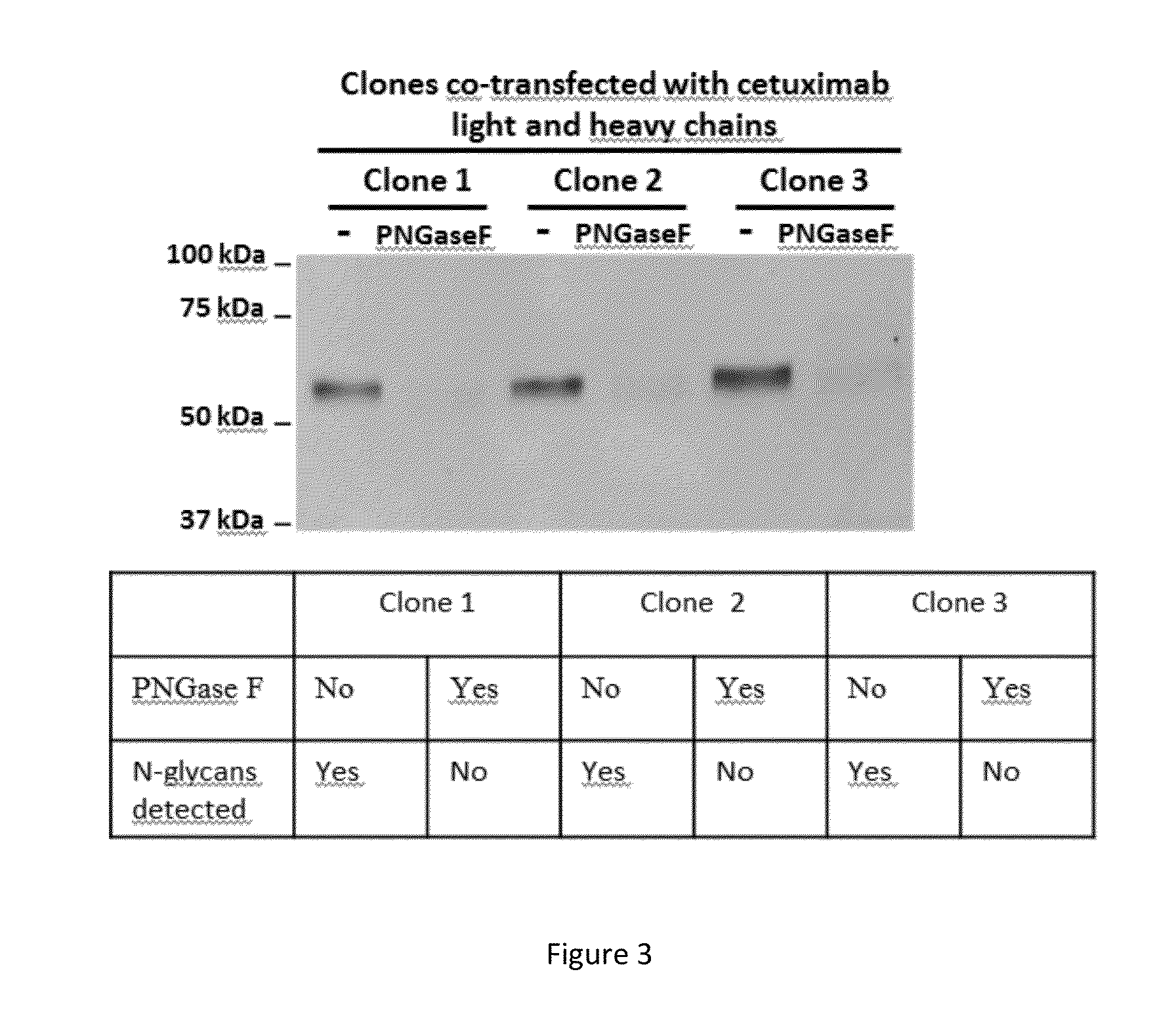
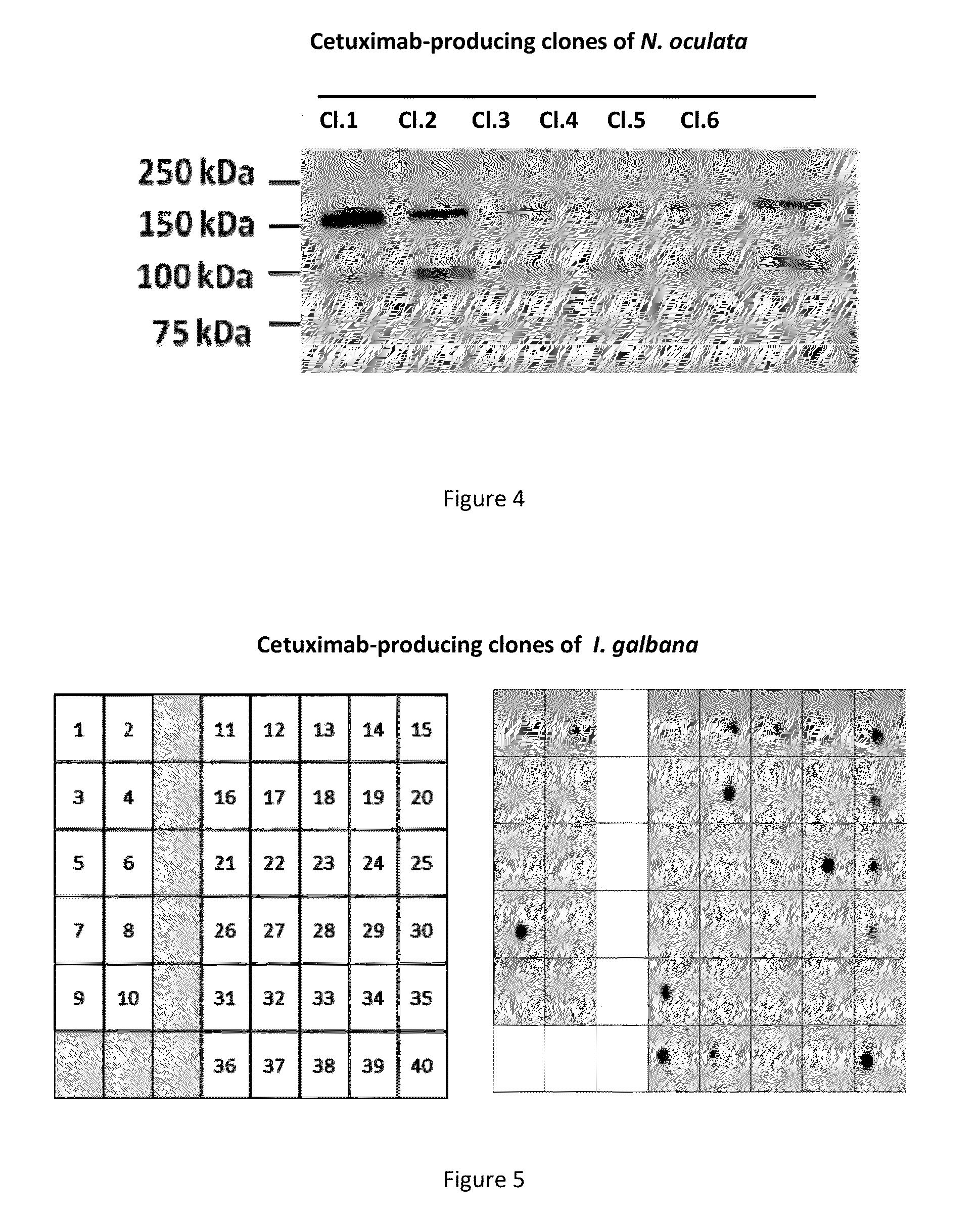
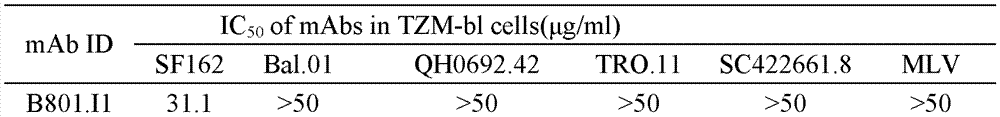Patents
Literature
30 results about "Cell-mediated cytotoxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
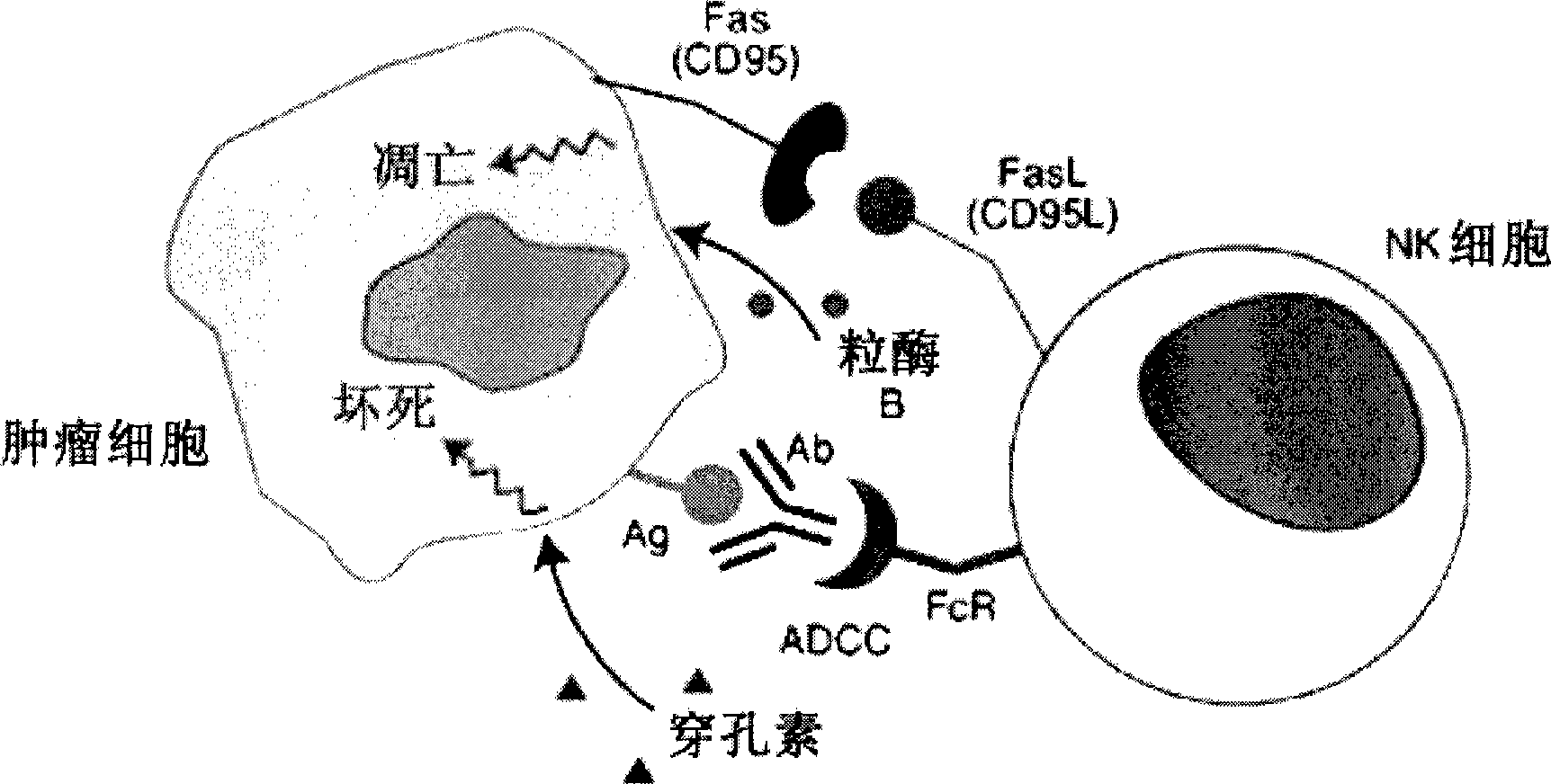
Antibody-dependent cell-mediated cytotoxicity (ADCC) (antibody-dependent cellular cytotoxicity) lysis of target cells coated with antibody by effector cells with cytolytic activity and specific immunoglobulin receptors called Fc receptors, including K cells, macrophages, and granulocytes.
Non-immunostimulatory antibody and compositions containing the same
ActiveUS20070148167A1Immunoglobulins against animals/humansEnzymologyTherapeutic antibodyFc-Gamma Receptor
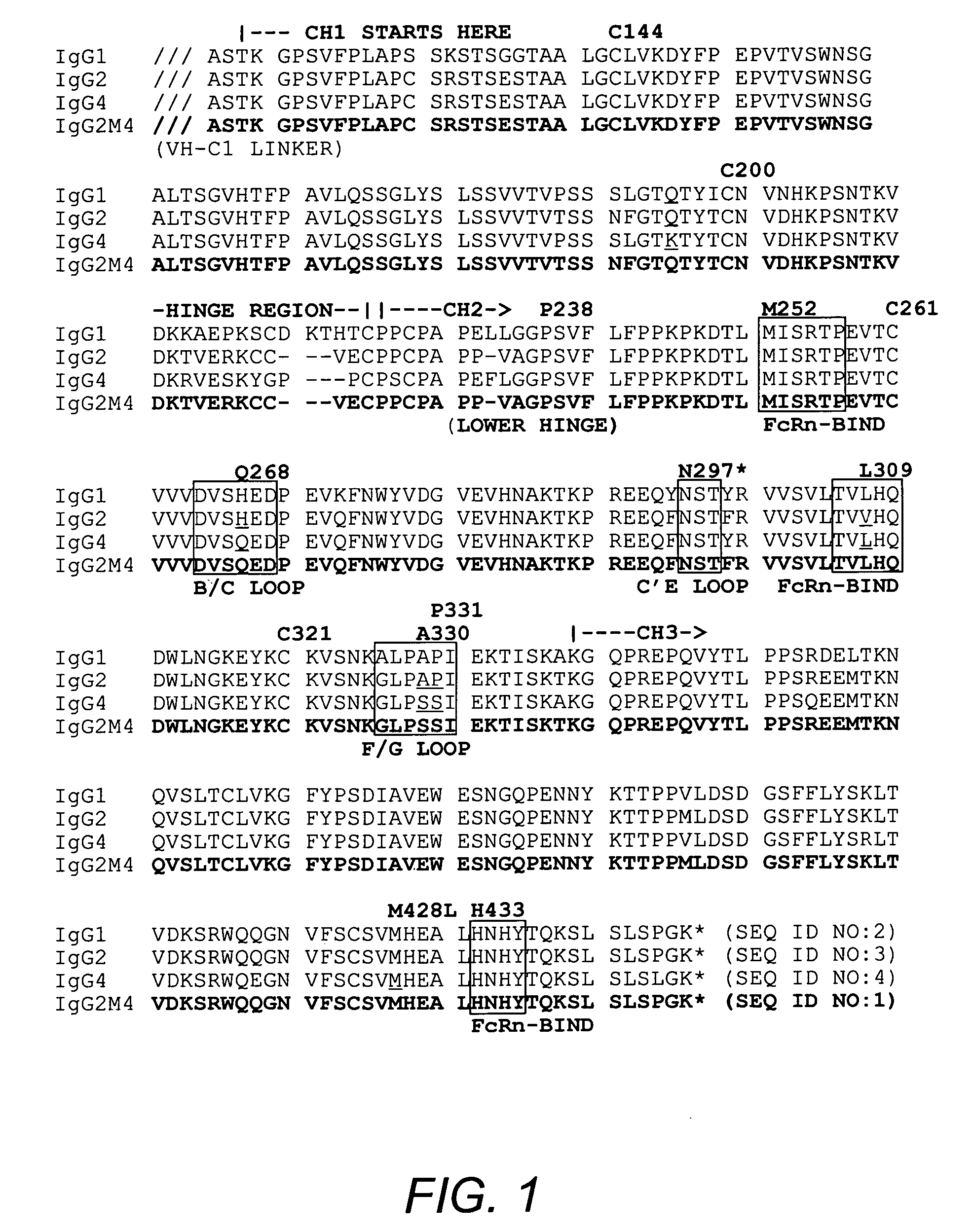
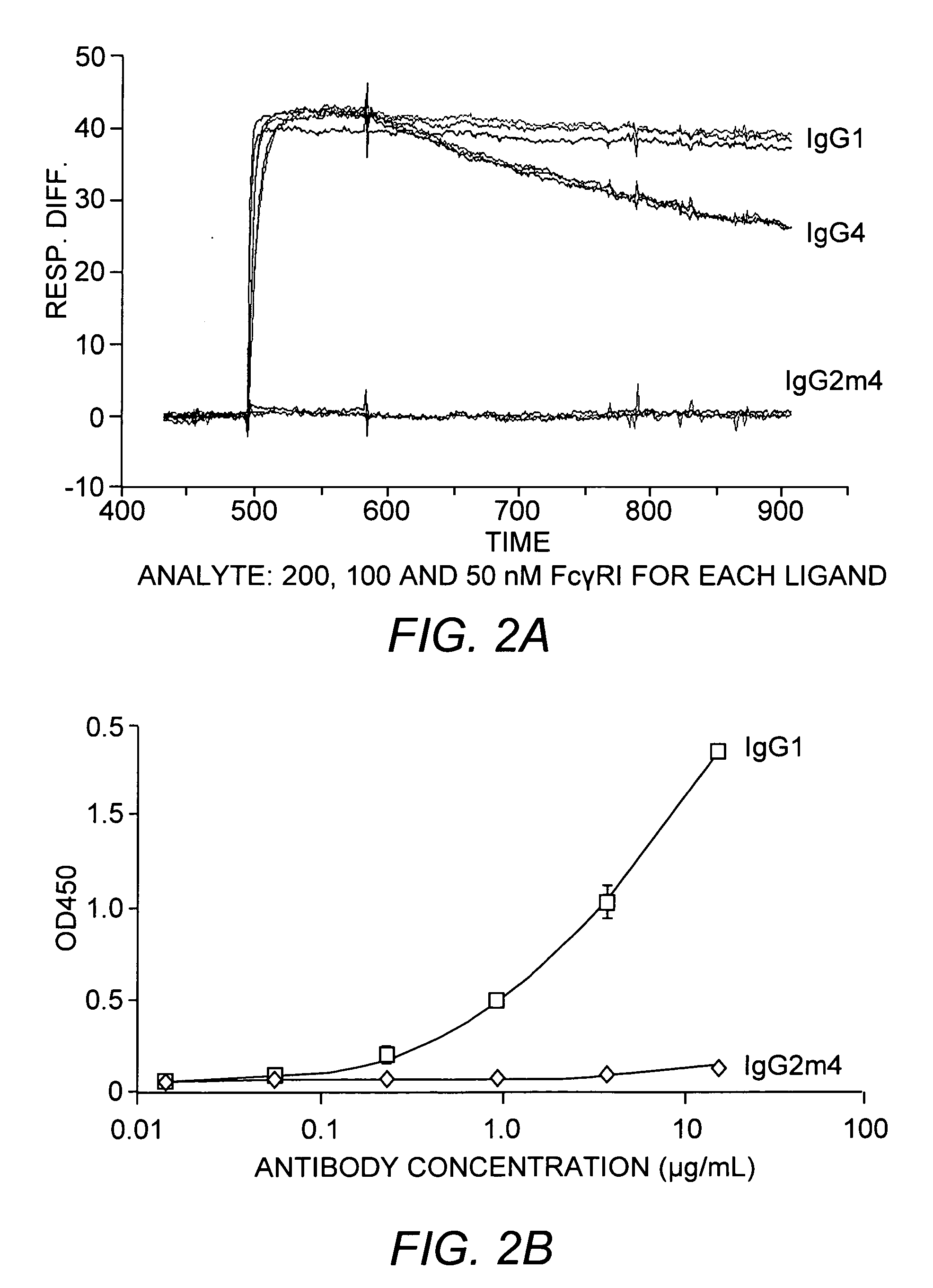
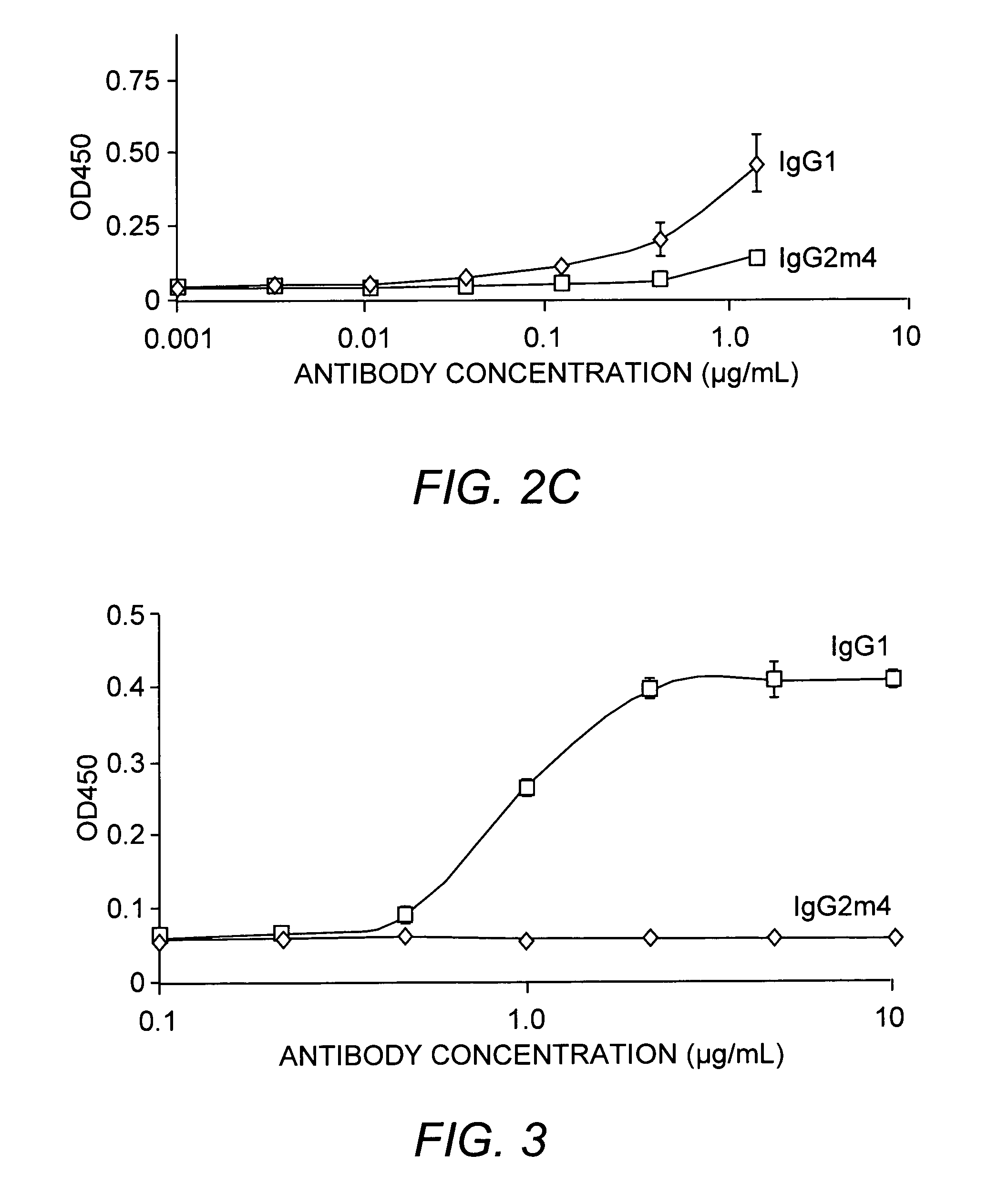
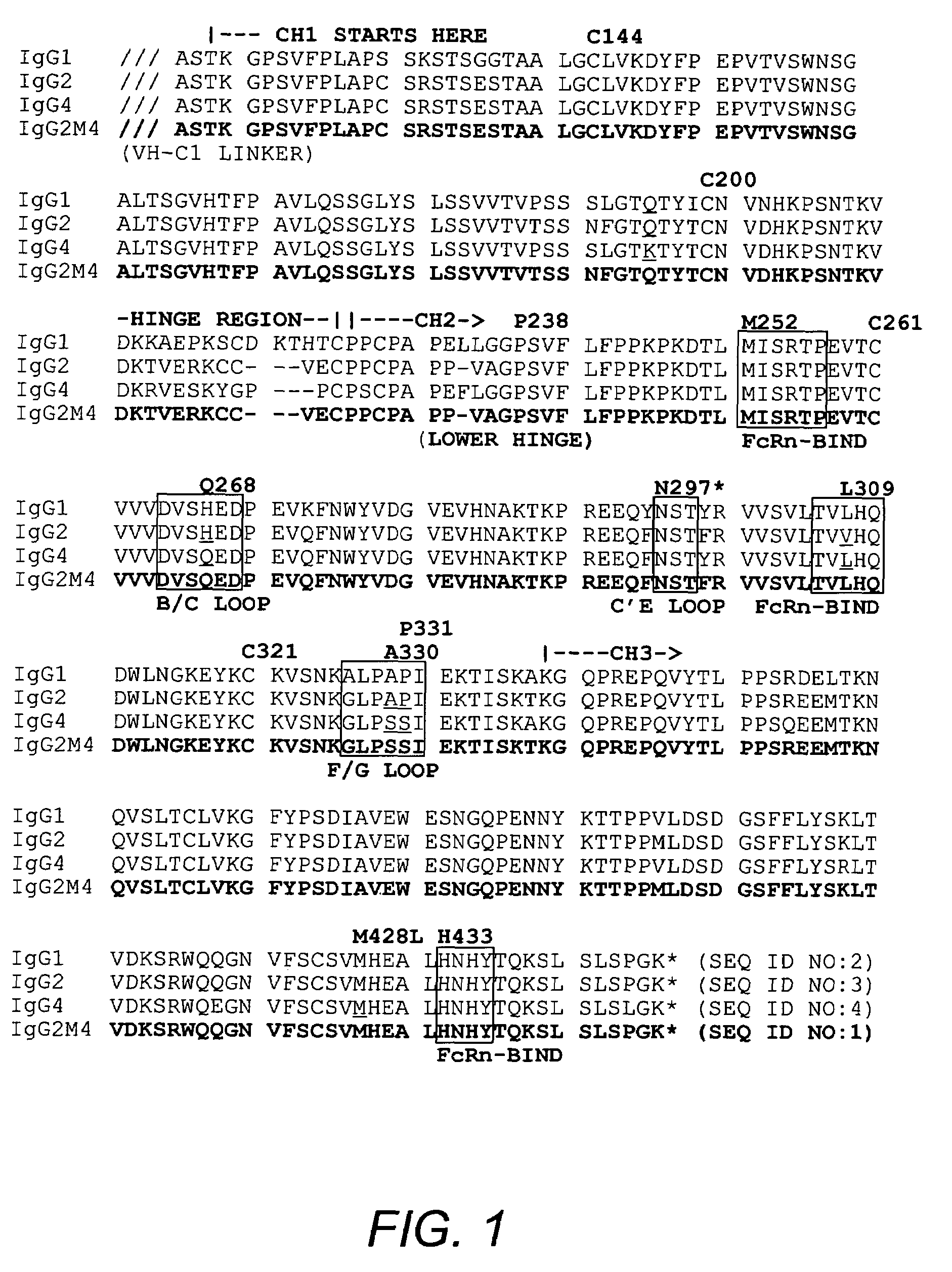
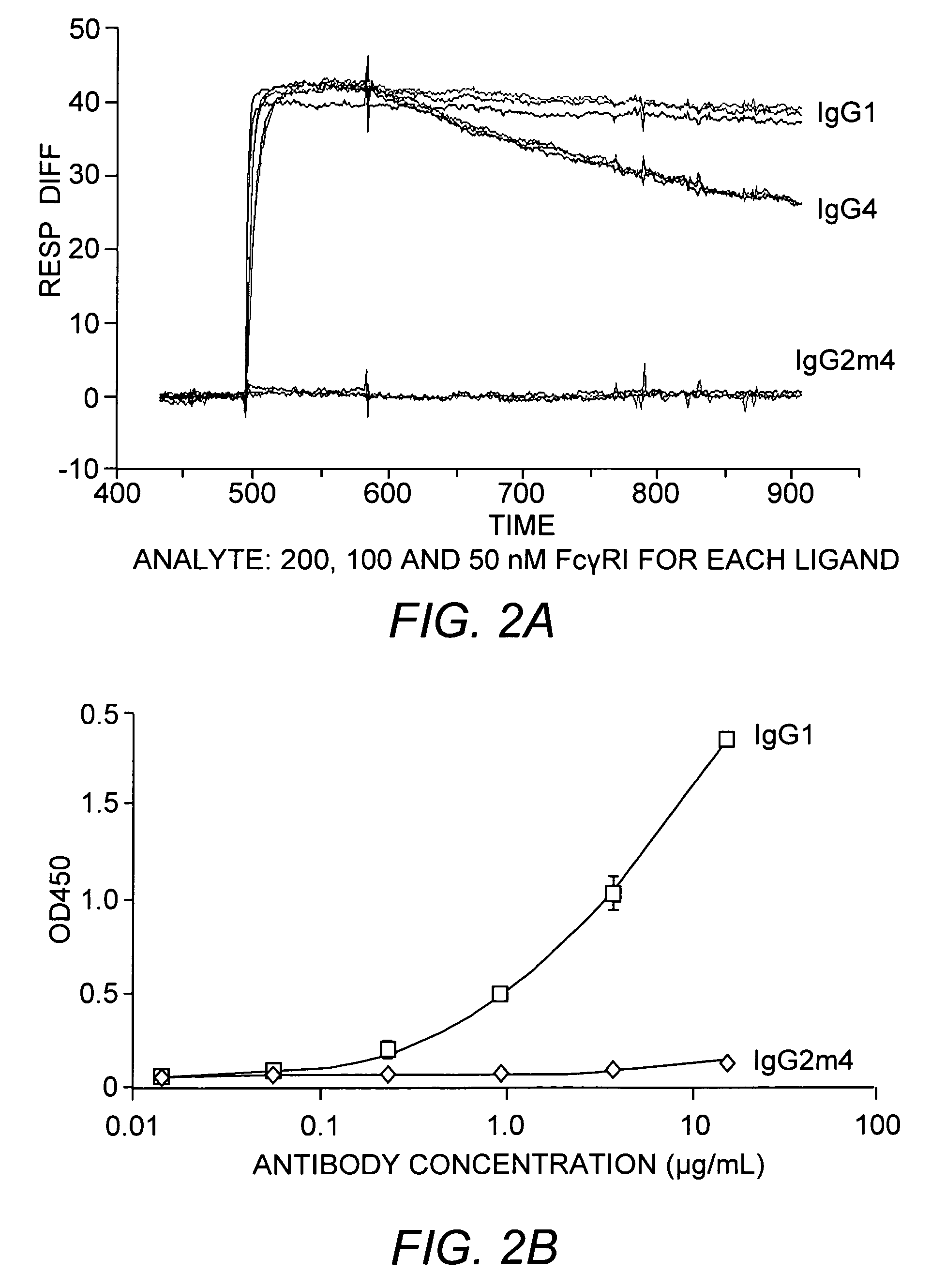
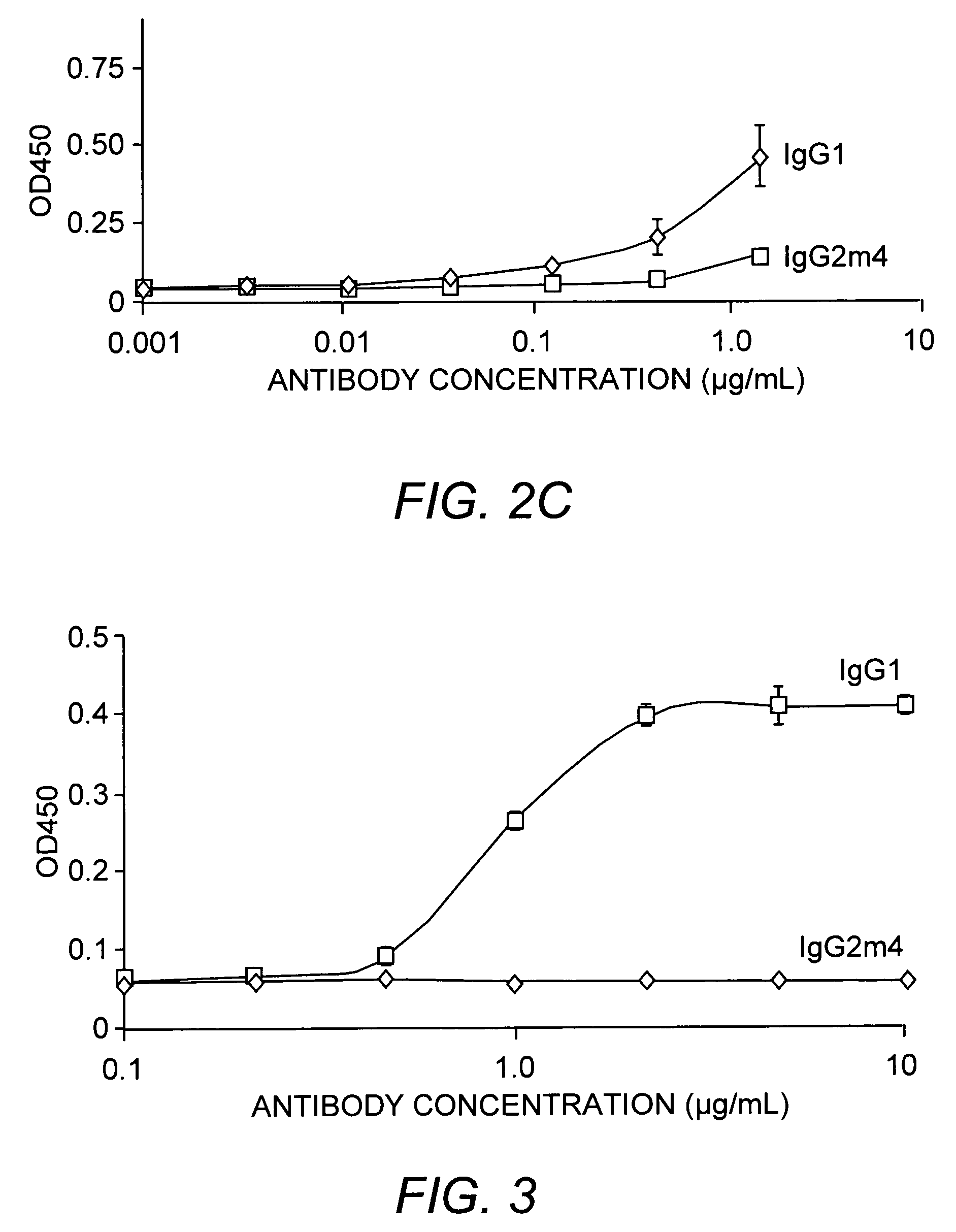
The present invention relates to a non-immunostimulatory antibody which lacks antibody-dependent cell-mediated cytotoxicity, Fc gamma receptor binding and complement-mediated cytotoxicity. In some embodiments, the antibody contains a modified immunoglobulin G2 (IgG2) Fc region with at least one substitution in the B / C loop, FcRn binding domain, and the F / G loop. The antibody of the invention is useful in the preparation of therapeutic antibodies and pharmaceutical compositions and kits containing the same.
Owner:MERCK SHARP & DOHME CORP
Chimeric receptors and uses thereof in immune therapy
ActiveUS20150139943A1Good curative effectEnhanced ADCC activityVirusesPeptide/protein ingredientsCytotoxicityCD8
Owner:COGENT BIOSCIENCES INC +2
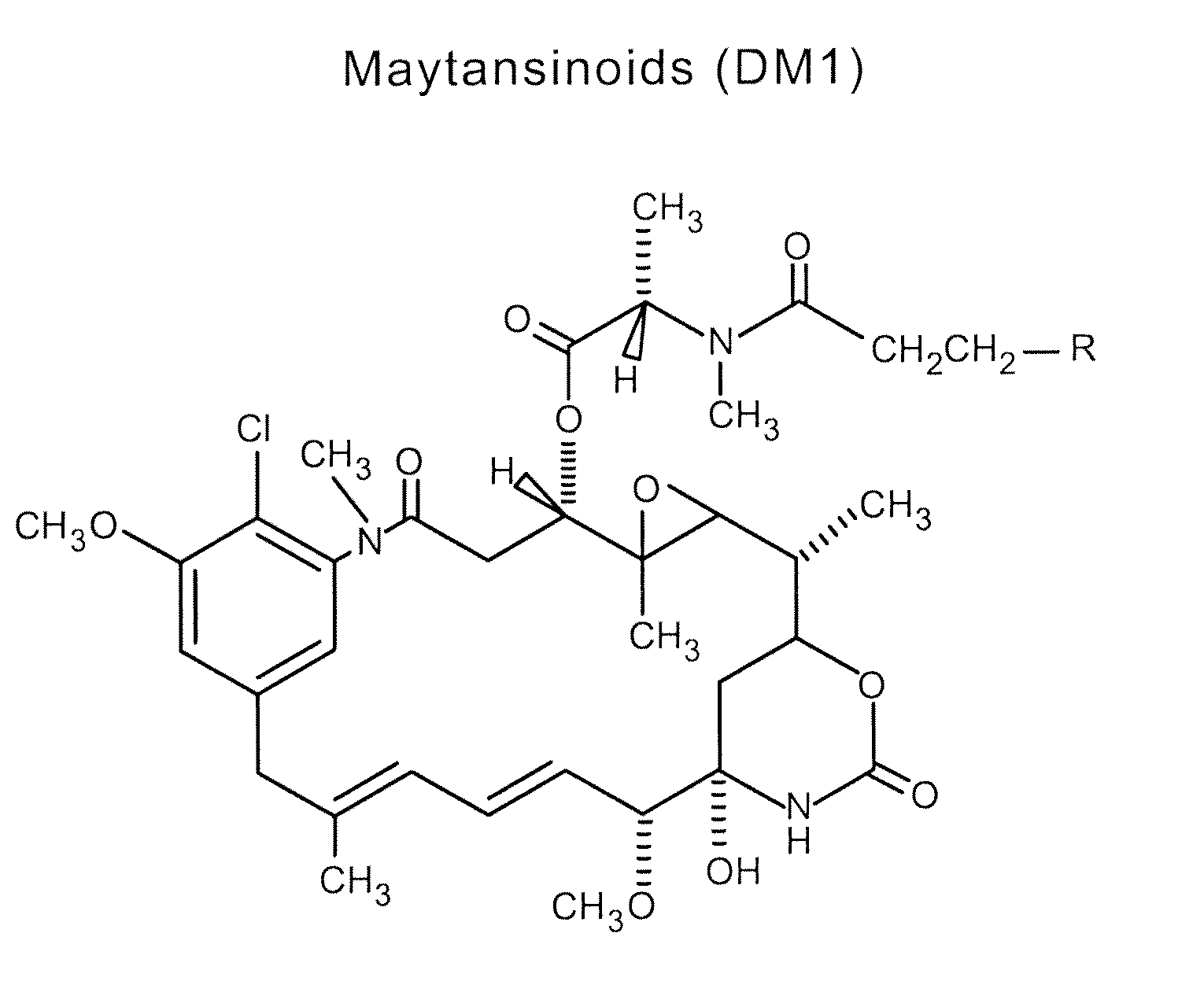
Methods for the identification of polypeptide antigens associated with disorders involving aberrant cell proliferation and compositions useful for the treatment of such disorders
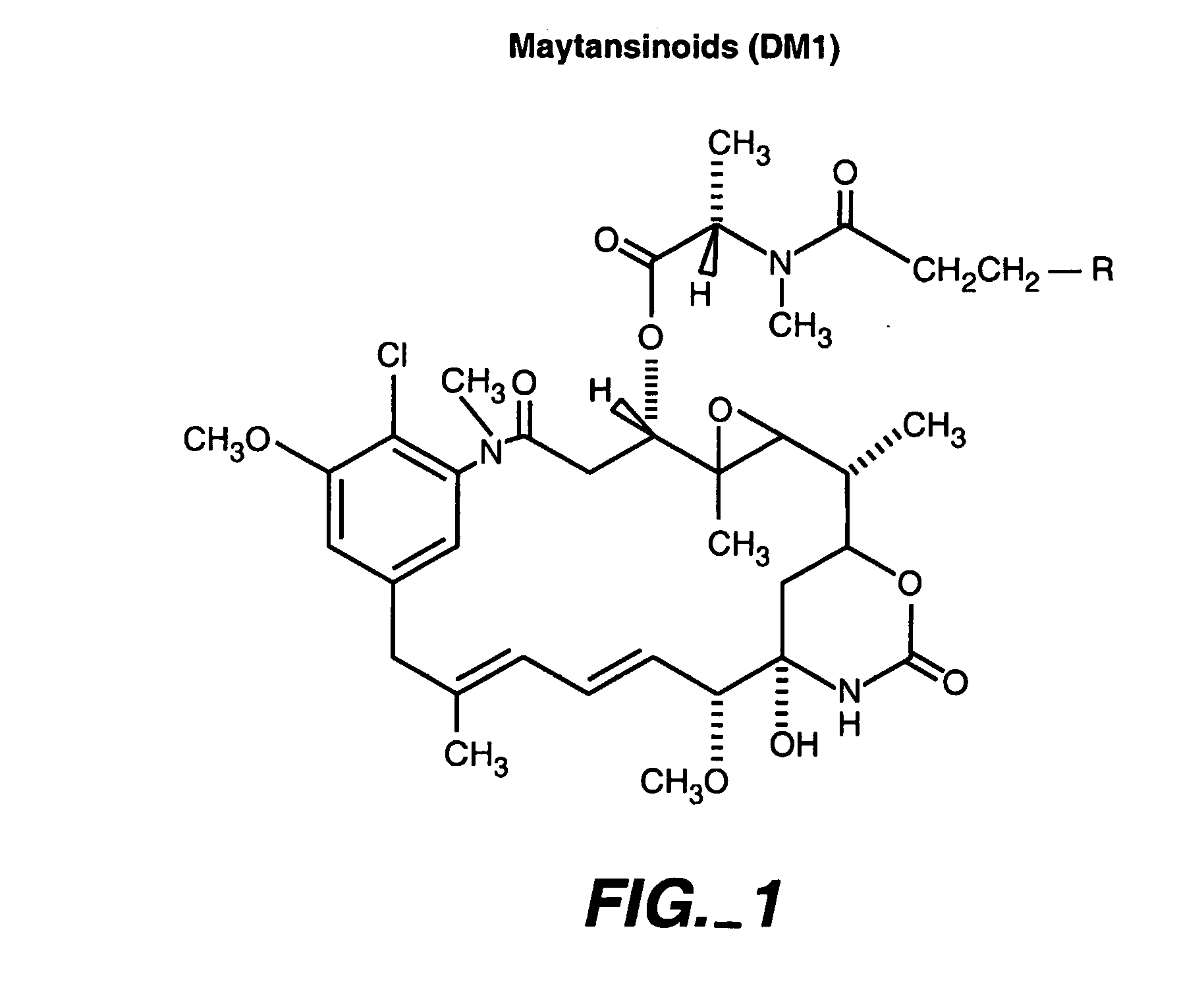
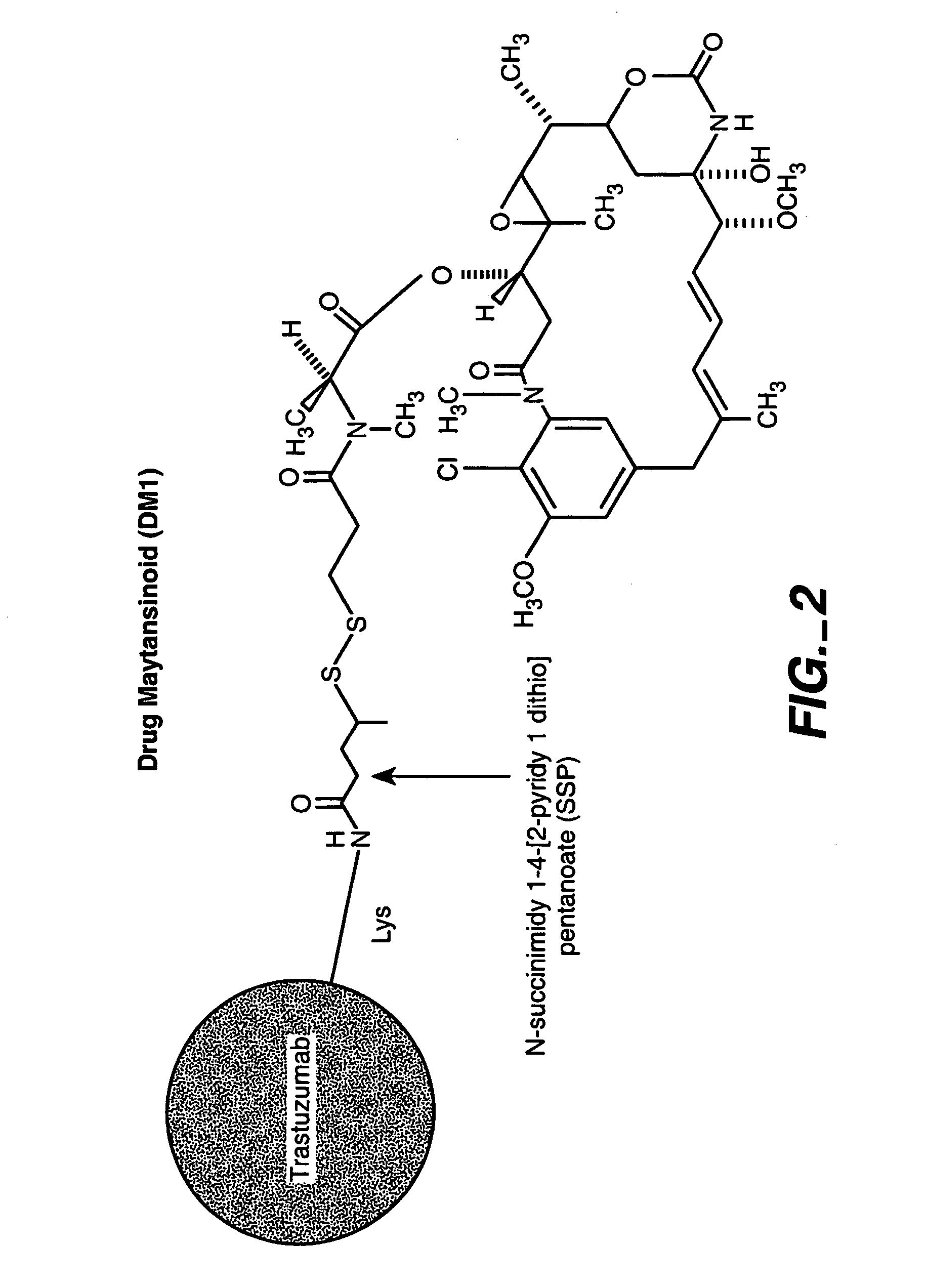
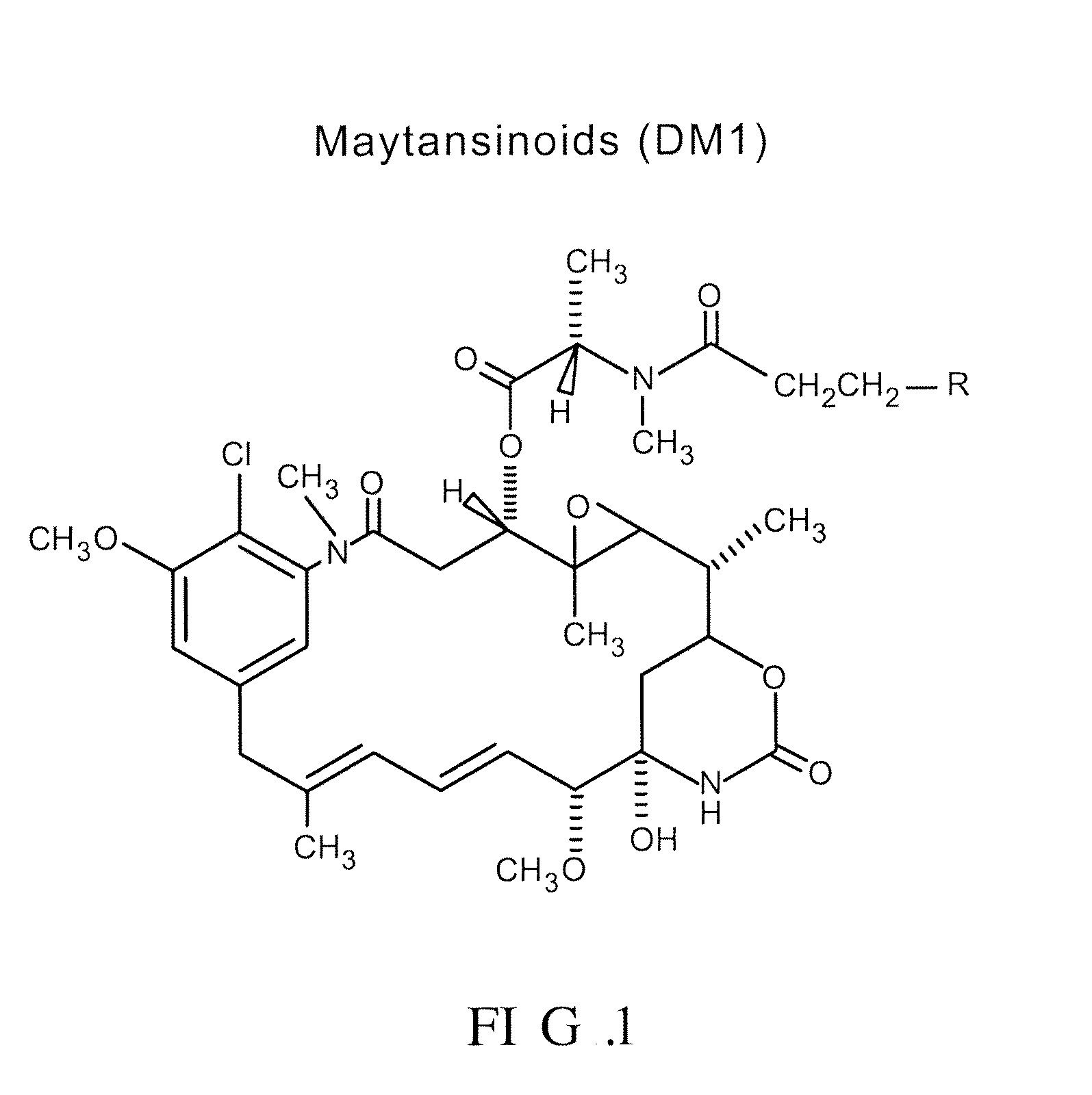
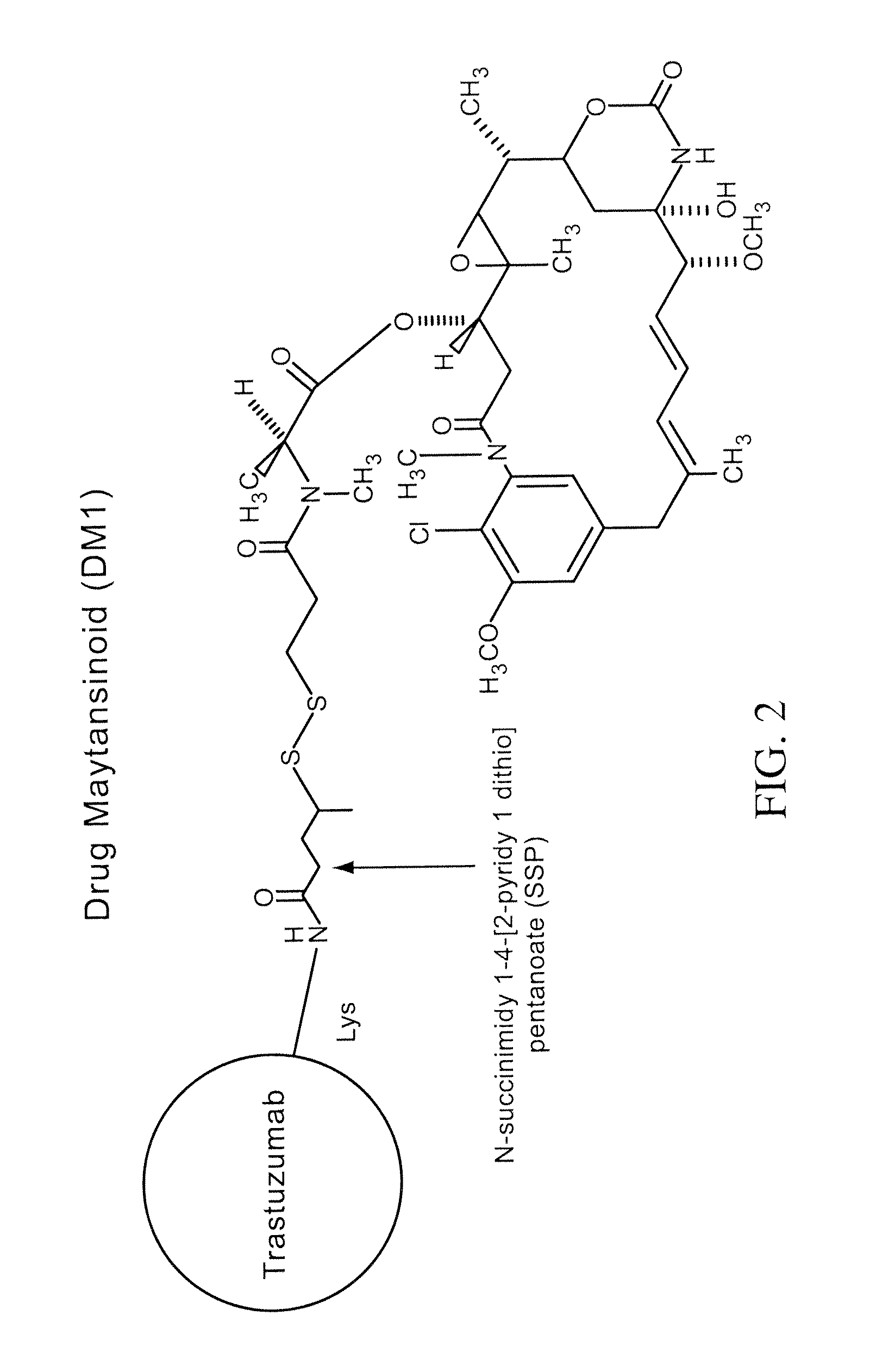
Methods and compositions for the development of effective cancer therapies using mitotic inhibitors which have limited general toxicity to normal, non-cancerous cells and tissues are provided. The methods and compositions utilize cytotoxic compounds comprised of a cell-binding agent (e.g., antibodies) conjugated to an anti-mitotic compound (e.g., maytansinoids). The invention further provides antibodies which are substantially incapable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC) and / or complement dependent cytotoxicity (CDC), thereby ensuring that the therapeutic effect is mediated primarily by the anti-mitotic component of the cytotoxic compound, rather than by indirect cell killing via ADCC and / or CDC. The antibodies of the invention further are capable of differentiating between polypeptide antigens which are more highly expressed on proliferating cancer cells as compared to proliferating non-cancer cells.
Owner:GENENTECH INC
Methods for the Treatment of Autoimmune Disorders Using Immunosuppressive Monoclonal Antibodies with Reduced Toxicity
ActiveUS20080095766A1Reduce the possibilityIncreasing concentration of antibodySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
Anti-cancer antibodies with reduced complement fixation
ActiveUS20050202021A1Reduce complement fixationReduce pain levelsPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthBlastoma
The invention provides modified antibodies directed against GD2 that have diminished complement fixation relative to antibody-dependent, cell-mediated cytotoxicity, which is maintained. The modified antibodies of the invention may be used in the treatment of tumors such as neuroblastoma, glioblastoma, melanoma, small-cell lung carcinoma, B-cell lymphoma, renal carcinoma, retinoblastoma, and other cancers of neuroectodermal origin.
Owner:MERCK PATENT GMBH
Non-immunostimulatory antibody and compositions containing the same
ActiveUS7700099B2Immunoglobulins against animals/humansEnzymologyTherapeutic antibodyFc-Gamma Receptor
Owner:MERCK SHARP & DOHME CORP
Anti-cancer antibodies with reduced complement fixation
ActiveUS7432357B2Easy to fixReduce pain levelsSugar derivativesPeptide/protein ingredientsMelanomaCell-mediated cytotoxicity
The invention provides modified antibodies directed against GD2 that have diminished complement fixation relative to antibody-dependent, cell-mediated cytotoxicity, which is maintained. The modified antibodies of the invention may be used in the treatment of tumors such as neuroblastoma, glioblastoma, melanoma, small-cell lung carcinoma, B-cell lymphoma, renal carcinoma, retinoblastoma, and other cancers of neuroectodermal origin.
Owner:MERCK PATENT GMBH
Antibody fusion protein targeting VEGFR2 as well as preparation and use thereof
InactiveCN104628866AEnhanced ADCC effectPeptide/protein ingredientsPeptide preparation methodsAbnormal tissue growthNatural Killer Cell Inhibitory Receptors
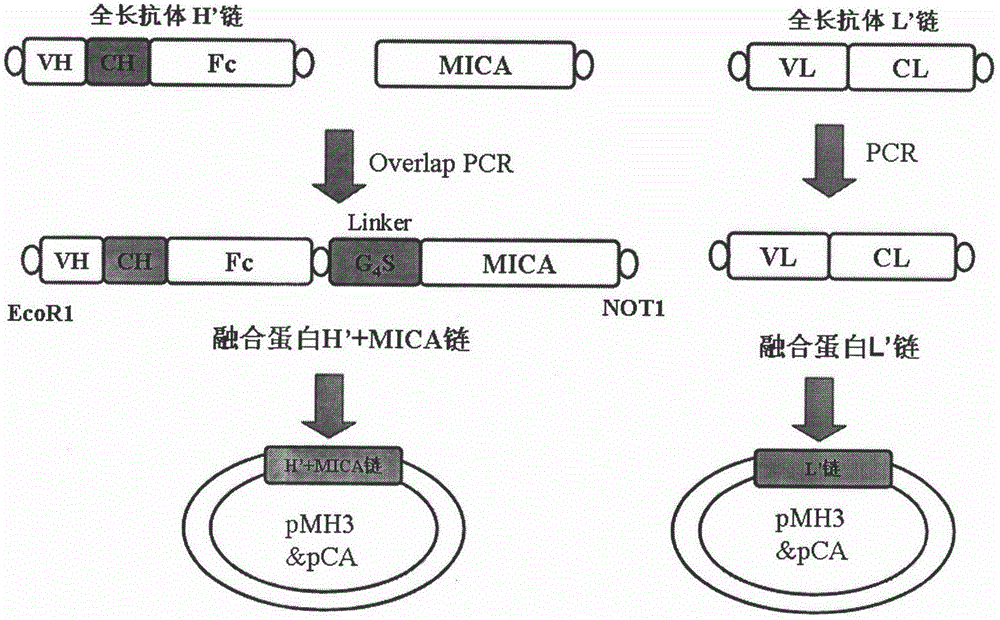
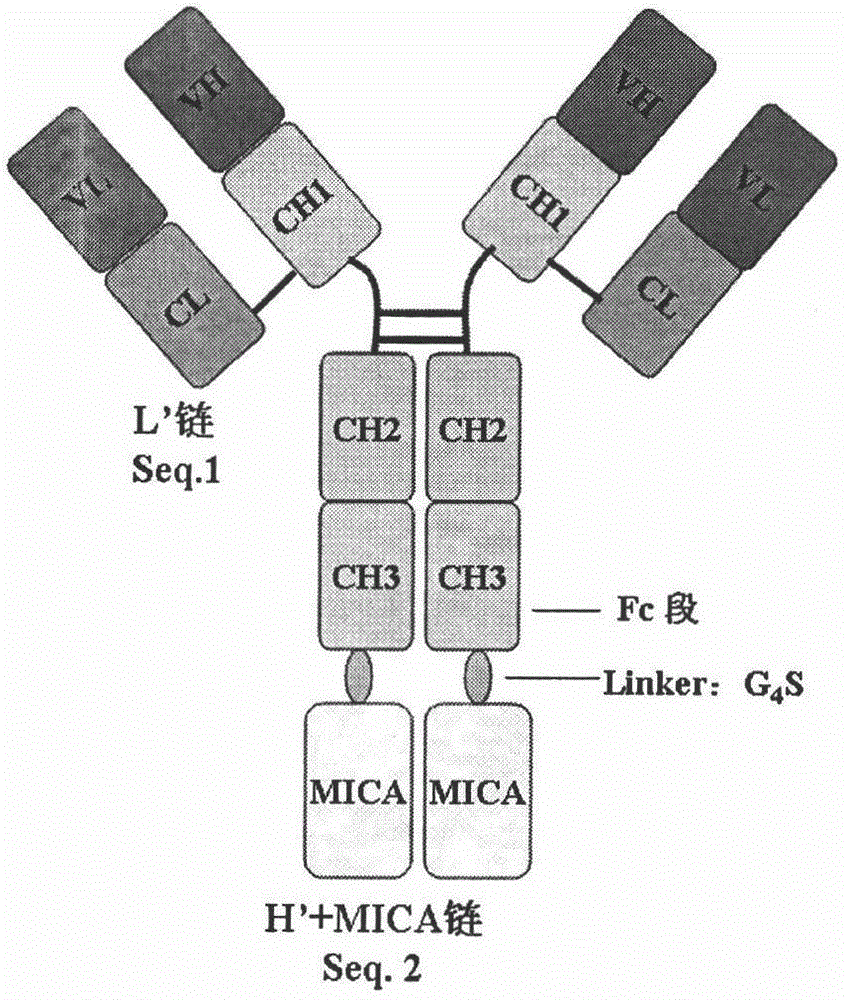
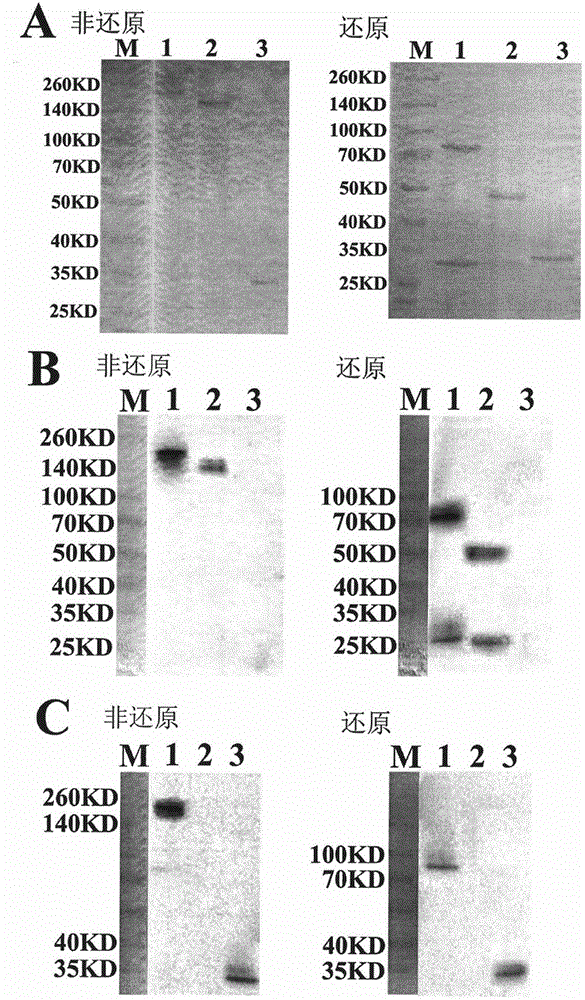
The invention belongs to the technical field of genetically engineered antibody, in particular relates to a fusion protein of tumor vascular endothelial growth factor receptor 2 (VEGFR2 / KDR3) antibody and MICA as well as a preparation method and use thereof. In the invention, a full-length antibody of VEGFR2 is linked with a ligand MHC I molecule associated protein A (MICA) of activated receptor of NK cell (NKG2D) through a flexible linker and expressed by CHO cells by utilizing the genetic engineering technology, and the formed fusion protein can act on VEGFR on the surface of a tumor cell to inhibit or kill tumor and inhibit or destroy tumor angiogenesis, and on the other hand, the formed fusion protein can increase the level of MICA on the surface of the tumor cell and stimulate the NK cells to kill tumor cells through identification of NKG2D on the surfaces of the NK cells, thereby reestablishing the immunological surveillance of an body activated by the NKG2D pathway, and enhancing effects of antibody-dependent cell-mediated cytotoxicity (ADCC) and the like mediated by the Fc region of the antibody to kill tumor cells.
Owner:CHINA PHARM UNIV
Chimeric receptors and uses thereof in immune therapy
ActiveUS10144770B2Good curative effectHigh activityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD8Immune therapy
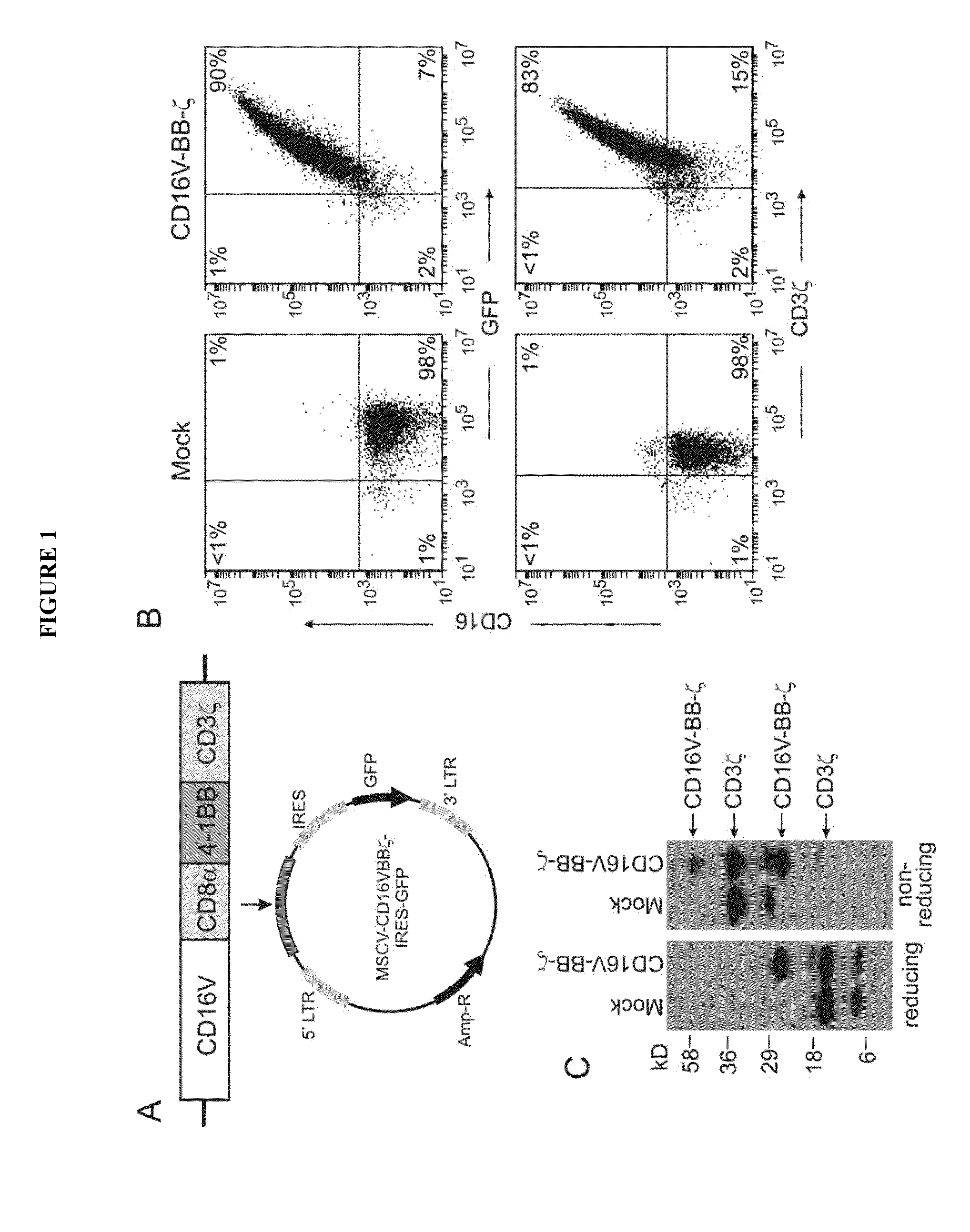
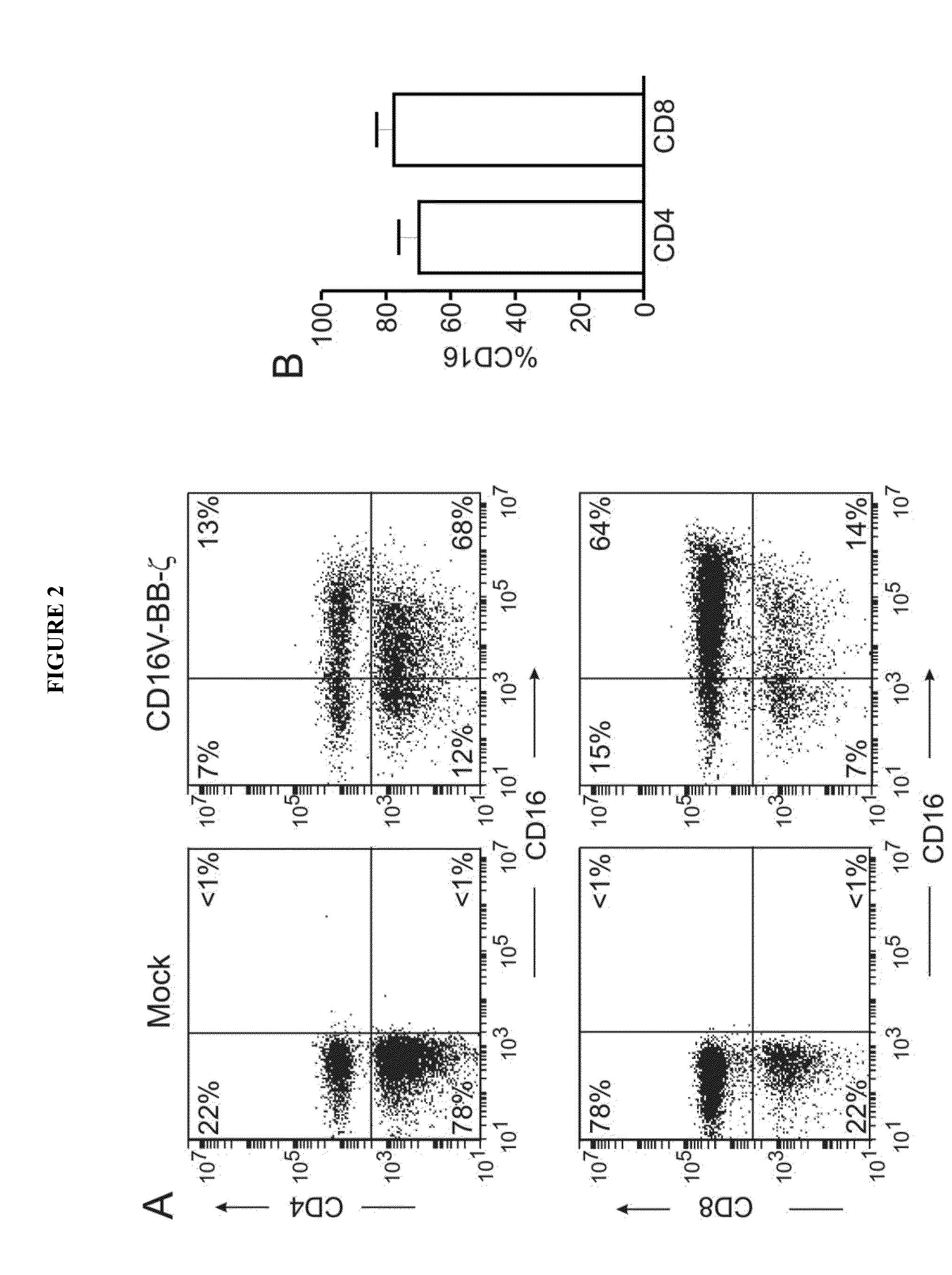
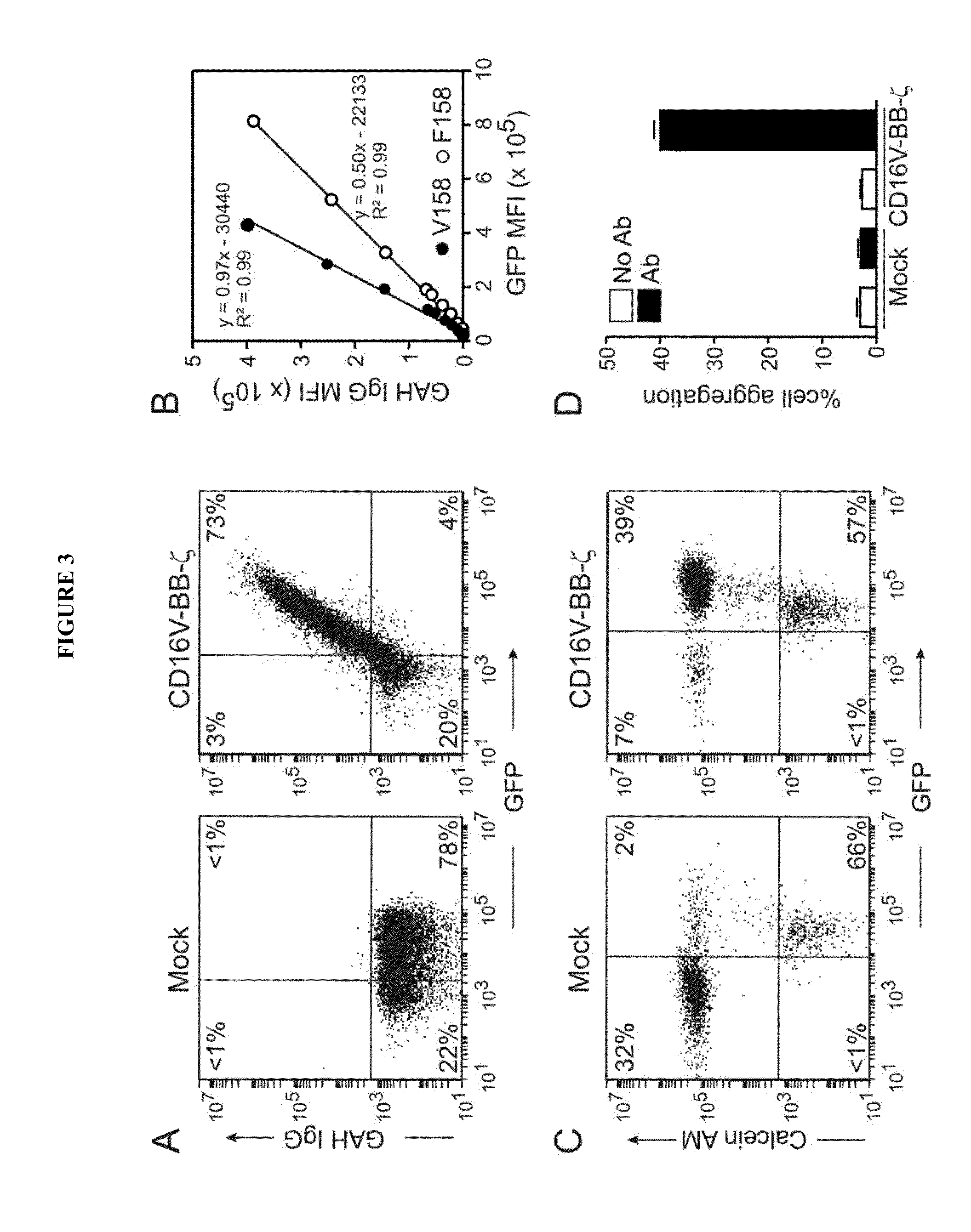
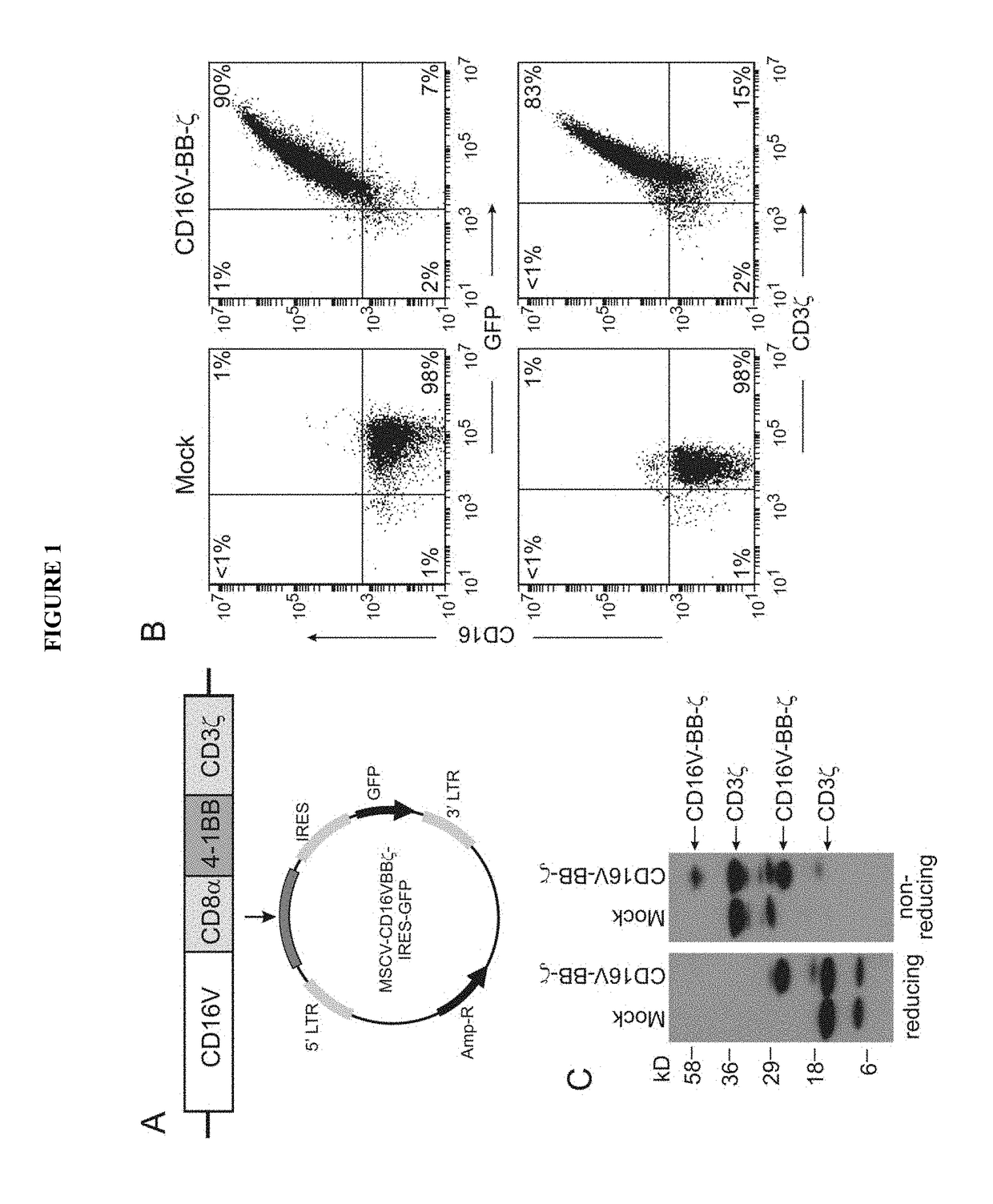
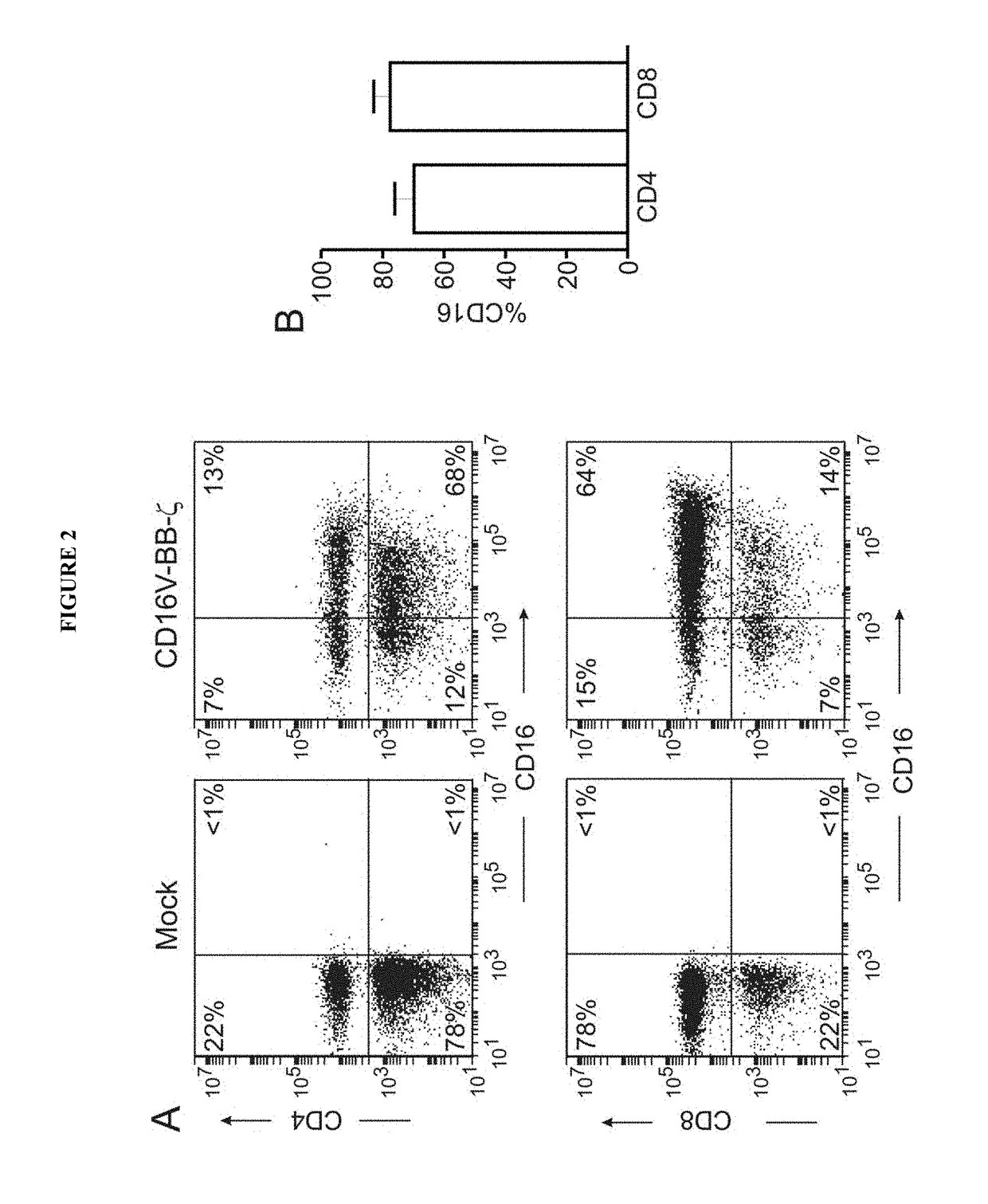
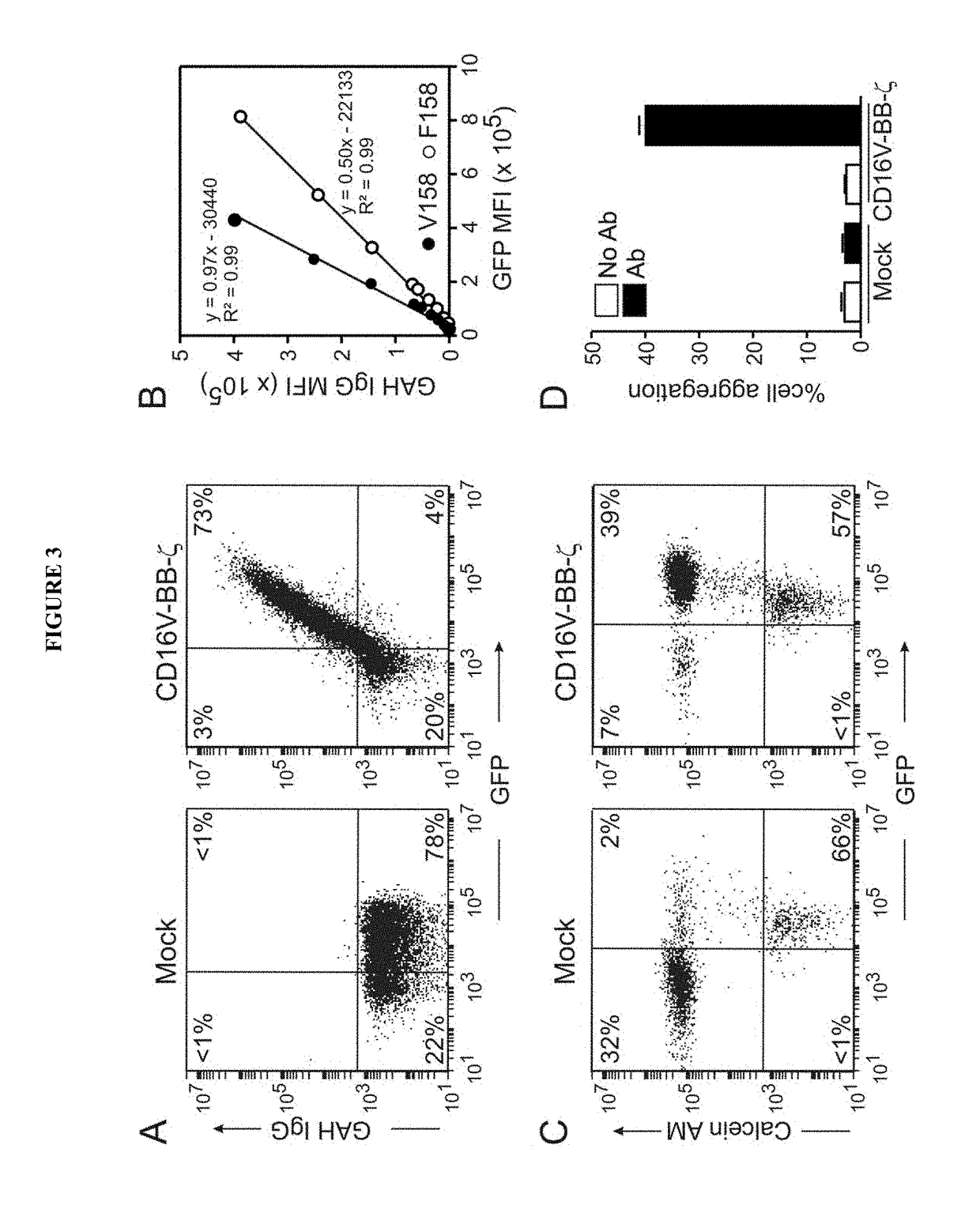
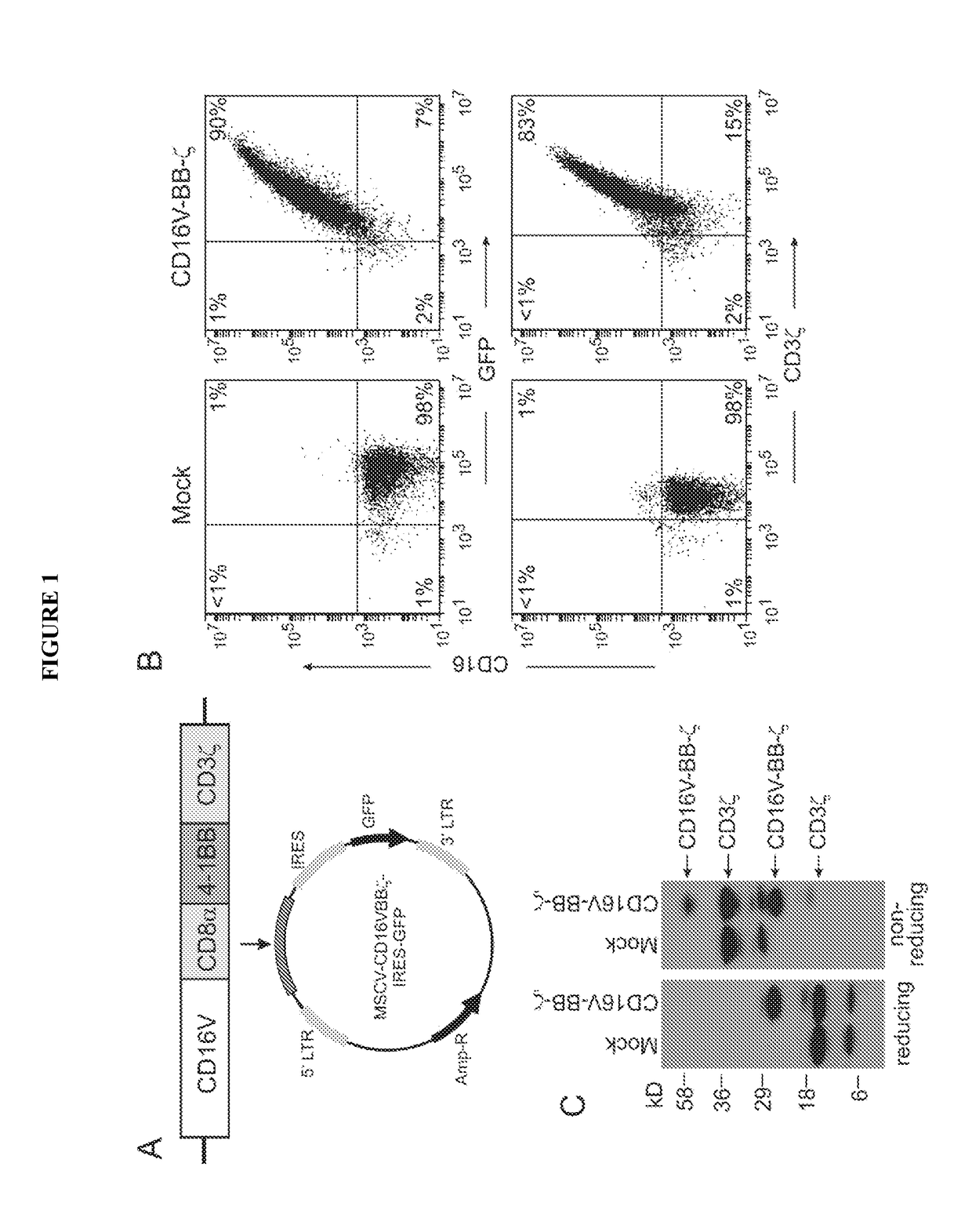
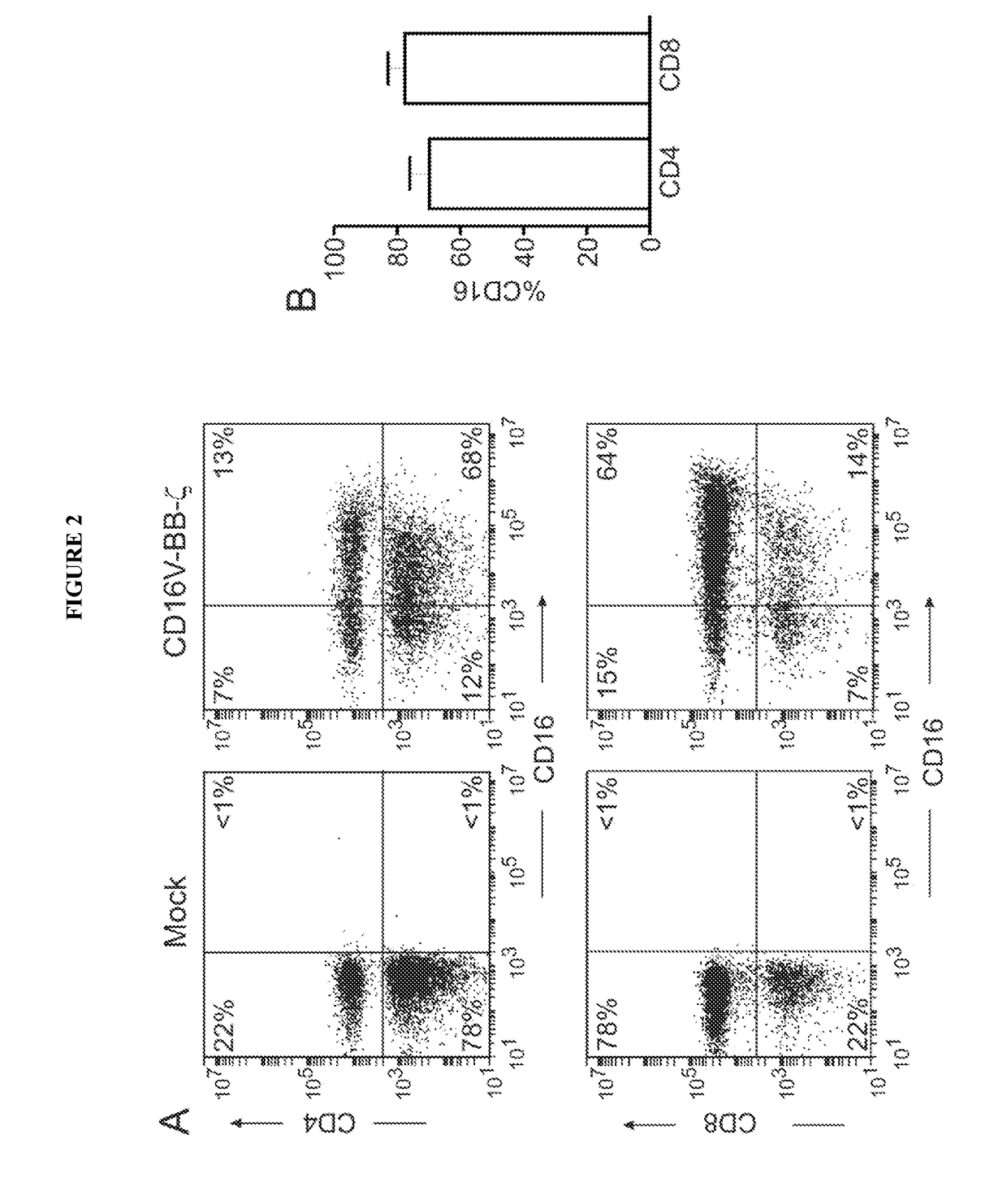
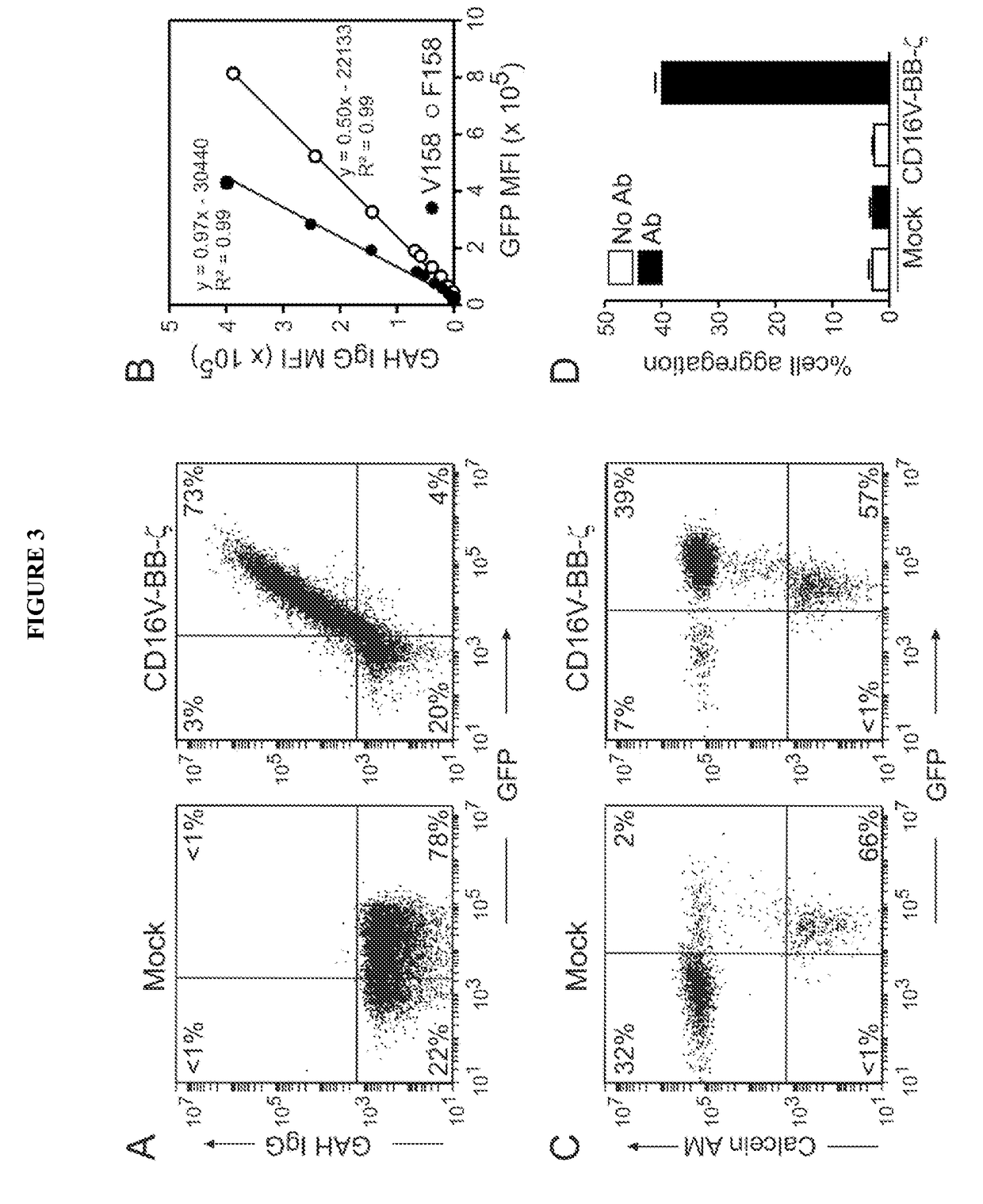
Disclosed herein are chimeric receptors comprising an extracellular domain with affinity and specific for the Fc portion of an immunoglobulin molecule (Ig) (e.g., an extracellular ligand-binding domain of F158 FCGR3A or V158 FCGR3A variant); a transmembrane domain (e.g., a transmembrane domain of CD8α); at least one co-stimulatory signaling domain (e.g., a co-stimulatory signaling domain of 4-1BB); and a cytoplasmic signaling domain comprising an immunoreceptor tyrosine-based activation motif (ITAM) (e.g., a cytoplasmic signaling domain of CD3ζ). Also provided herein are nucleic acids encoding such chimeric receptors and immune cells expressing the chimeric receptors. Such immune cells can be used to enhance antibody-dependent cell-mediated cytotoxicity and / or to enhance antibody-based immunotherapy, such as cancer immunotherapy.
Owner:COGENT BIOSCIENCES INC +2
Chimeric receptors and uses thereof in immune therapy
InactiveUS20170281682A1Good curative effectEnhanced ADCC activityAntibody mimetics/scaffoldsMammal material medical ingredientsFc bindingCytotoxicity
Disclosed herein are chimeric receptors comprising an extracellular domain with affinity and specific for the Fc portion of an immunoglobulin molecule (Ig), an Fc-binding domain; a transmembrane domain; at least one co-stimulatory signaling domain; and a cytoplasmic signaling domain comprising an immunoreceptor tyrosine-based activation motif (ITAM). Also provided herein are nucleic acids encoding such chimeric receptors and immune cells expressing the chimeric receptors. Such immune cells can be used to enhance antibody-dependent cell-mediated cytotoxicity and / or to enhance antibody-based immunotherapy, such as cancer immunotherapy.
Owner:UNUM THERAPEUTICS INC
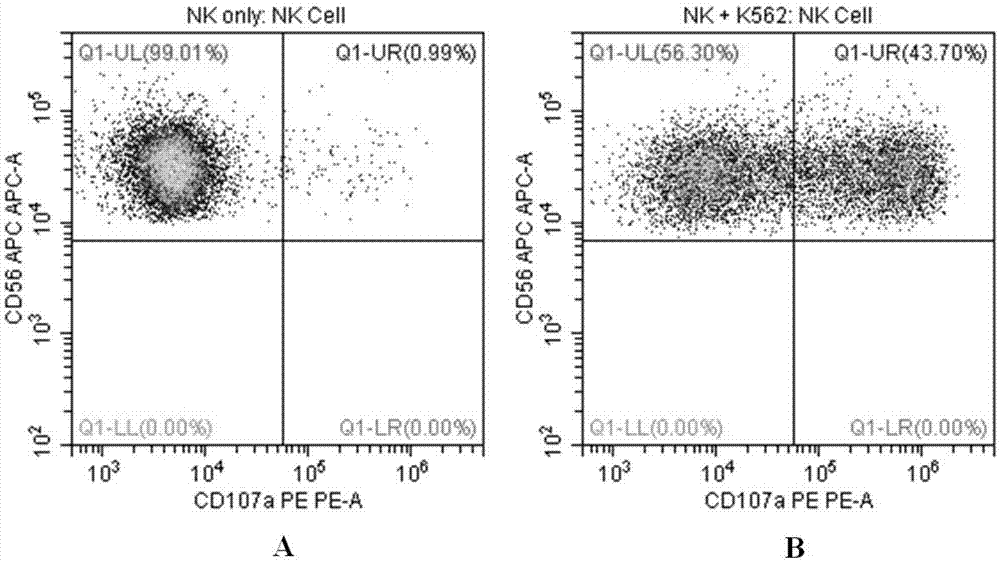
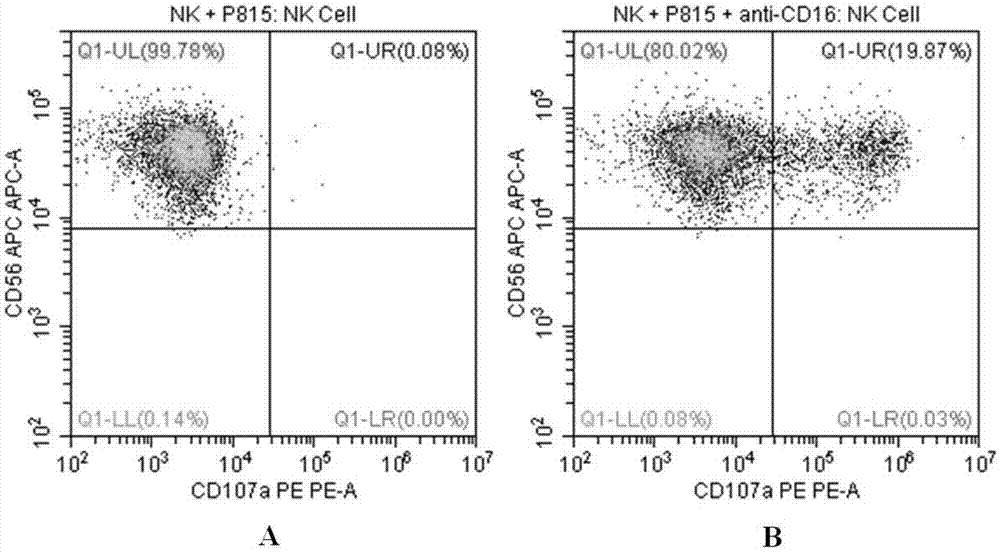
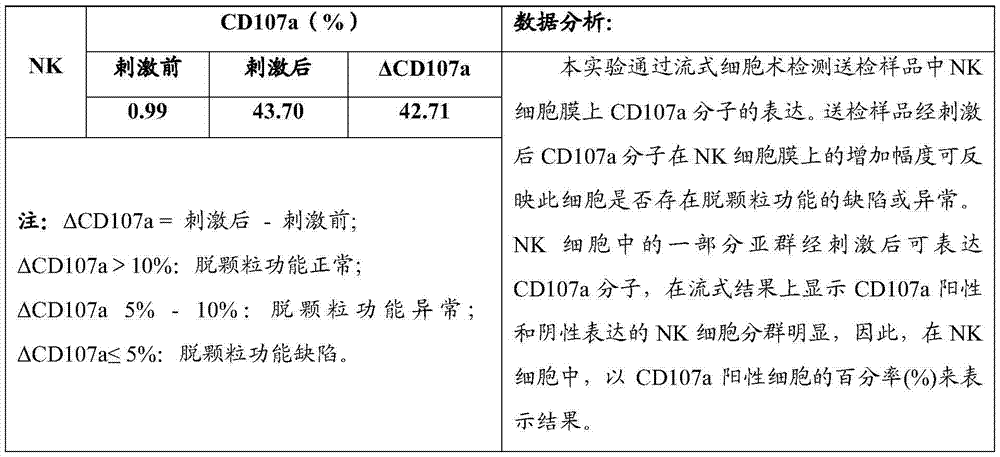
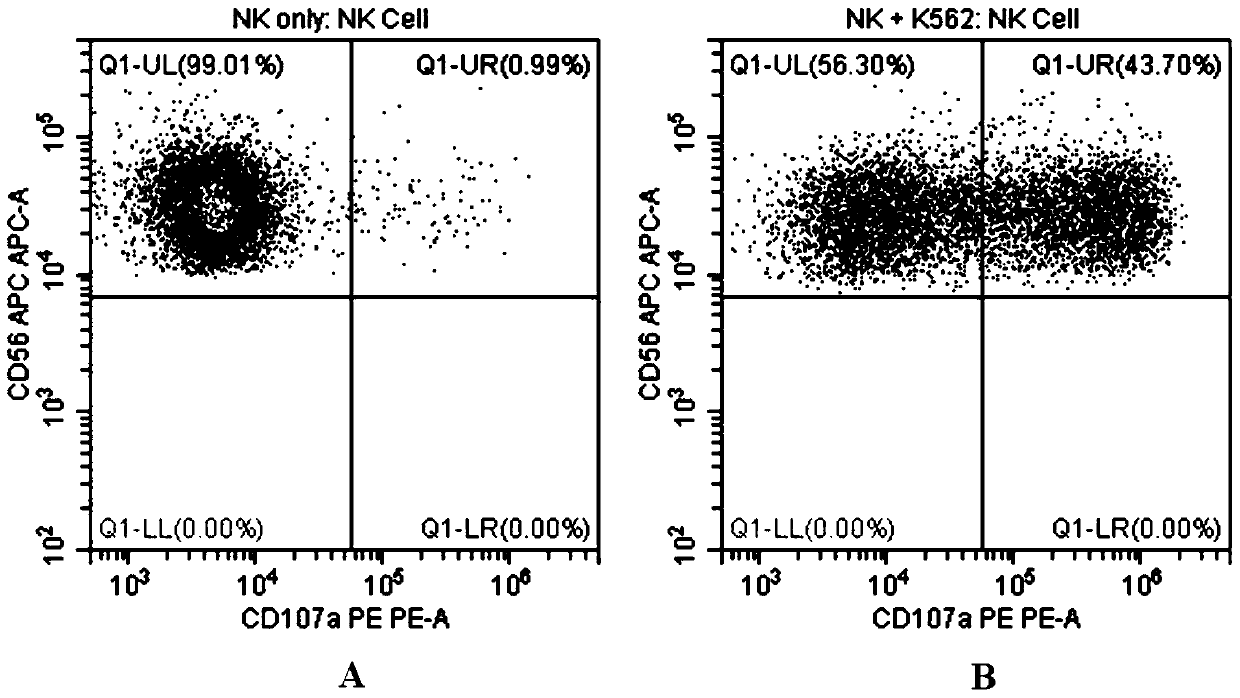
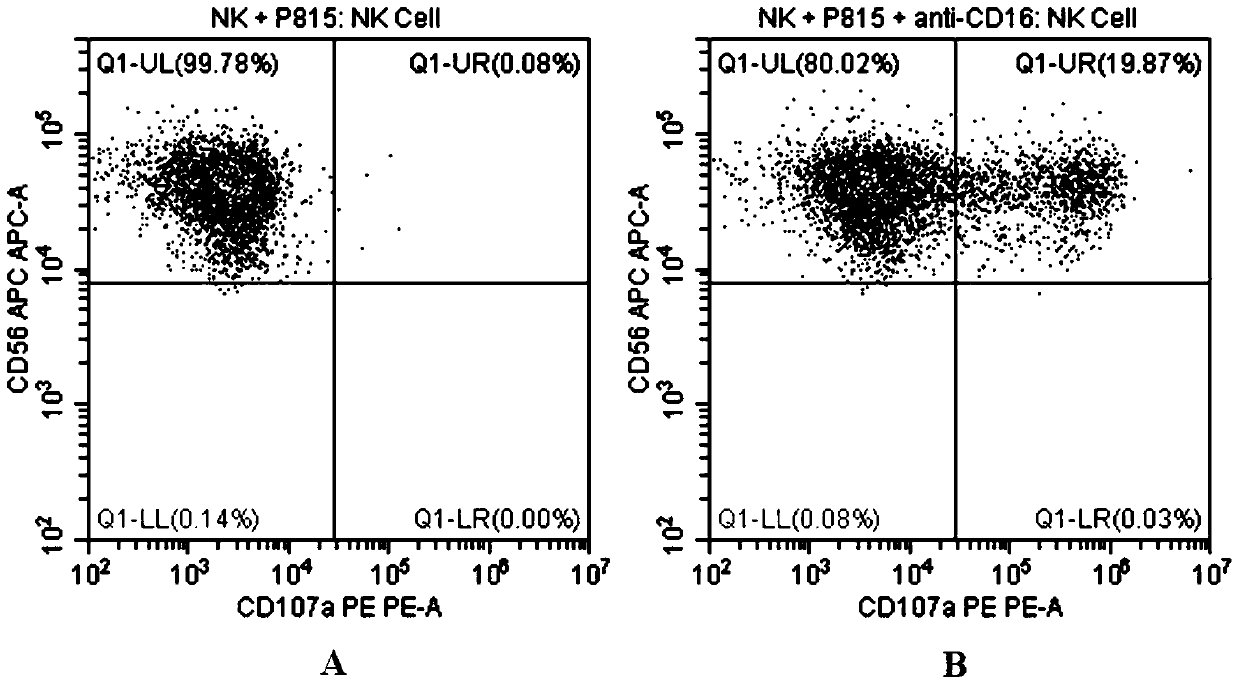
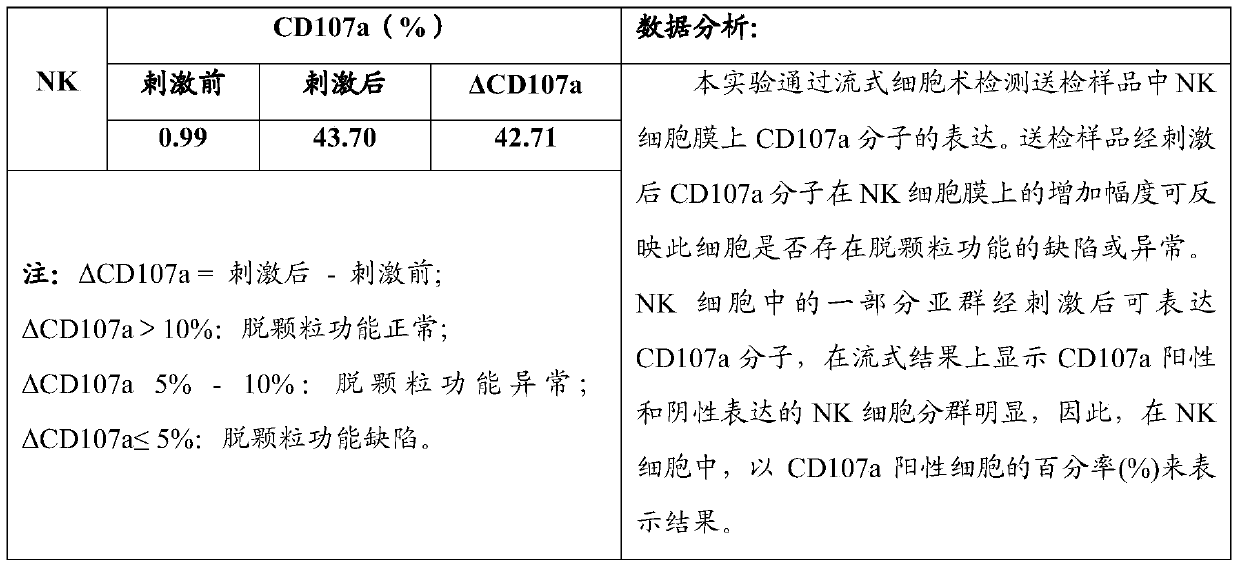
Flow cytometry detection method of natural killer cell degranulation
ActiveCN105445171AQuick checkImprove throughputIndividual particle analysisCell-mediated cytotoxicityStimulant
The invention relates to the field of biotechnology, in particular to a flow cytometry detection method of natural killer cell degranulation. The method comprises the following steps of stimulating natural killer cell degranulation through a natural killing effect or an antibody-dependent cell-mediated cytotoxicity effect; then using flow cytometry to detect the rangeability of the proportion of vesicle membrane protein marker CD107a positive cells in natural killer cells in the total natural killer cells before and after stimulating degranulation so as to evaluate the degranulation capacity. The flow cytometry detection method of natural killer cell degranulation provided by the invention is quick in detection, large in flux, high in accuracy, and smaller in man-made influences. The stable and regulated technique process is established through optimization limitation carried out on key technology details such as natural stimulant selection, effect target cell matching and incubating time.
Owner:倍科为(天津)生物技术有限公司
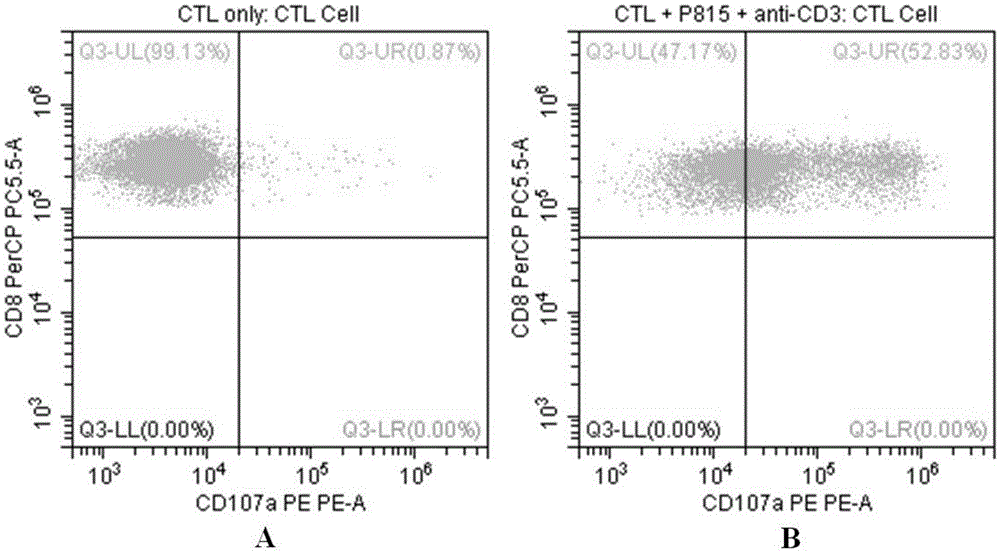
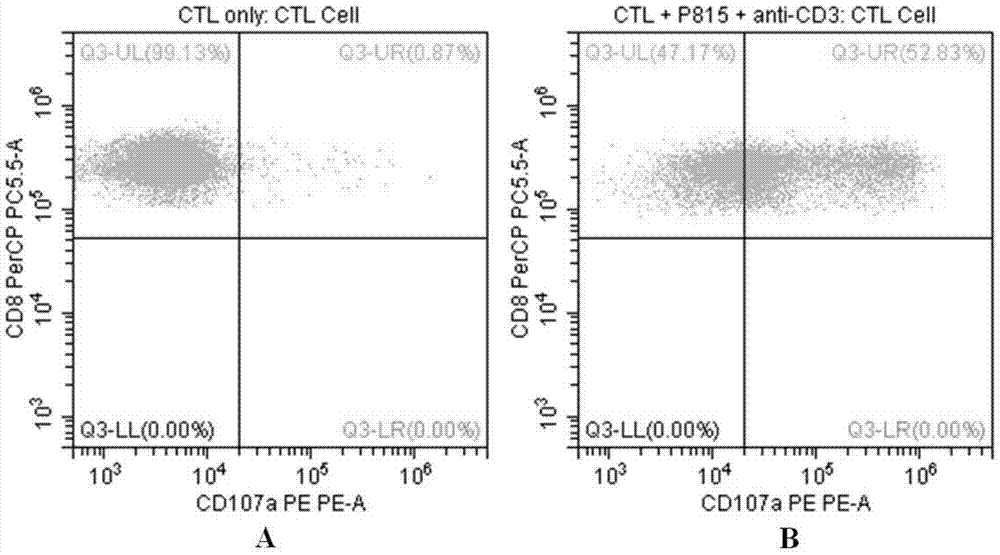
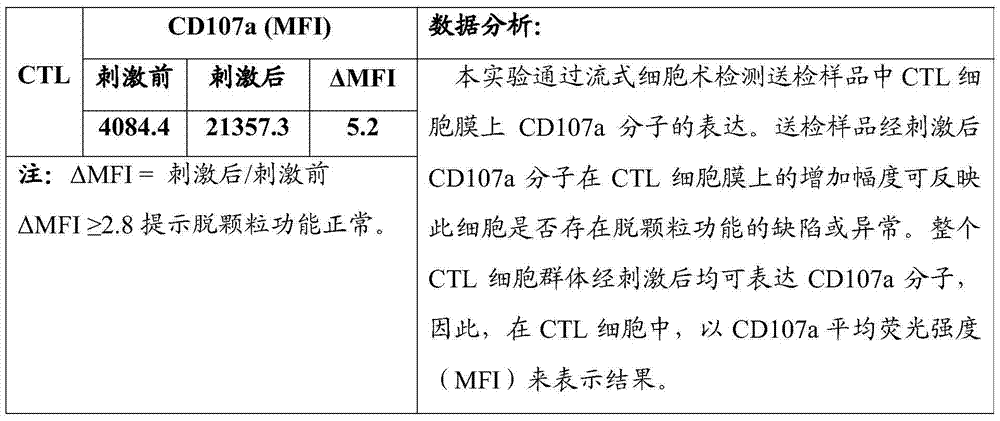
Flow-cytometry detecting method of cytotoxic-T-lymphocyte degranulation
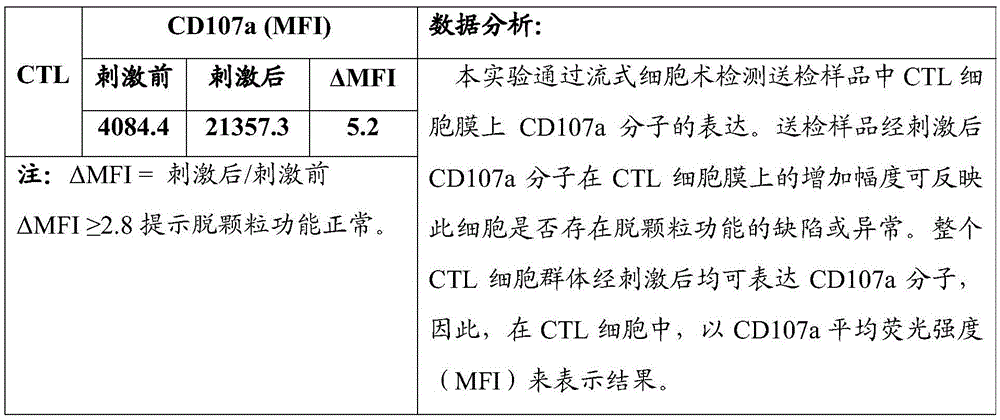
ActiveCN105547971AQuick checkImprove throughputIndividual particle analysisCell-mediated cytotoxicityMean fluorescence intensity
The invention relates to the technical field of biology, in particular to a flow-cytometry detecting method of cytotoxic-T-lymphocyte degranulation. The flow-cytometry detecting method includes the steps that cytotoxic-T-lymphocyte degranulation is excited through the antibody-dependent cell-mediated cytotoxic effect, the ratio of the average fluorescence intensity of vesicle-membrane-protein markers CD107a in cytotoxic T lymphocytes after exciting to the average fluorescence intensity before exciting is detected through flow cytometry to evaluate the degranulation function of the cytotoxic T lymphocytes, and if the ratio is larger than or equal to 2.8, the degranulation function is normal. The flow-cytometry detecting method of cytotoxic-T-lymphocyte degranulation is rapid in detection, large in flux, high in accuracy and small in human-factor influence. As key technology details such as natural irritant selecting, the effector target cell ratio and the incubating time are optimized and limited, the stable and normative technical process is built.
Owner:倍科为(天津)生物技术有限公司
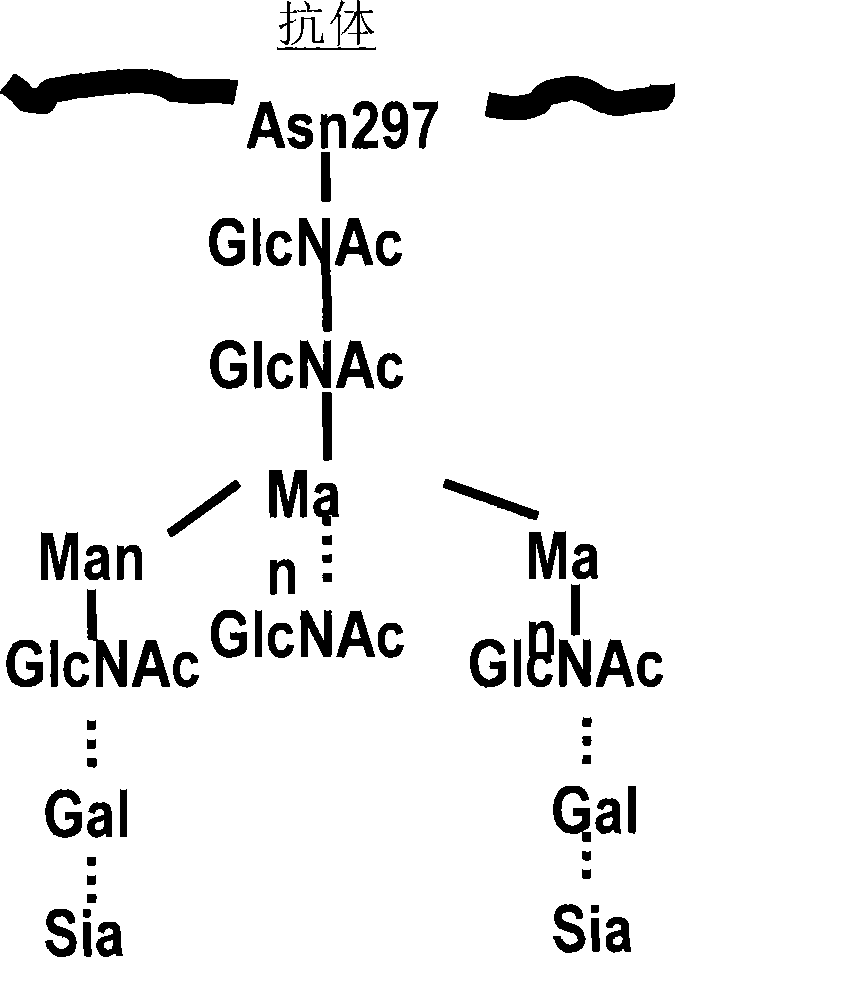
Glycosylation engineered antibody therapy
The instant invention is drawn to methods of generating a glycosylation- engineered antibody, and using the glycosylation-engineered antibody for treating a patient, particularly a cancer patient or a patient with an immune disease or disorder. The instant invention is also drawn to methods of generating a glycosylation-engineered antibody for use in the treatment of patients having a polymorphism that does not respond to conventional antibody therapy. The instant invention is also drawn to methods of improving the biological activity of an antibody by glycosylation engineering. The instant invention is also drawn to methods of modulating antibody-dependent cell-mediated cytoxicity (ADCC) using a glycosylation- engineered antibody.
Owner:UNIV OF MARYLAND BALTIMORE +1
CD38 protein antibody and application thereof
ActiveCN110144008AOrganic active ingredientsGenetic material ingredientsAntigenCell-mediated cytotoxicity
Owner:HANGZHOU SUMGEN BIOTECH CO LTD +1
Real-time monitor system with function of evaluating specific CTL immunostimulatory effect of cytokines on EBV-LMP2
InactiveCN111647558ADetermine the optimum concentrationDefining the impact of kill functionDiagnosticsMicrobiological testing/measurementCell-mediated cytotoxicitySpecific immunity
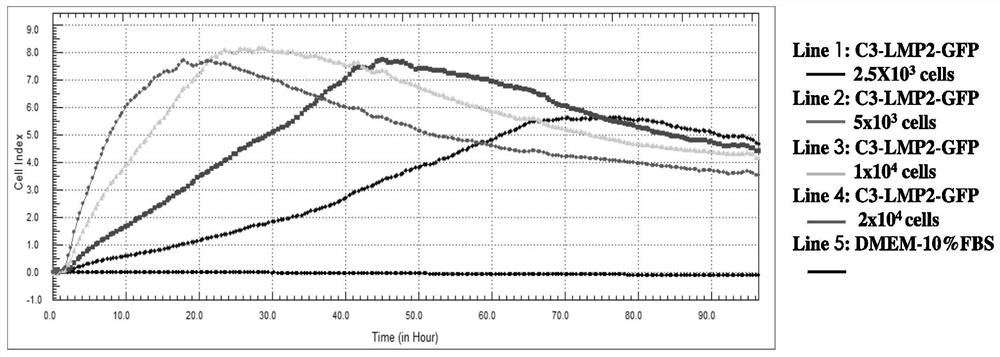
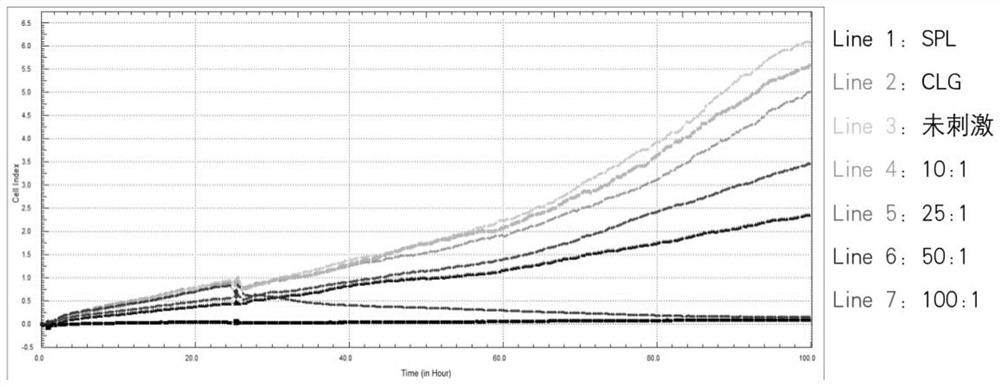
The invention provides a real-time monitor system with a function of evaluating the specific CTL immunostimulatory effect of cytokines on EBV-LMP2 and belongs to the field of cell culture in vitro. The real-time monitor system can be used for continuously and dynamically monitoring the immunological enhancement effect of IL-2 and IL-21 on T cell mediated cytotoxicity in real-time. The method detection results indicate that the highest killing efficiency enhancing effect on the EBV-LMP2 specific CD8+T cell is achieved when the IL-2 concentration is 60U / mL and the IL-21 concentration is 10ng / mL;and a remarkable immunological enhancing effect can be obtained by individually adding IL-21 with the final concentration of 10ng / mL. Results indicate that RTCA can be used for monitoring the immunological enhancing effect of the IL-2 and IL-21 on T cell functions in real time.
Owner:BEIJING UNIV OF TECH
De-fucosylated fully humanized monoclonal antibody and applications thereof
InactiveCN106478814AStrong cytotoxicityImmunoglobulins against virusesAntiviralsFucosylationGlycosyltransferase activity
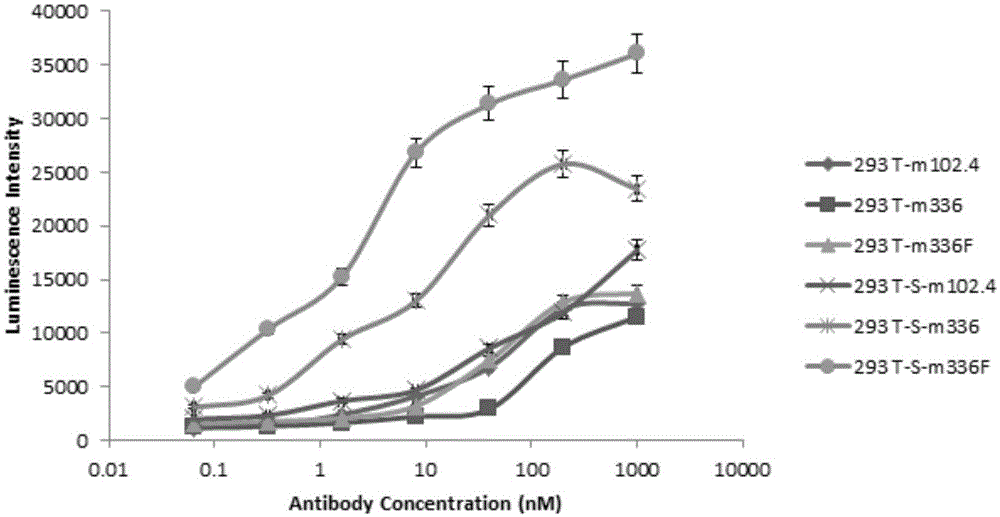
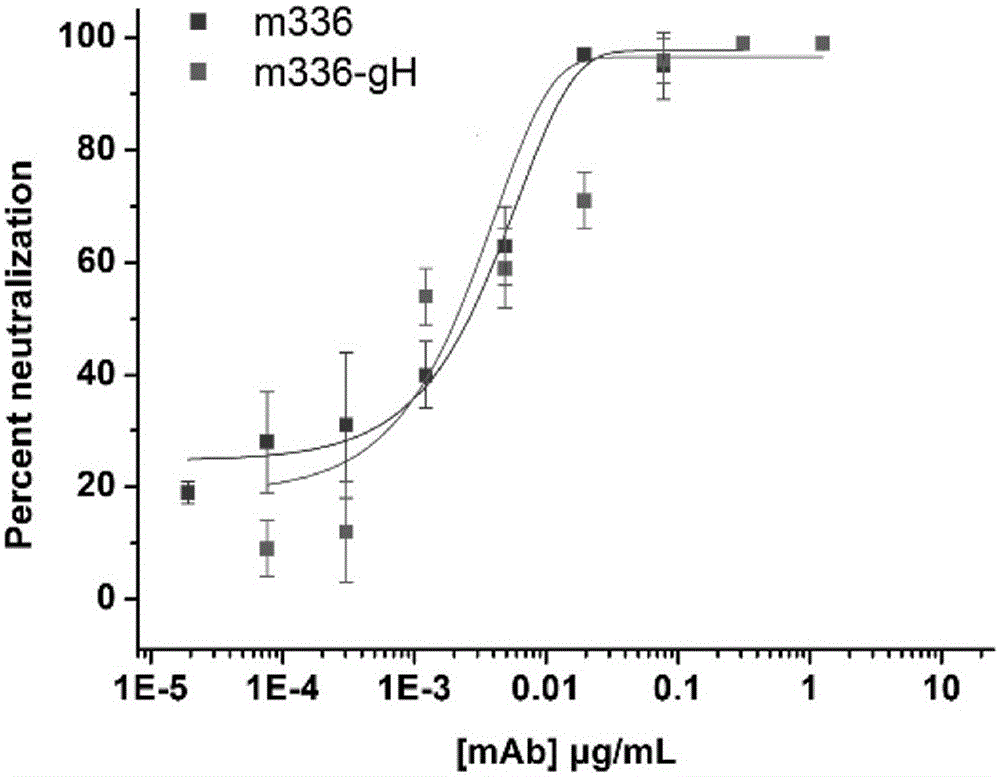
The present invention belongs to the technical field of biology, and relates to a de-fucosylated fully humanized monoclonal antibody for Middle East Respiratory Syndrome Coronavirus (MERS-CoV), an antigen binding fragment, and applications thereof, wherein the de-fucosylated fully humanized monoclonal antibody is mainly characterized in that the de-fucosylated fully humanized monoclonal antibody has an enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) effect, wherein the effect comprises cleaving the cells expressing MERS-CoV S protein. The present invention further relates to a method for making cells express the de-fucosylated MERS-CoV fully humanized monoclonal antibody by inhibiting fucosyltransferase activity. According to the present invention, by using the antibody, the Middle Eastern respiratory syndrome can be clinically prevented or treated.
Owner:FUDAN UNIV
Extractive of compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup and application thereof in preparing medicament for inhibiting H460 cell proliferation
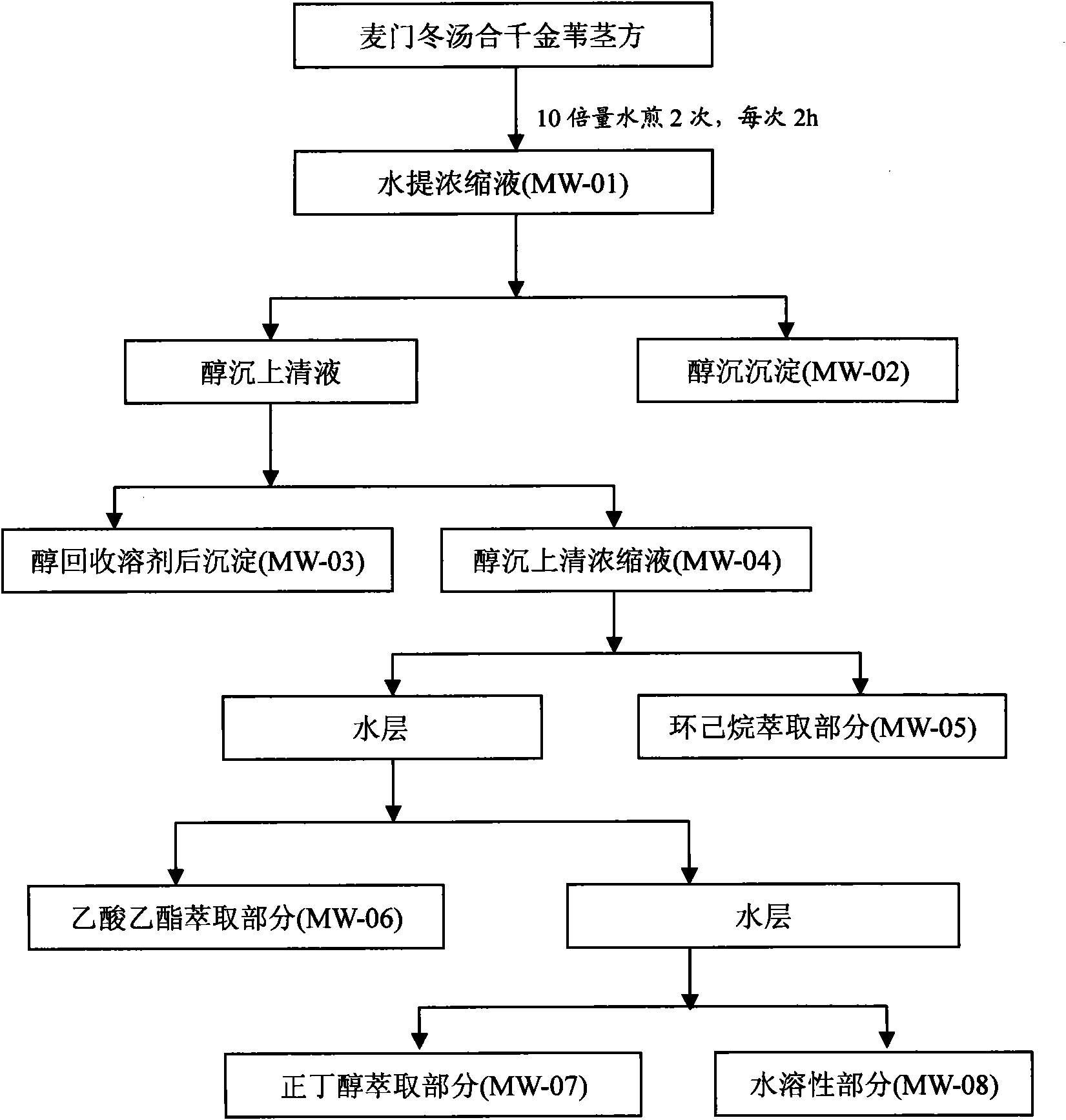
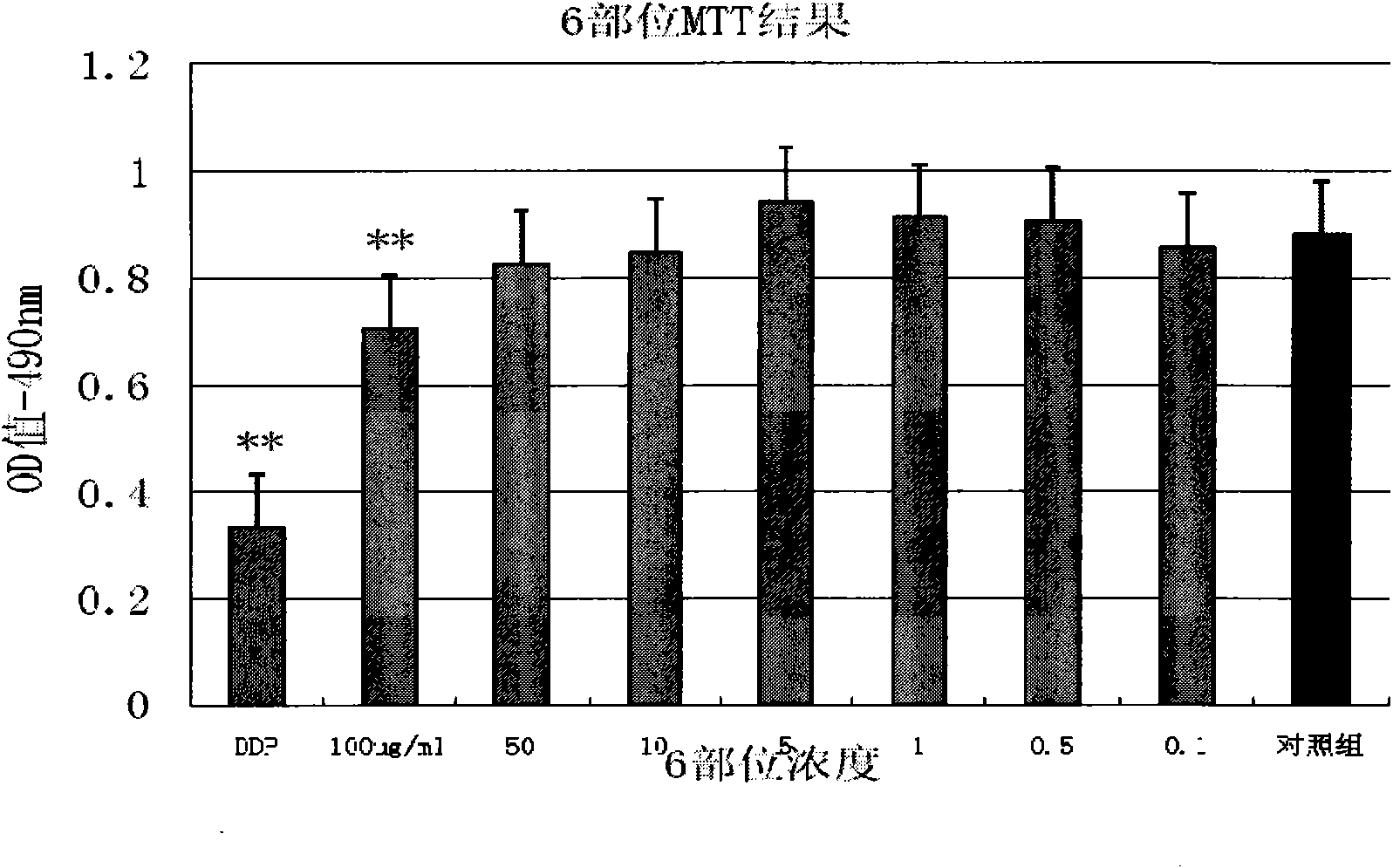
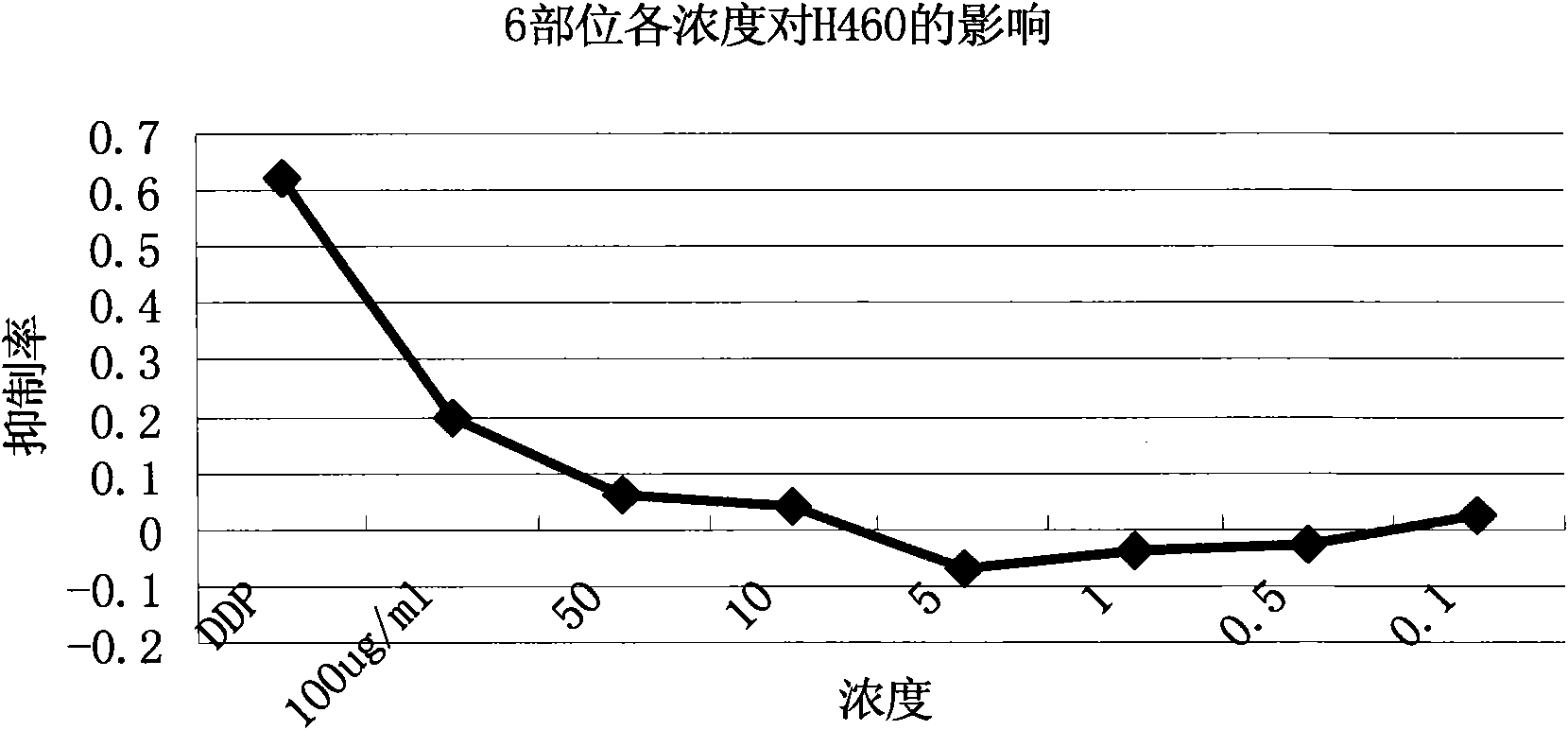
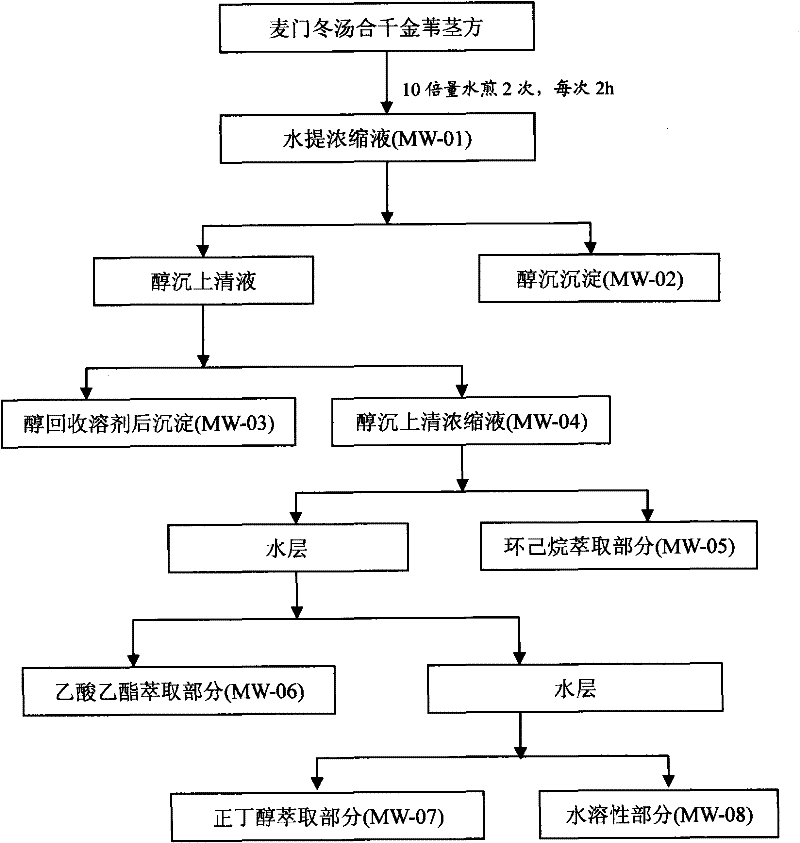
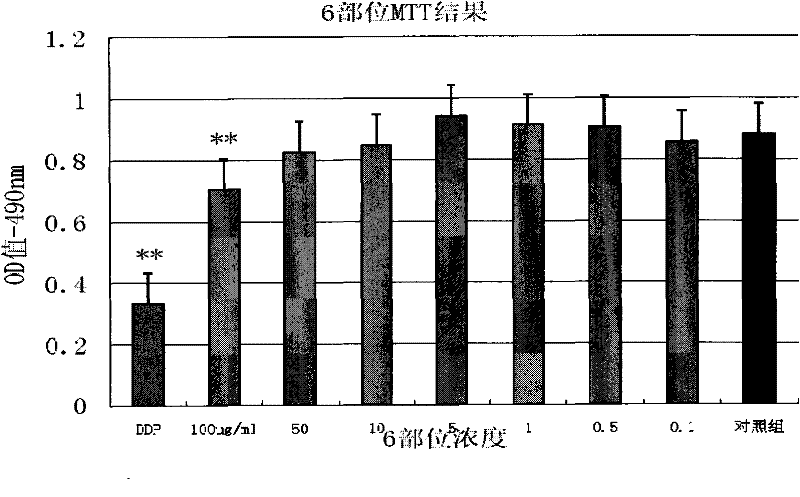
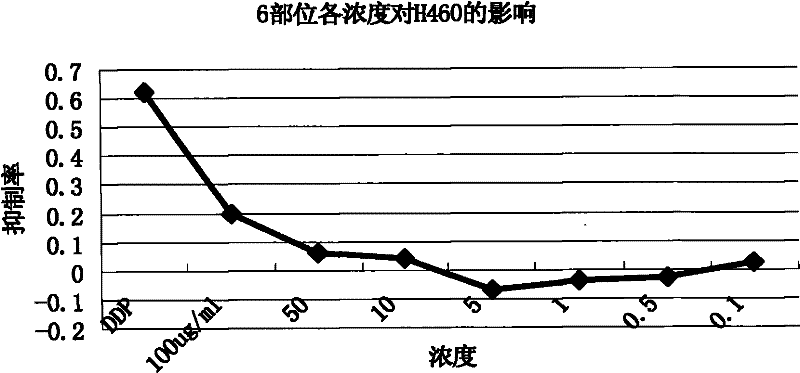
InactiveCN101647970AGrowth inhibitionCytotoxicAntineoplastic agentsPlant ingredientsAdemetionineEthyl acetate
The invention discloses an extractive of a compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup. The extractive is thick paste which is obtained by treating a compoundformula of ophiopogon decoction and caper euphorbia seed and reed stem soup by a series of steps of water extraction, alcohol precipitation and extraction of cyclohexane and ethylacetate. The invention discloses a preparation method of the extractive and also discloses the application of the extractive in preparing a medicament for inhibiting H460 cell proliferation. The extractive of a compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup is characterized in that the extraction of the ethylacetate can obviously inhibit the growth of human lung giant cell carcinoma H460 and has no obvious influences on human normal cells, and the extraction has the effect of cell mediated cytotoxicity and can further separate and screen components and monomeric compounds forinhibiting the proliferation of the human lung giant cell carcinoma H460.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Anti-human CD38 humanized monoclonal antibody and application thereof
ActiveCN114075285AHighly expressed sequenceHigh expressionNervous disorderAntipyreticAntigenCell-mediated cytotoxicity
The invention provides a monoclonal antibody combined with a CD38 antigen and application of the monoclonal antibody. The humanized monoclonal antibody provided by the invention can specifically recognize a CD38 antigen, has relatively good affinity, and also has antibody-dependent cell-mediated cytotoxicity (ADCC) activity and complement-dependent cytotoxicity (CDC) activity, so that CD38 + cells are killed. The invention also provides application of the monoclonal antibody or the variant in prevention and / or treatment of tumors.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
Oncofetal antigen binding proteins and related compositions and methods
ActiveUS20190382503A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenProtein molecules
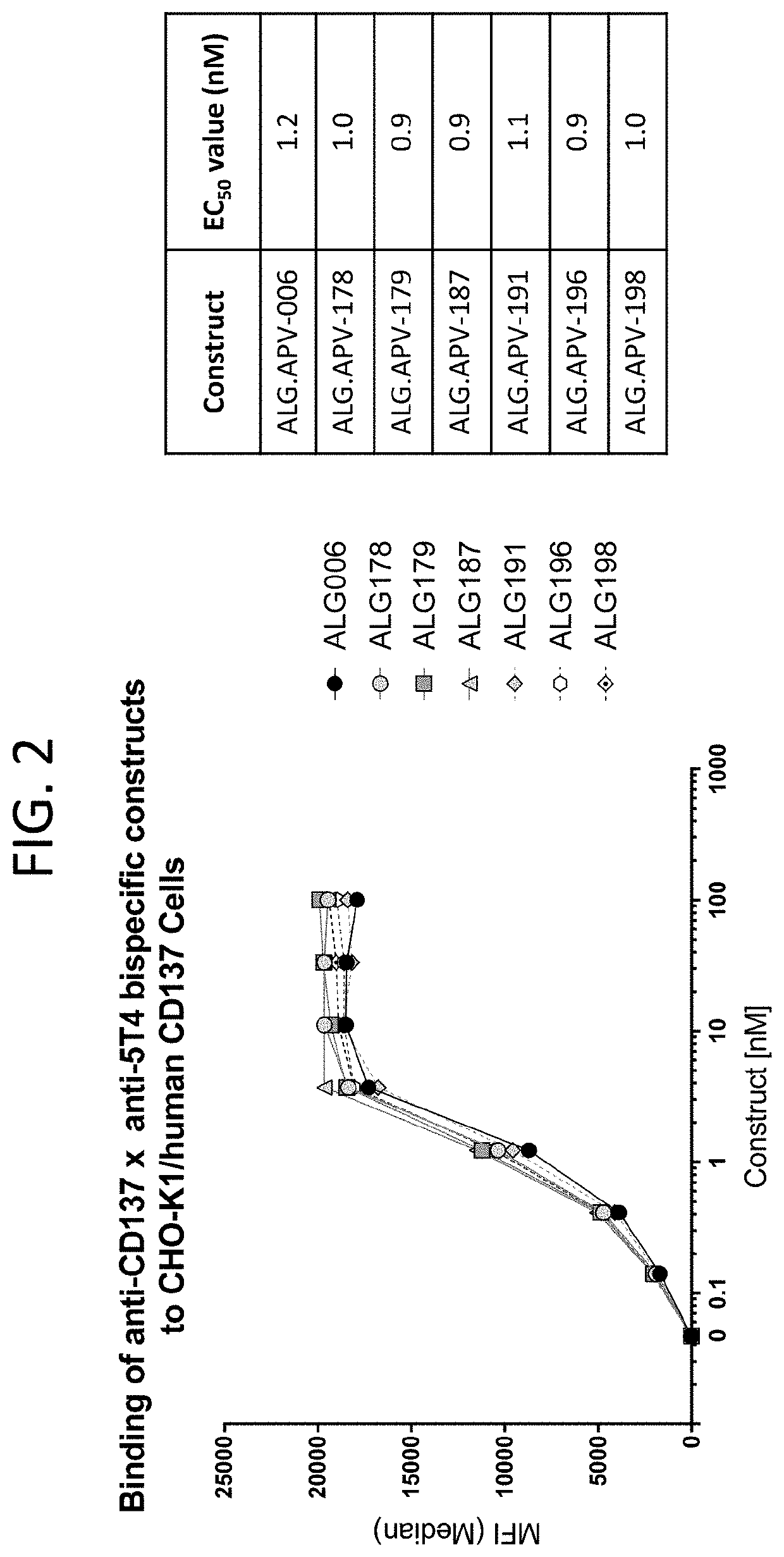
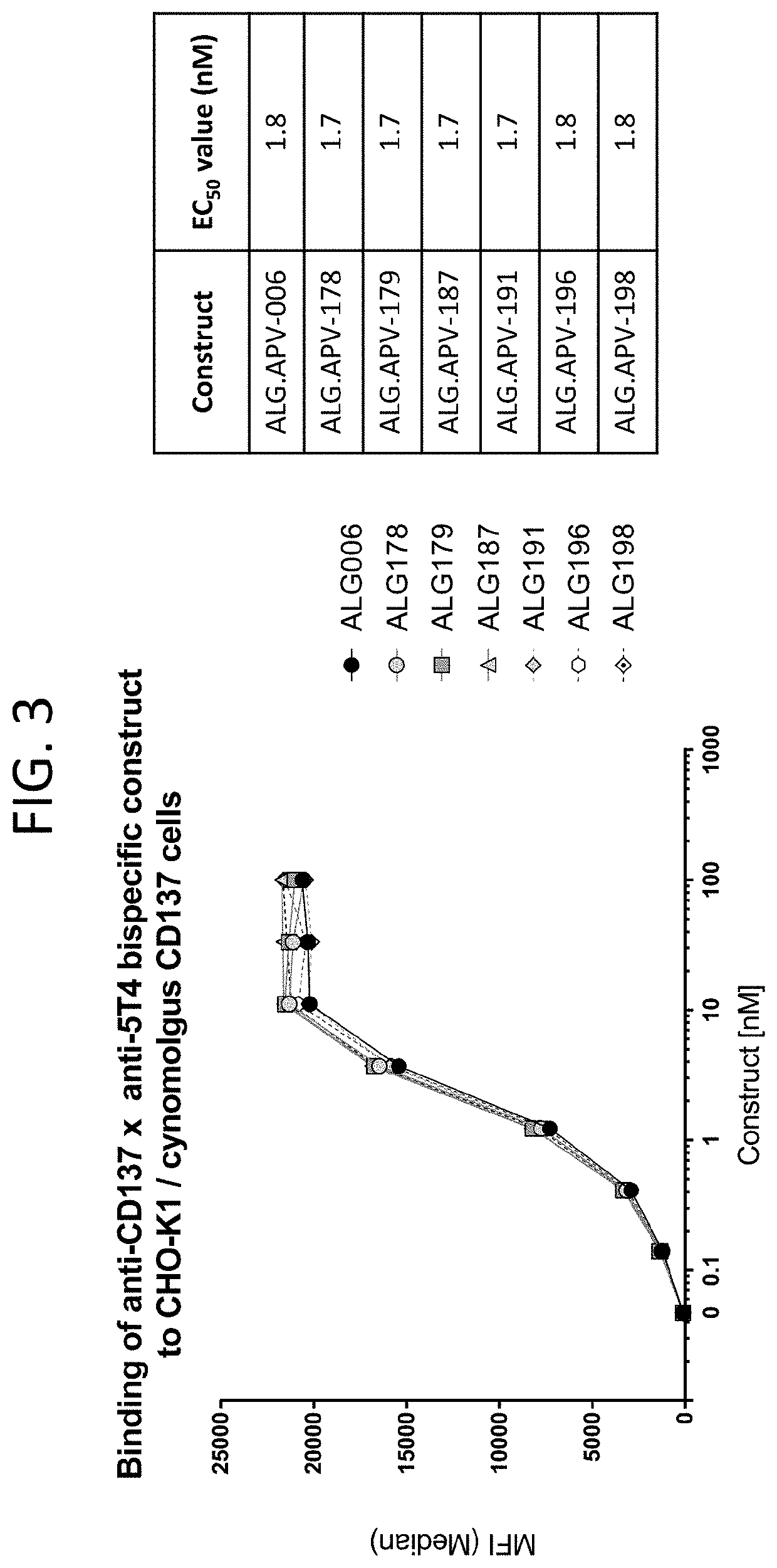
The present disclosure relates to protein molecules that specifically bind to 5T4 and / or 4-1BB. The molecules may have at least one humanized 5T4-binding or 4-1BB-binding domain. Such molecules are useful for the treatment of cancer. The protein molecule binding to 5T4 or 4-1BB may have a second binding domain that binds to another target. The molecules may bind both 5T4-expressing cells and a cell-surface molecule expressed by an effector cell to enhance effector cell activation, proliferation, survival and / or effector-cell mediated cytotoxicity. The disclosure also provides pharmaceutical compositions comprising the 5T4-binding or 4-1BB-binding polypeptide or protein molecules, nucleic acid molecules encoding these polypeptides and methods of making and using these molecules.
Owner:APTEVO RES & DEV LLC +1
Application of genkwanin in preparation of drug for resisting Listeria monocytogenes infection
InactiveCN110812353ANo drug resistanceHigh cure rateAntibacterial agentsOrganic active ingredientsGenkwaninErythroid cell
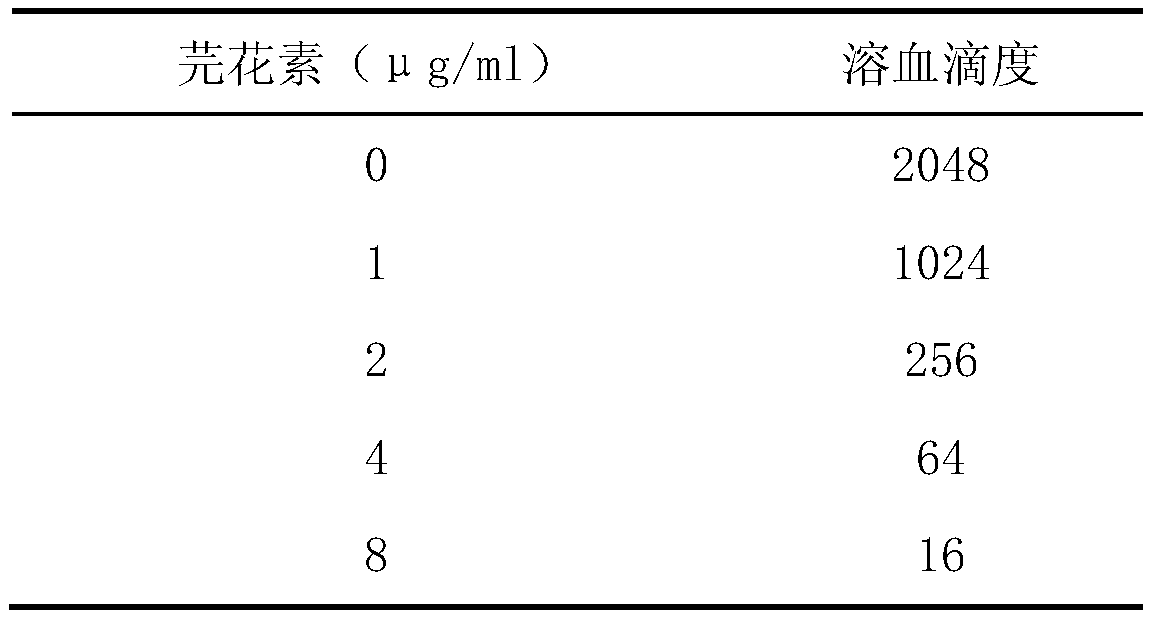
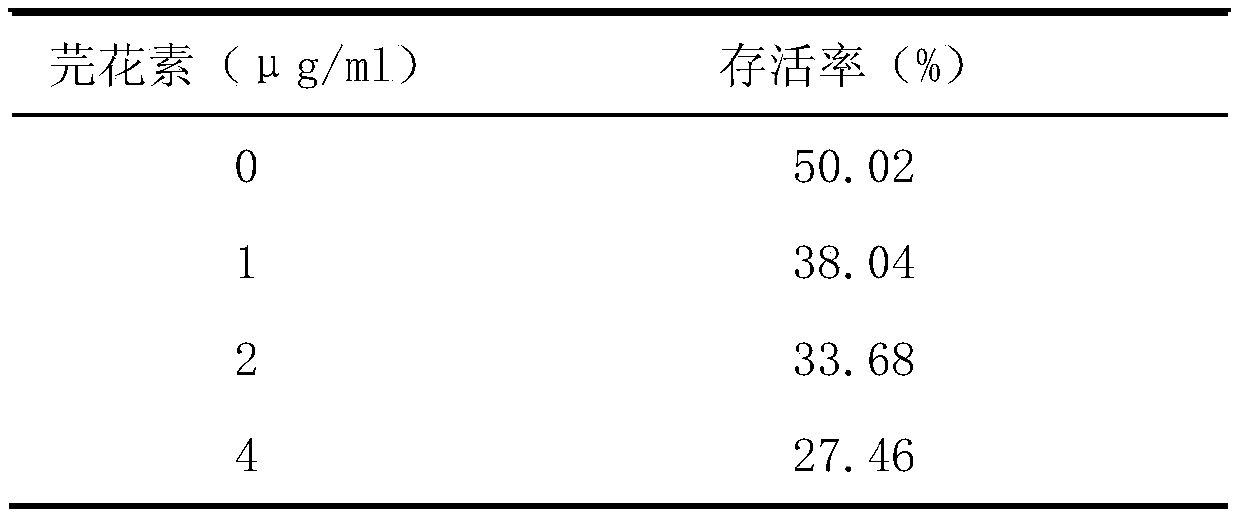
The invention provides an application of genkwanin in preparation of a drug for resisting Listeria monocytogenes infection. Rabbit erythrocyte hemolysis test, J774 cell protection effect test and mouse infection experiment therapeutics test prove that the genkwanin can effectively inhibit the pore-forming activity of Listeria monocytogenes hemolysin at an extremely low concentration, can inhibit the cytotoxic effect of Listeria monocytogenes on J774 cell mediation, and has a good treatment effect on mouse infection caused by the Listeria monocytogenes. According to the invention, Listeria monocytogenes infection is treated by the genkwanin. Compared with the traditional antibiotic treatment, the genkwanin is not easy to induce the generation of drug resistance, has lower selection pressureon bacteria, has no obvious toxic effect in the test, and has higher cure rate. Therefore, the genkwanin can be used for new drug development and has important significance for drug target confirmation.
Owner:JILIN UNIV
Markers selectively deregulated in tumor-infiltrating regulatory t cells
InactiveUS20190391152A1Easy to understandSafe modulationOrganic active ingredientsTumor rejection antigen precursorsRegulatory T cellCell-mediated cytotoxicity
The present invention discloses a number of markers selectively deregulated in tumor-infiltrating regulatory T cells. The invention relates to molecules able to modulate the expression and / or function of at least one such marker for use in the prevention and / or treatment of the tumor. Preferably the molecule specifically binds to the marker and induces antibody-dependent cell-mediated cytotoxicity (ADCC). The invention further relates to a molecule able to modulate the expression and / or function of at least one such marker for use in a method for in vivo depleting tumor-infiltrating regulatory T cell in a subject, or for use in a method to enhance tumor immunity in a subject. Corresponding pharmaceutical compositions are also contemplated.
Owner:CHECKMAB SRL
Flow Cytometry Assay for Cytotoxic T Cell Degranulation
ActiveCN105547971BQuick checkImprove throughputIndividual particle analysisCell-mediated cytotoxicityMean fluorescence intensity
The invention relates to the technical field of biology, in particular to a flow-cytometry detecting method of cytotoxic-T-lymphocyte degranulation. The flow-cytometry detecting method includes the steps that cytotoxic-T-lymphocyte degranulation is excited through the antibody-dependent cell-mediated cytotoxic effect, the ratio of the average fluorescence intensity of vesicle-membrane-protein markers CD107a in cytotoxic T lymphocytes after exciting to the average fluorescence intensity before exciting is detected through flow cytometry to evaluate the degranulation function of the cytotoxic T lymphocytes, and if the ratio is larger than or equal to 2.8, the degranulation function is normal. The flow-cytometry detecting method of cytotoxic-T-lymphocyte degranulation is rapid in detection, large in flux, high in accuracy and small in human-factor influence. As key technology details such as natural irritant selecting, the effector target cell ratio and the incubating time are optimized and limited, the stable and normative technical process is built.
Owner:倍科为(天津)生物技术有限公司
Flow Cytometry Assay for Natural Killer Cell Degranulation
ActiveCN105445171BQuick checkImprove throughputIndividual particle analysisCell-mediated cytotoxicityStimulant
The invention relates to the field of biotechnology, in particular to a flow cytometry detection method of natural killer cell degranulation. The method comprises the following steps of stimulating natural killer cell degranulation through a natural killing effect or an antibody-dependent cell-mediated cytotoxicity effect; then using flow cytometry to detect the rangeability of the proportion of vesicle membrane protein marker CD107a positive cells in natural killer cells in the total natural killer cells before and after stimulating degranulation so as to evaluate the degranulation capacity. The flow cytometry detection method of natural killer cell degranulation provided by the invention is quick in detection, large in flux, high in accuracy, and smaller in man-made influences. The stable and regulated technique process is established through optimization limitation carried out on key technology details such as natural stimulant selection, effect target cell matching and incubating time.
Owner:倍科为(天津)生物技术有限公司
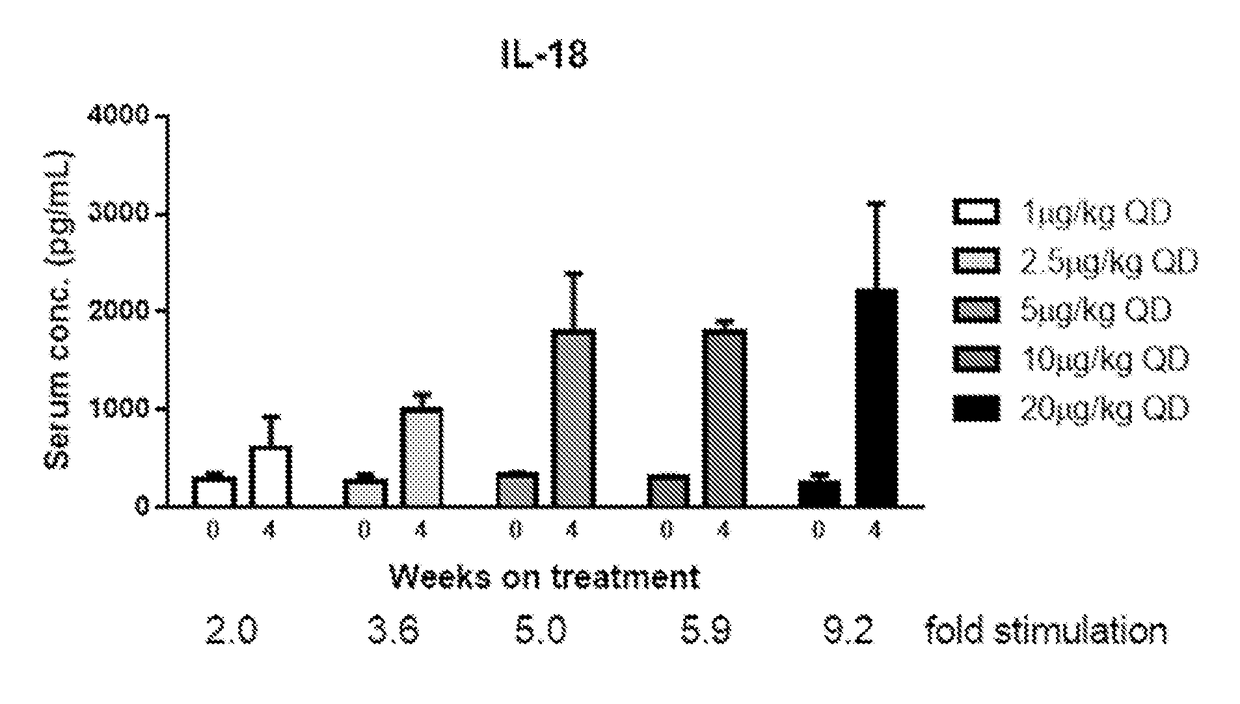
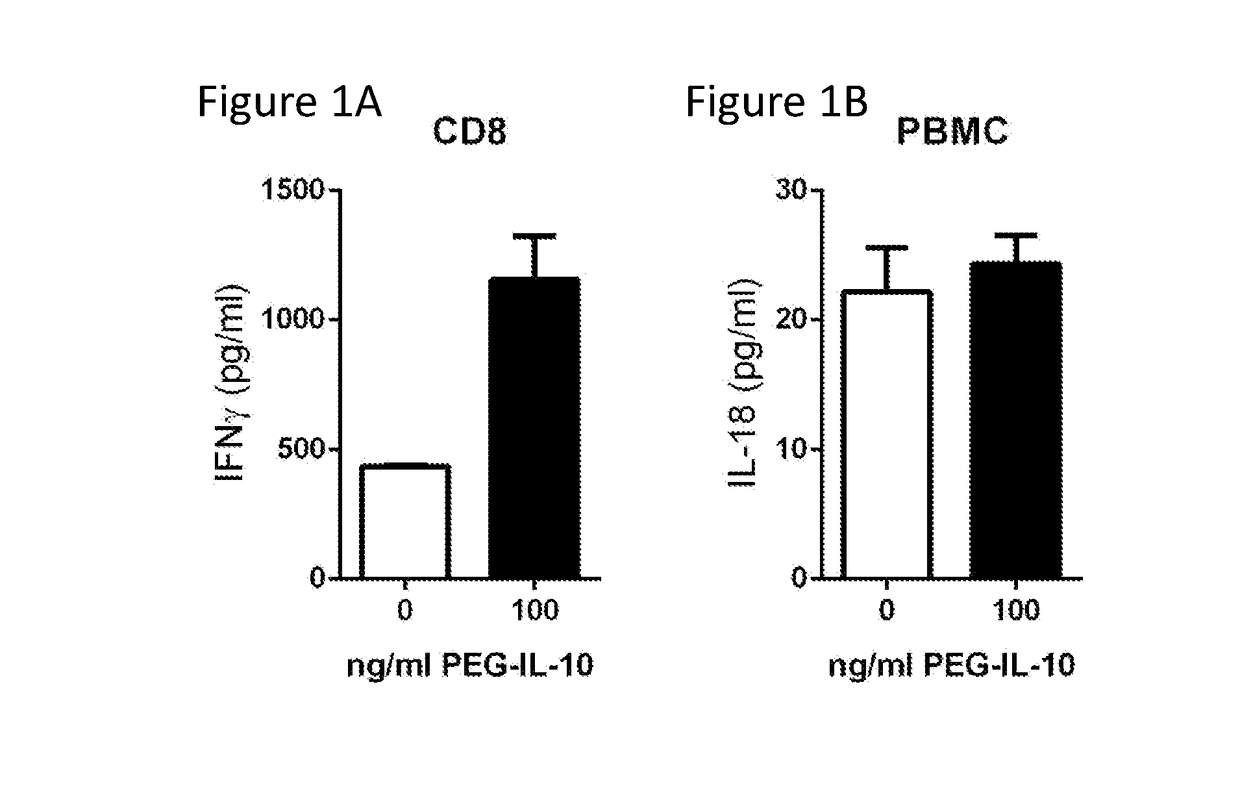
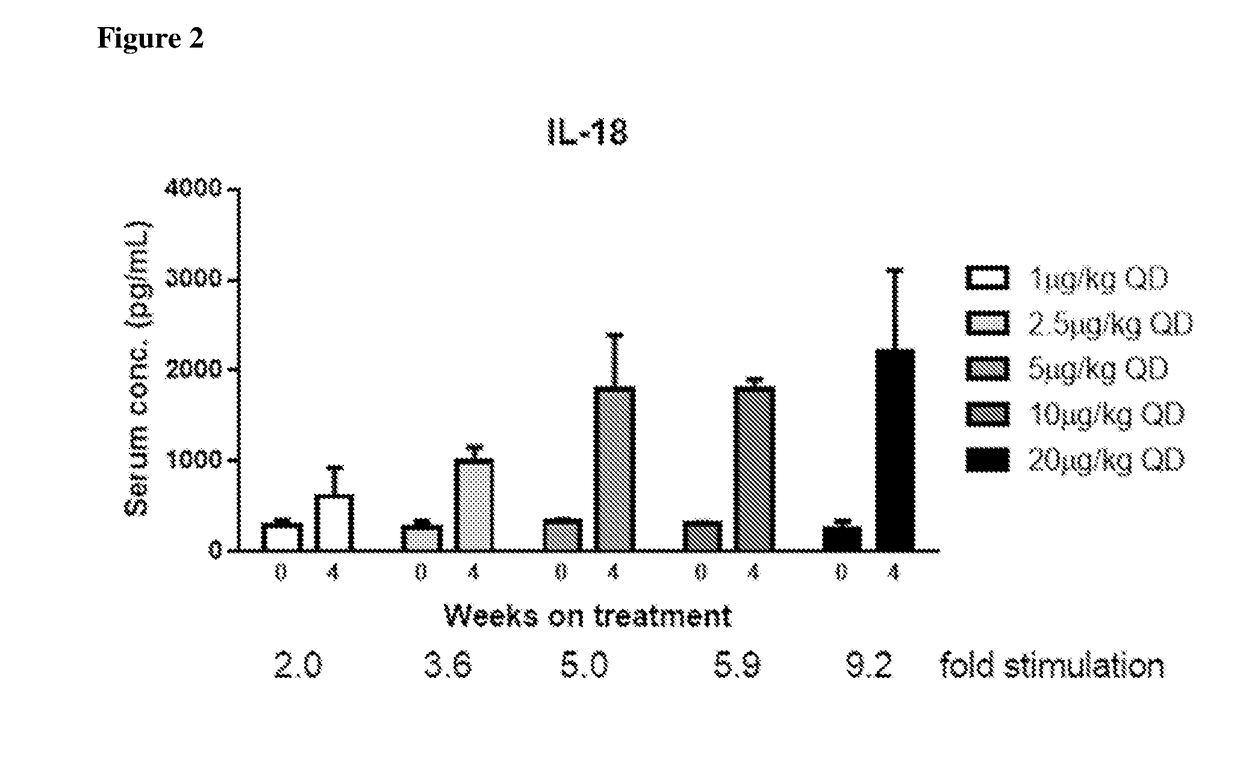
Methods of Using Interleukin-10 for Treating Diseases and Disorders
ActiveUS20180002434A1Reduce the amount requiredReduce frequencyAntibacterial agentsPeptide/protein ingredientsDiseaseCell-mediated cytotoxicity
Methods of treating subjects having a proliferative disease, disorder, or condition, including cancer, via the administration of an IL-10 agent, including pegylated IL-10, in combination with an antibody capable of inducing anti-body-dependent cell-mediated cytotoxicity, are provided.
Owner:ARMO BIOSCI
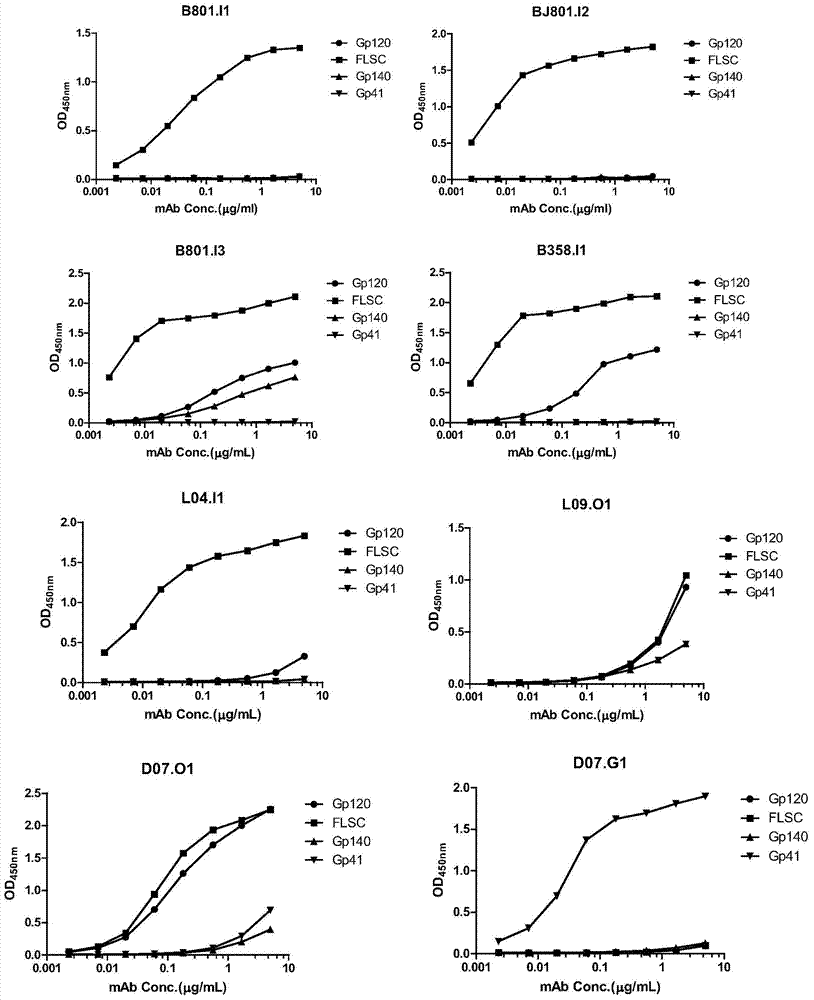
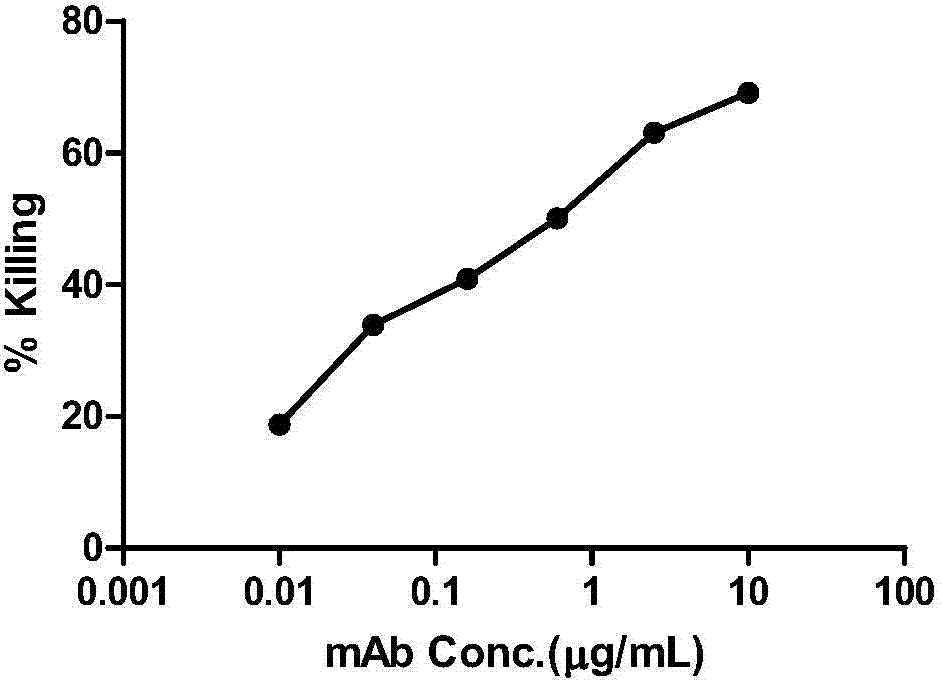
HIV-1 Env-specific fully human monoclonal antibody
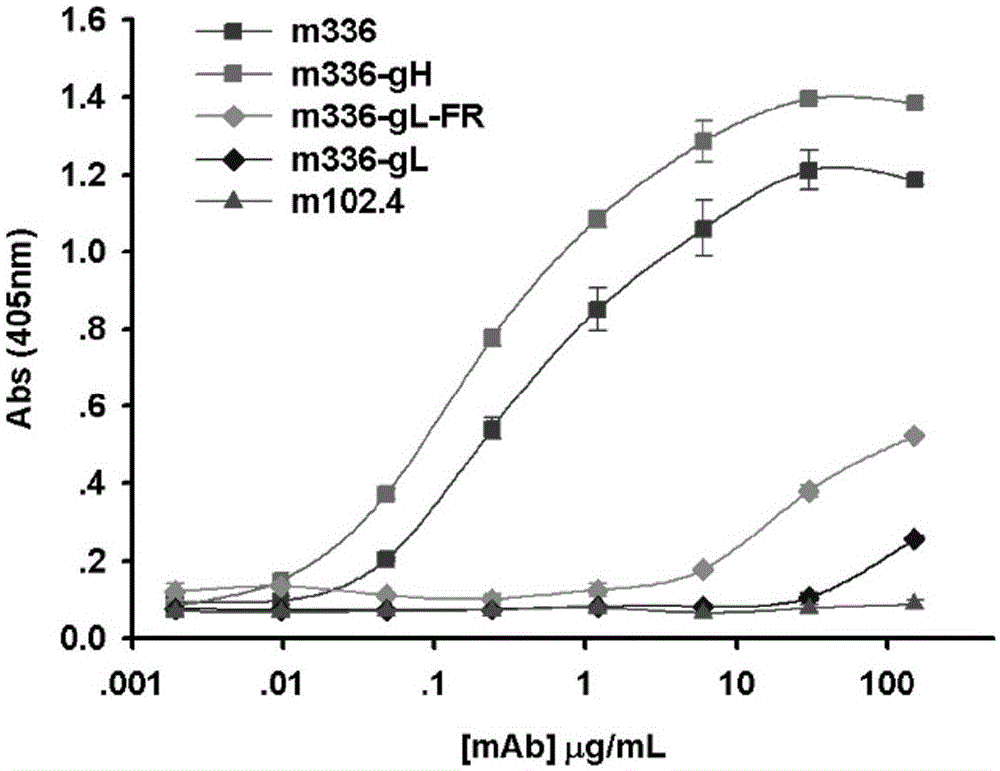
ActiveCN103755805BLow immunogenicityHigh affinityNervous disorderGenetic material ingredientsCell-mediated cytotoxicityGene engineering
The invention belongs to the technical field of gene engineering, and particularly relates to a human monoclonal antibody with an anti-HIV Env specificity. The antibody molecules have a neutralizing activity and antibody-dependent cell-mediated cytotoxicity (ADCC) on HIV-1. The antibodies can be applied singly or combinedly to prevent infection, and further can eliminate more viruses possibly in treatment; moreover, HIV-1 monoclonal antibodies can be further applied in diagnosis and treatment of HIV-1 infection.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Methods for the identification of polypeptide antigens associated with disorders involving aberrant cell proliferation and compositions useful for the treatment of such disorders
Methods and compositions for the development of effective cancer therapies using mitotic inhibitors which have limited general toxicity to normal, non-cancerous cells and tissues are provided. The methods and compositions utilize cytotoxic compounds comprised of a cell-binding agent (e.g., antibodies) conjugated to an anti-mitotic compound (e.g., maytansinoids). The invention further provides antibodies which are substantially incapable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC) and / or complement dependent cytotoxicity (CDC), thereby ensuring that the therapeutic effect is mediated primarily by the anti-mitotic component of the cytotoxic compound, rather than by indirect cell killing via ADCC and / or CDC. The antibodies of the invention further are capable of differentiating between polypeptide antigens which are more highly expressed on proliferating cancer cells as compared to proliferating non-cancer cells.
Owner:LEVINSON ARTHUR D
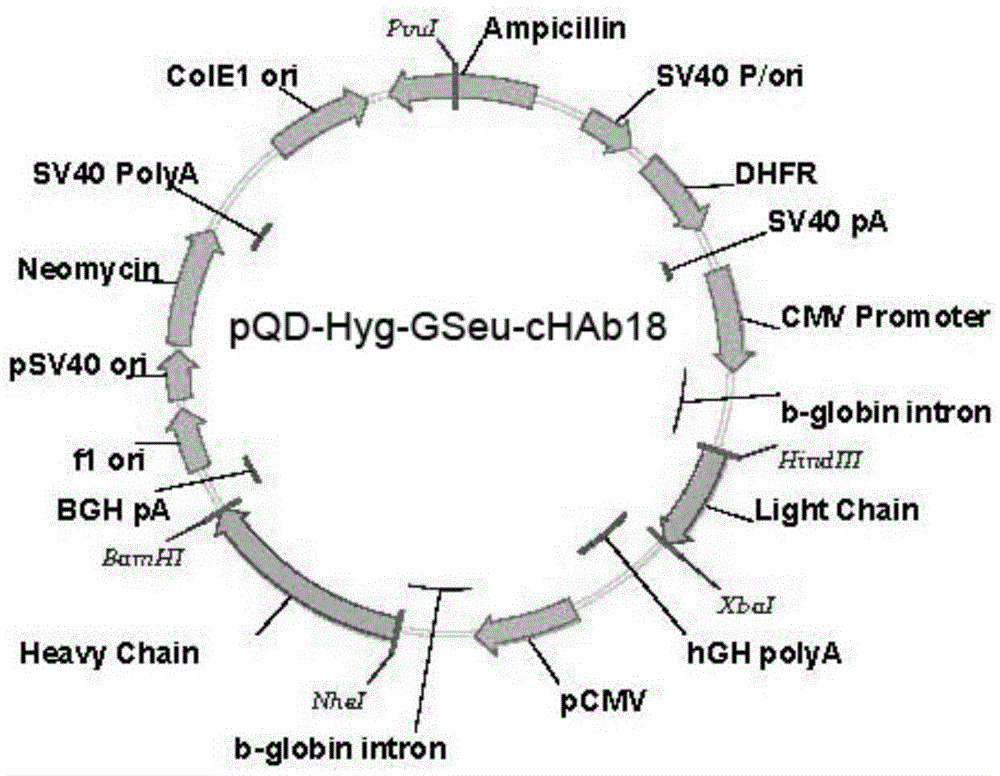
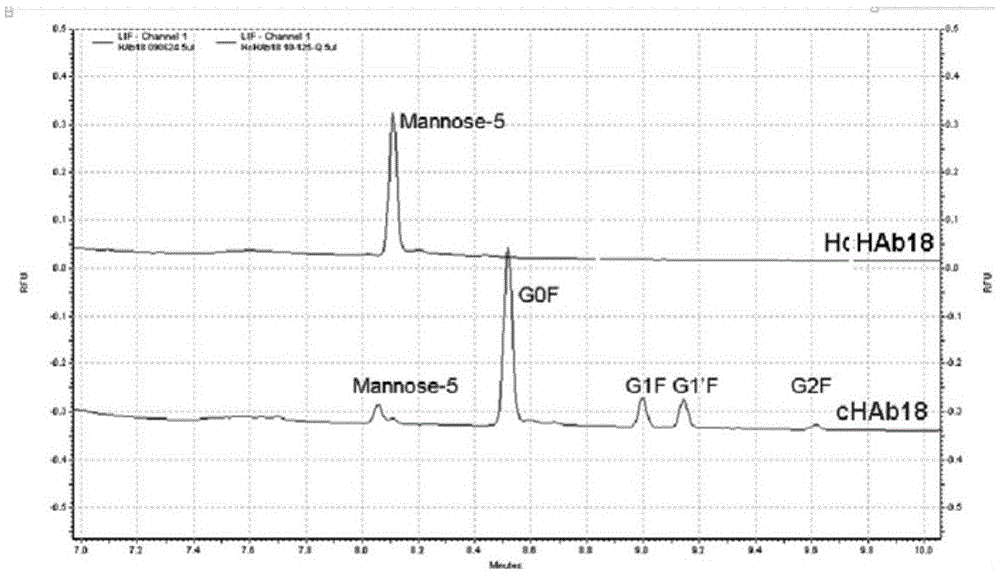
Humanized modified anti-CD147 chimeric antibody hchab18 and its application
ActiveCN104086654BAbility to inhibit invasion and migrationGrowth inhibitory effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFhit geneChimeric antibody
The invention provides a humanized modified anti-CD147 chimeric antibody HcHAb18 and its application. Specifically, on the basis of obtaining the hybridoma cell of anti-human CD147 monoclonal antibody and its antibody HAb18 gene, an anti-CD147 human-mouse chimeric antibody was prepared, and its light chain variable region has the expression shown in SEQ ID NO:1 The amino acid sequence of its heavy chain variable region has the amino acid sequence shown in SEQ ID NO: 2. In specific host cells, fucose is not bound to N-acetylglucosamine in the reducing end of the sugar chain of the Fc segment of the antibody, sugar The type is mannose (MAN5), and the antibody has antibody-mediated cell-dependent cytotoxicity (ADCC). The present invention also includes the expression vector of the anti-CD147 chimeric antibody, the engineered cell line and its application as a cancer treatment drug.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Production of secreted therapeutic antibodies in microalgae
InactiveUS20150112045A1Strong cytotoxicityUnicellular algaeMicrobiological testing/measurementVolvocalesNucleic acid sequence
Disclosed is a transformed microalga including a nucleic acid sequence operatively linked to a promoter, wherein the nucleic acid sequence encodes an amino acid sequence including (i) an heterologous signal peptide; and (ii) a therapeutic antibody, a functional fragment or a derivative thereof, the transformed microalga expressing the therapeutic antibody, functional fragment or derivative thereof secreted in the extracellular media and the microalga being selected among green algae except Volvocales, and among red algae, chromalveolates, and euglenids. Preferably, therapeutic antibody, functional fragment or derivative thereof has an increased antibody-dependant cell-mediated cytotoxicity (ADCC) and a low fucose content. The present invention also relates to a method for producing therapeutic antibody, a functional fragment or a derivative thereof, a functional fragment or a derivative thereof in the extracellular medium, to a therapeutic antibody, a functional fragment or a derivative thereof produced and secreted in the extracellular medium of microalgae.
Owner:ALGENICS
Extractive of compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup and application thereof in preparing medicament for inhibiting H460 cell proliferation
InactiveCN101647970BGrowth inhibitionCytotoxicAntineoplastic agentsPlant ingredientsCell-mediated cytotoxicityChemical compound
The invention discloses an extractive of a compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup. The extractive is thick paste which is obtained by treating a compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup by a series of steps of water extraction, alcohol precipitation and extraction of cyclohexane and ethylacetate. The invention discloses a preparation method of the extractive and also discloses the application of the extractive in preparing a medicament for inhibiting H460 cell proliferation. The extractive of a compound formula of ophiopogon decoction and caper euphorbia seed and reed stem soup is characterized in that the extraction of the ethylacetate can obviously inhibit the growth of human lung giant cell carcinoma H460 and has no obvious influences on human normal cells, and the extraction has the effect of cell mediated cytotoxicity and can further separate and screen components and monomeric compounds forinhibiting the proliferation of the human lung giant cell carcinoma H460.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
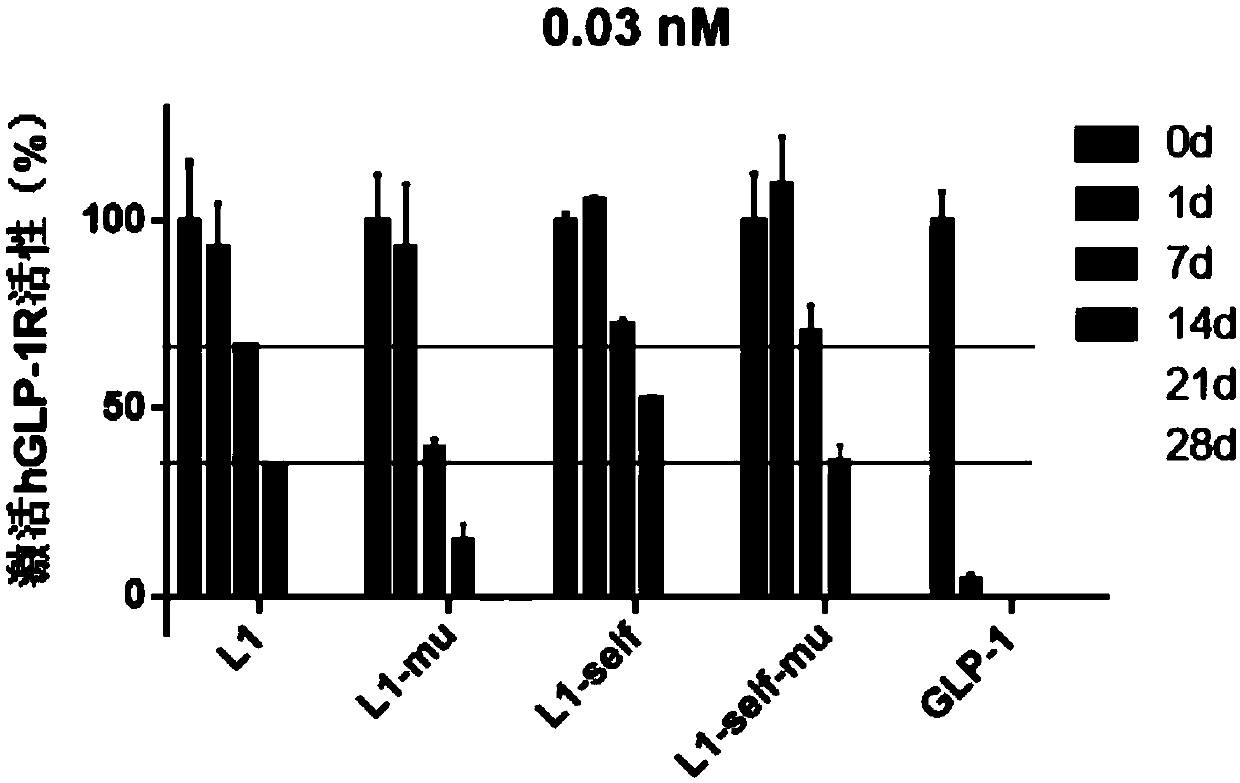
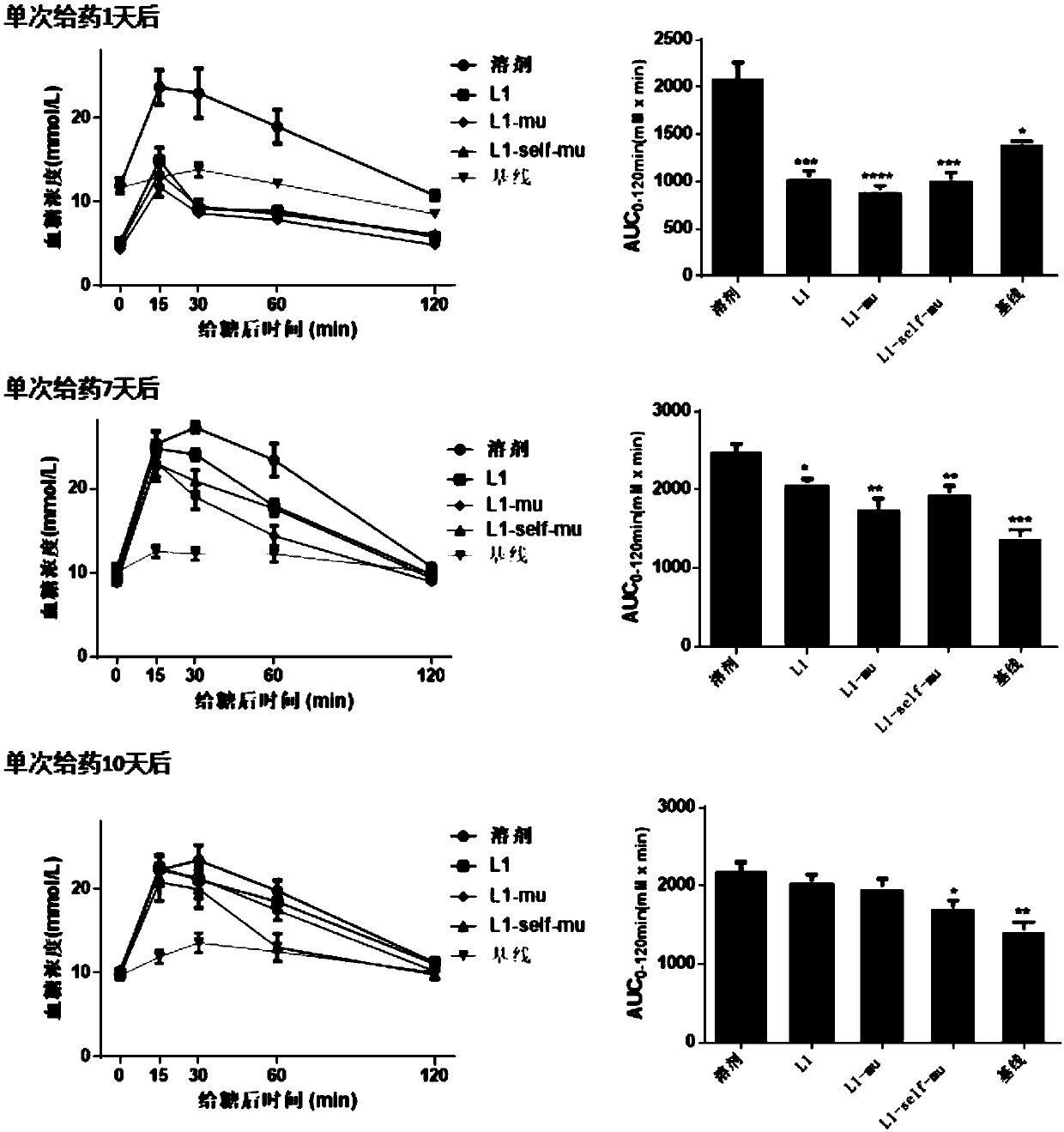
Long-acting recombinant GLP1-Fc-CD47 protein as well as preparation method and application thereof
ActiveCN110878127AImprove biological activityReduce formationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyDimerIn vivo
Owner:ZHEJIANG PALOALTO PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com