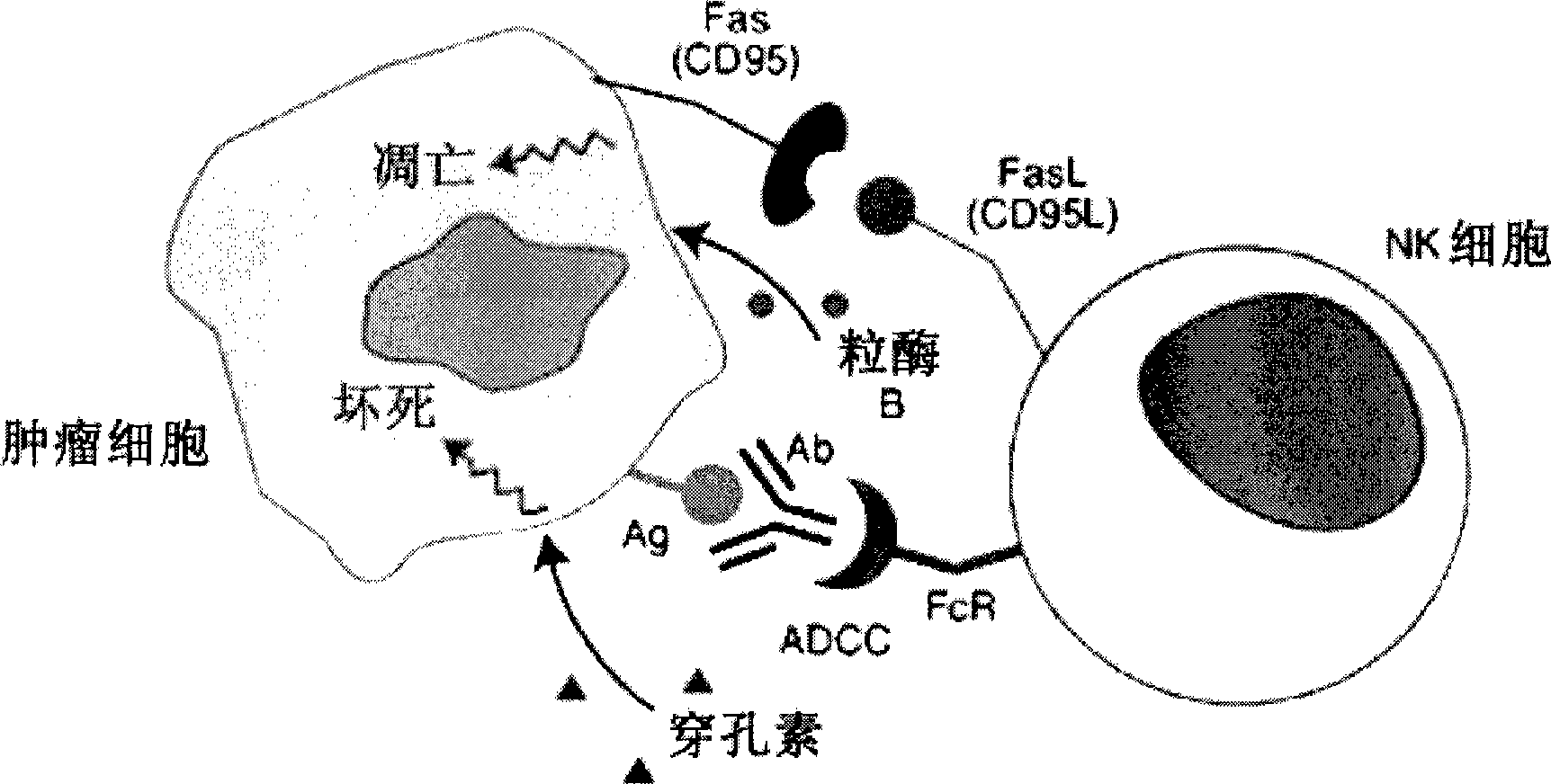
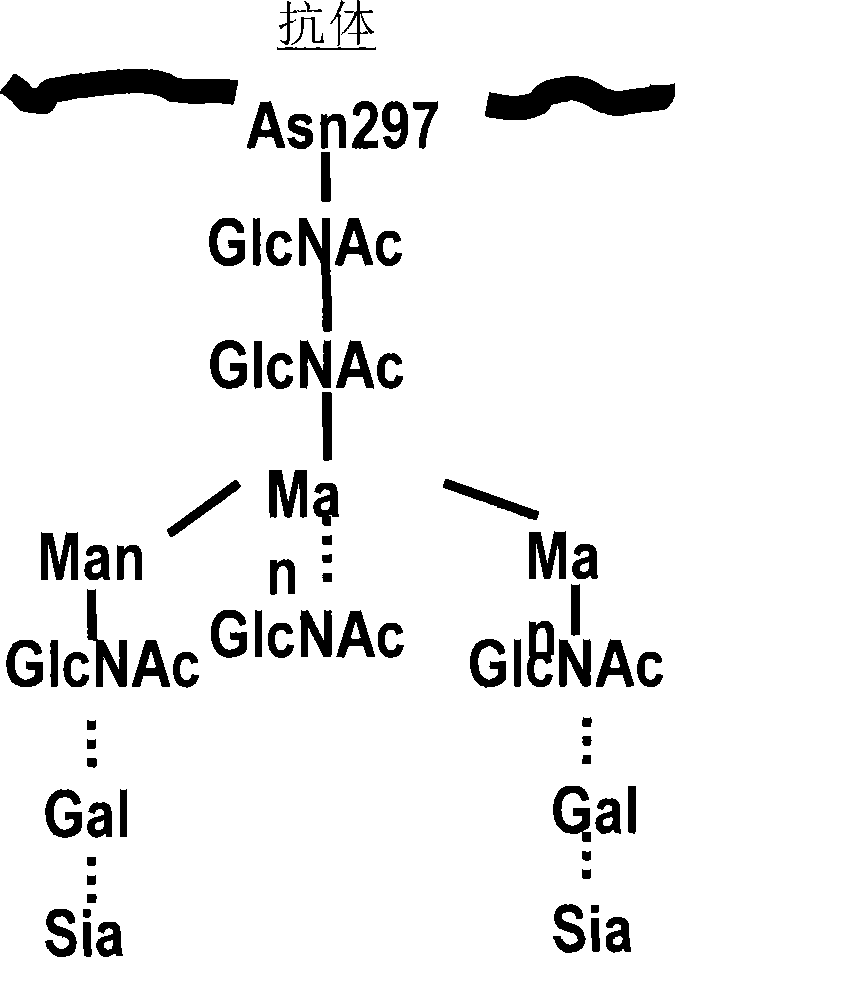
Glycosylation engineered antibody therapy
A glycosylation, antibody technology, applied in fucosylation and ,N-, can solve the problems of unstable expression efficiency and low host system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
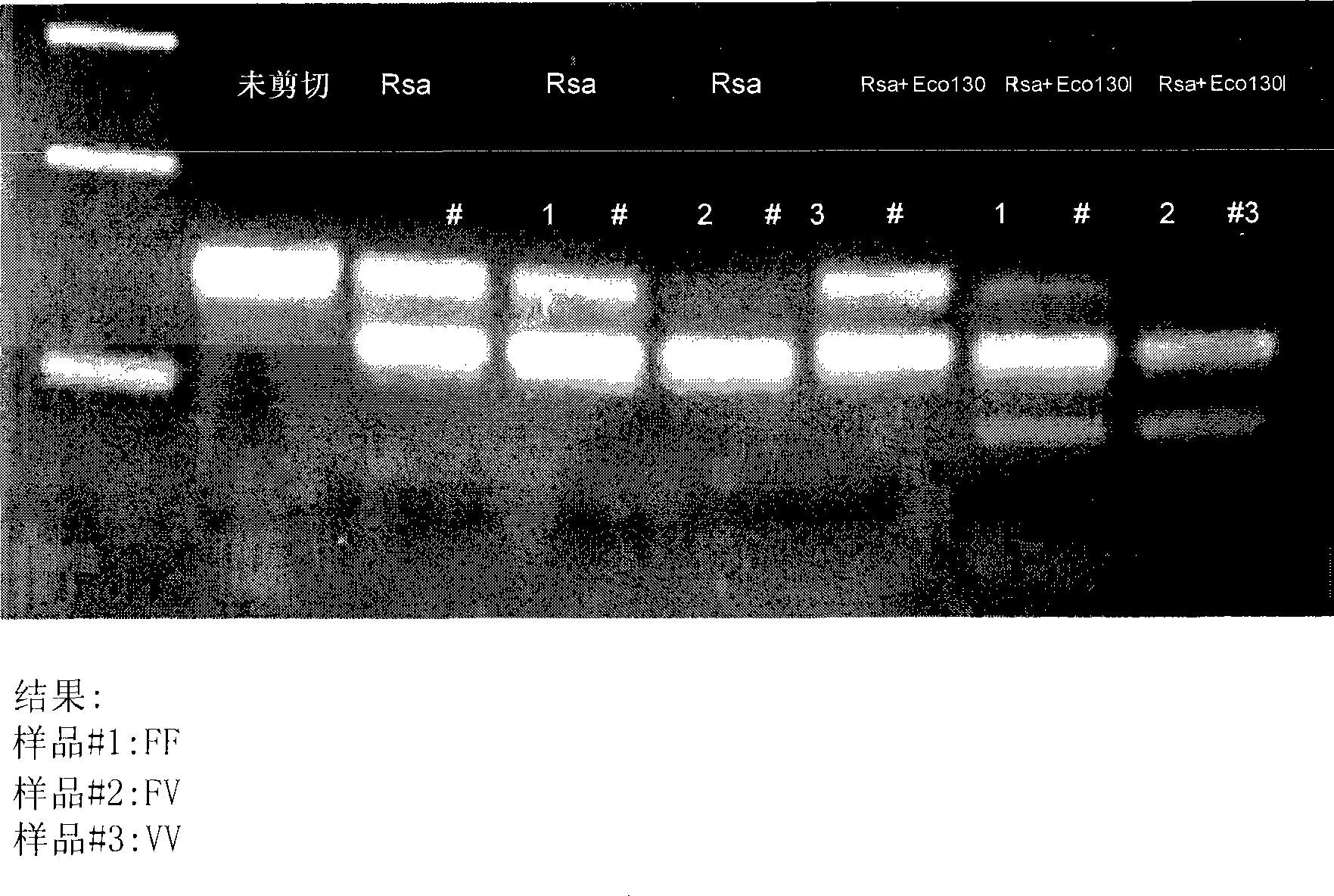
[0085] Example 1: Detection of FcgRIIIa receptor (CD16a) and FcgRIIa (CD32) allelic polymorphism
[0086] To determine the ability of glycoengineered mAbs to induce ADCC in patients with various genotypes or to determine the responsiveness of non-glycoengineered mAbs, the allelic variants at position 131 of FcgRIIa and position 158 of FcgRIIIa were characterized using, for example, a PCR-based strategy. body. First, genomic DNA isolated from human tumor cell lines, human saliva, human PBMC, or paraffin-embedded tissue was used as a template for PCR amplification.
[0087] A. Detection of polymorphisms in the FcgIIIa receptor (CD16a) allele using PCR amplification and restriction enzyme digestion.
[0088] Primer design was performed according to the sequence available in GenBank (accession number X52645 for FcgRIIIa, Nieto et al., 2000). This protocol uses a primer that introduces a new RsaI site to one end of all amplification products and a second primer that creates a new...
Embodiment 2
[0094] Prophetic Example 2: Homogeneous Preparation of Antibodies.
[0095] To obtain homogenous preparations of mAbs with specific glycosylation states, systems combining high cell expression yields and in vitro glycoengineering using chemoenzymatic transglycosylation were utilized [27-30]. Combined with the power of chemically synthesizing oligosaccharide oxazoline substrates for endonucleases, this method allows the preparation of a range of defined glycosylation states (native or non-native) of mAbs or their IgG-Fc domains, which in turn allows the systematic Analysis of the structure-activity relationship of IgG glycosylation and ADCC activity. Following the seminal work of Jeffries et al., using hingeless human IgG-Fc i.e. (aa231-447) as a model system in which the hinge region of the Fc has been deleted [7, 31]. Using this truncated Fc form rather than fully human antibody IgG or IgG-Fc as a model system greatly simplifies synthesis and subsequent study of structure-...
Embodiment 3
[0097] Embodiment 3: Design and synthetic example of sugar oxazoline.
[0098]ENGases are a class of endoglycosidases that hydrolyze β-1,4-glycosidic linkages in the core N,N'-diacetylchitobiose moiety of N-glycoproteins to release the N-glycans. However, some ENG enzymes, such as endo-A of Arthrobacter protophormiae and endo-M of Mucor hiemalis, possess transglycosylation activity and are able to convert released N-glycans Transfer to GlcNAc-peptide receptors to form new glycopeptides. Endo-A and endo-M can transfer large intact oligosaccharides to GlcNAc-peptide acceptors in one step to form new glycopeptides, allowing highly focused synthesis of glycopeptides without protecting groups. Chemoenzymatic methods suffer from low transglycosylation yields (typically 5-20%), product hydrolysis, and the limitations of using only native N-glycans as donor substrates. To address these issues, we used synthetic oligosaccharide oxazolines (mimetics of putative oxazolinium ion interme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com