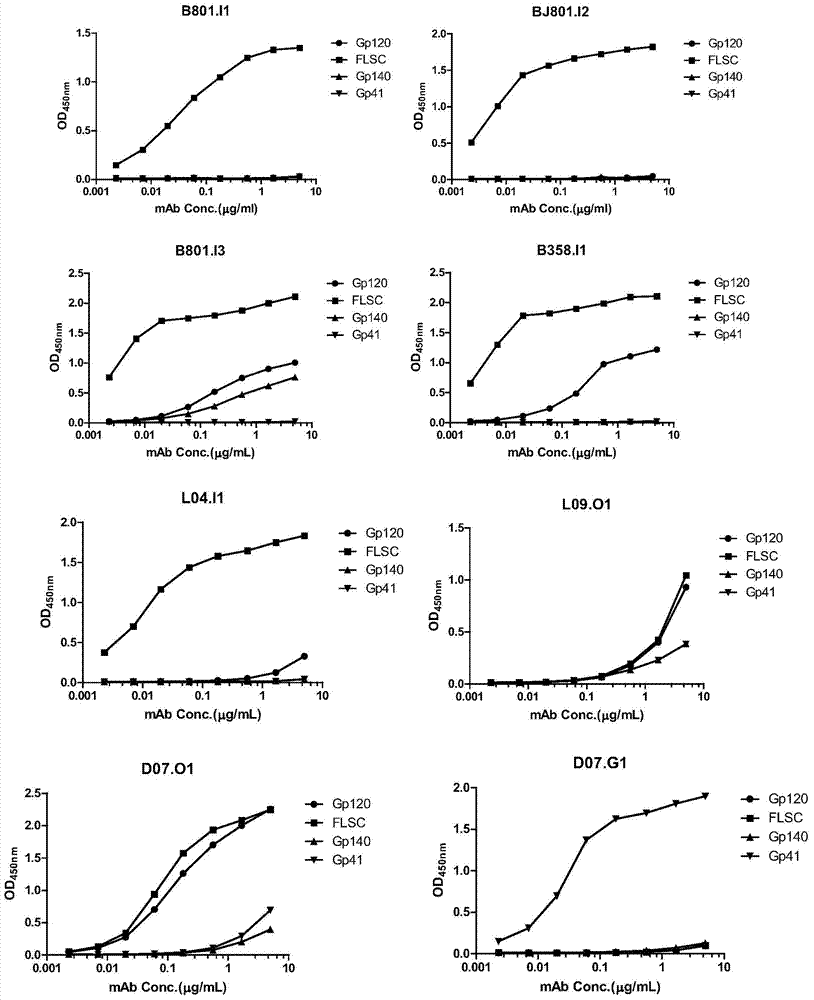
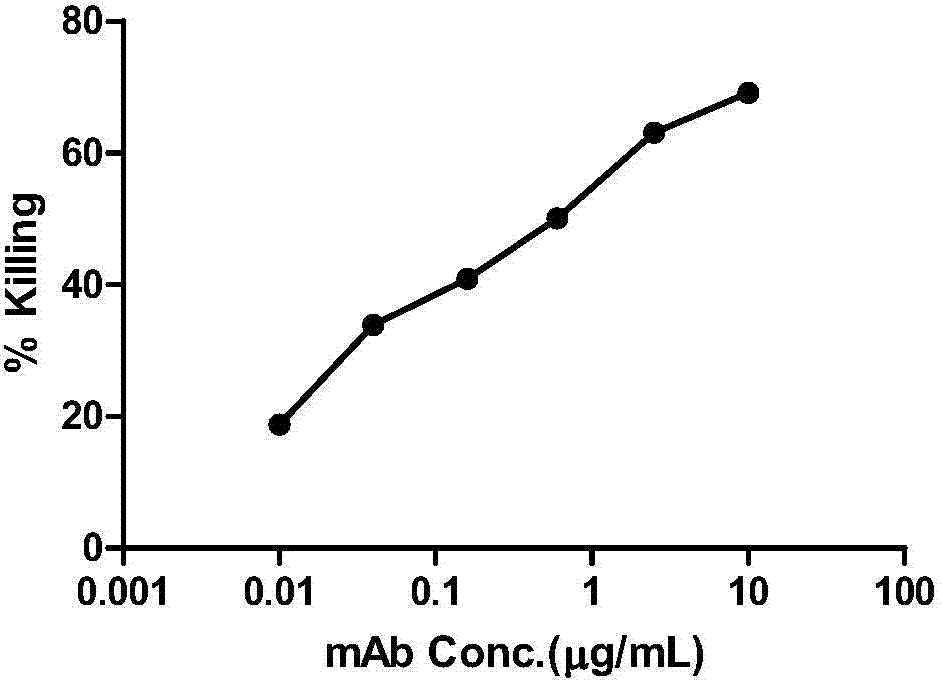
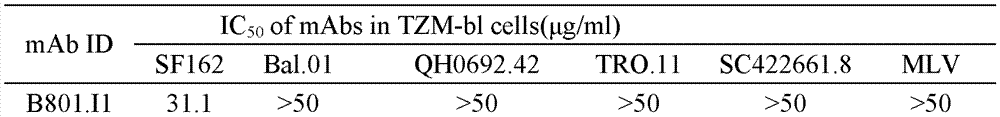HIV-1 Env-specific fully human monoclonal antibody
A specific, antibody technology, applied in the direction of antibodies, antiviral agents, antitumor drugs, etc., to achieve the effect of reducing immunogenicity, good application prospects, and improving antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] The whole human monoclonal antibody preparation and antibody characteristic analysis process of the present invention is divided into two parts: using B cell culture and RT-PCR method to obtain antibody gene and antibody expression; and antibody characteristic analysis.
[0012] 1 Antibody gene screening and antibody expression and purification
[0013] 1.1 Isolation and purification of memory B cells
[0014] After testing the plasma neutralizing activity of HIV-1 infected patients, the infected patients with better plasma neutralizing activity were screened out, and 20ml of EDTA anticoagulated whole blood samples were taken after obtaining the informed consent of the infected patients. Add 20ml of fresh anticoagulated whole blood into a human lymphocyte separation tube (purchased from Shenzhen Dakowei Co., Ltd.) at a rate of 5ml / tube, centrifuge at 2200r / min for 15min, and absorb the white lymphocyte layer in the middle, which is human peripheral blood mononuclear cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com