Anti-human CD38 humanized monoclonal antibody and application thereof
A monoclonal antibody and variant technology, applied in the field of biomedicine, can solve the problems of complex production process of ADC products, poor stability, high maturity of BCMA targets, and achieve good thermal stability and solution stability, less impurities, and more. Beneficial for mass production and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1 monoclonal antibody preparation
[0080] The DNA nucleotide sequence of the heavy chain of RB0021-S1 is:
[0081] CAAGTGACACTGAAAGAGAGCGGCCCTACACTGGTGAAGCCCACCCAGACACTGACACTGACATGCACCACCAGCGGCTACACATTCACCTCCCACGGCATCAACTGGGTGAGACAGCCCCCCGGCAAGGCCCTCGAGTGGATCGGCTACATCTACATCGGCAACGGCTACACCGAGTACAACGAGAAGTTCAAGGGAAGAGCTACACTGACCAGCGACACCAGCAAGAATCAAGCCGTGCTGACCATGACCAACATGGACCCCGTGGATACCGCCACCTACTTCTGCGCTAGATCCCACTACGACAGCTCCAGCTGGTTTGCTTACTGGGGCCAAGGCACACTGGTGACCGTGAGCTCCGCCAGCACCAAGGGACCTAGCGTGTTTCCTCTGGCCCCTTCTAGCAAGAGCACAAGCGGAGGAACAGCCGCTCTGGGCTGTCTGGTGAAAGACTACTTTCCCGAGCCCGTGACCGTGTCTTGGAATTCAGGAGCCCTGACCAGCGGAGTGCACACATTTCCAGCCGTGCTGCAGAGCAGCGGACTGTATAGCCTGAGCAGCGTGGTGACCGTGCCTTCTTCTTCTCTGGGCACCCAGACCTACATCTGCAACGTGAACCACAAGCCCAGCAACACCAAGGTGGACAAGAAGGTGGAGCCCAAGTCTTGCGACAAGACCCACACTTGCCCCCCTTGTCCAGCTCCAGAACTCCTGGGAGGACCTAGCGTGTTCCTGTTCCCTCCCAAGCCTAAGGACACCCTGATGATCAGCCGGACCCCAGAAGTGACTTGCGTGGTGGTGGACGTGTCCCACGAAGACCCCGAGGTCAAGTTCAATTGGTACGTGGACGGAGTGGAGG...
Embodiment 2
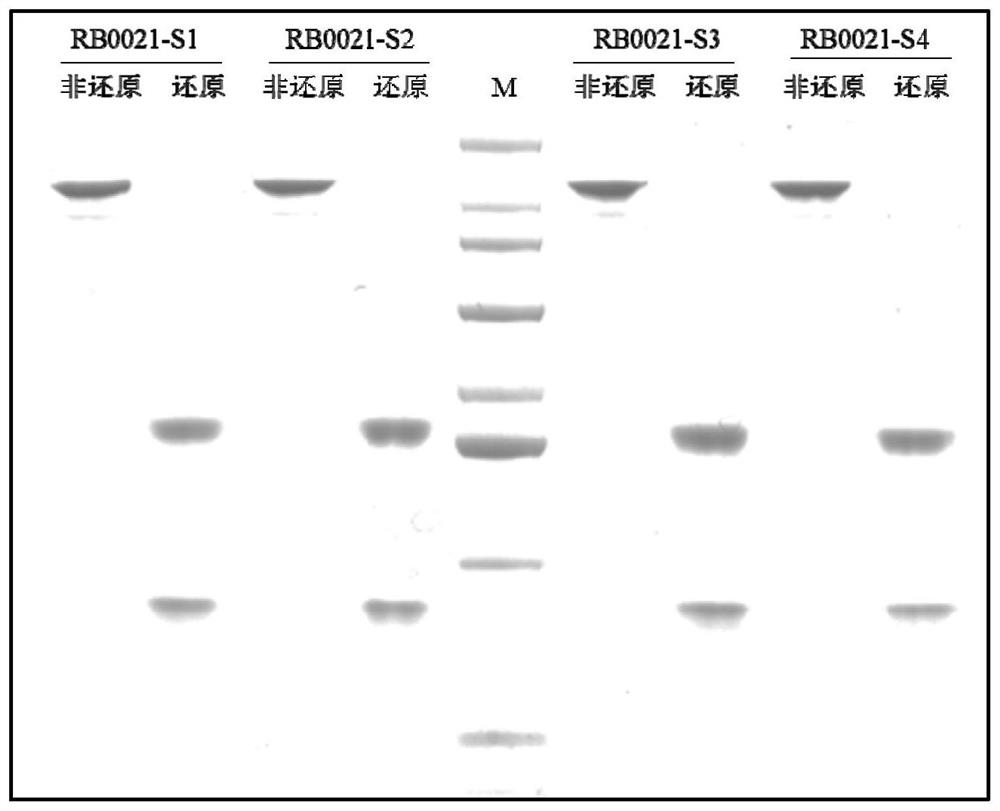
[0093] Embodiment 2 Purification and detection of monoclonal antibody
[0094] 1. Affinity chromatography
[0095] Flush the system with 0.5M NaOH, flush the filler Mabselect Sure with 0.1M NaOH, and control endotoxin for 1 hour each, and balance the filler with 20mM PB, 150mM NaCl (PH7.2). Filter the RB0021 PC (Daratumumab), RB0021-S1, RB0021-S2, RB0021-S3, RB0021-S4 transient expression supernatant and load the sample at a flow rate of 1mL / min. After loading, wash with 20mM PB and 150mM NaCl To the level of UV280 peak change, then washed with 20mM PB, 1M NaCl (PH6.5) to the level of peak change, finally eluted with 20mM Cit-Na3Cit (PH3.5), and neutralized with 1M Tris (PH9.0).
[0096] 2. Cation chromatography
[0097] Flush the system with 0.5M NaOH, flush the filler SP-HP with 0.1M NaOH, treat each for 2 hours to control endotoxin, and use 20mMPB (pH6.0) to balance the filler. Adjust the pH of the RB0021 PC (Daratumumab), RB0021-S1, RB0021-S2, RB0021-S3, and RB0021-S4 s...
Embodiment 3
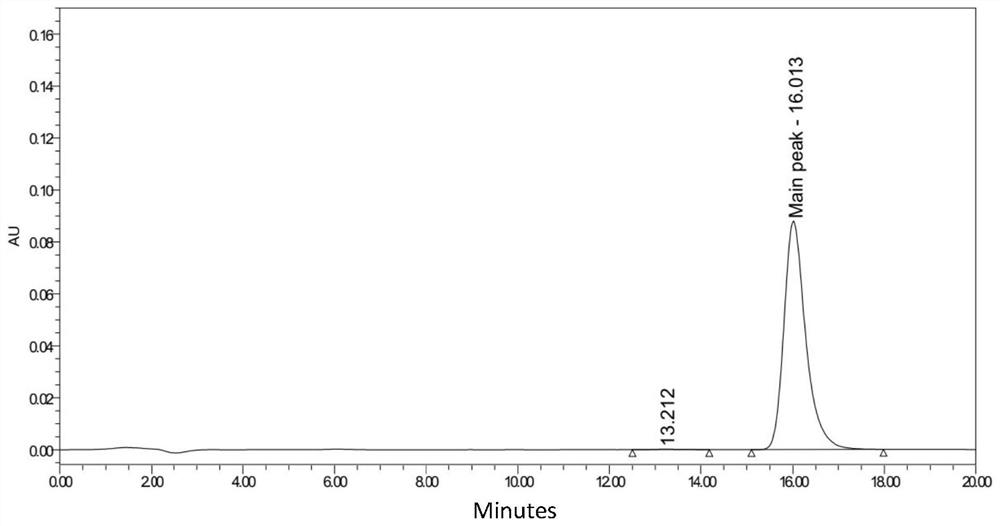
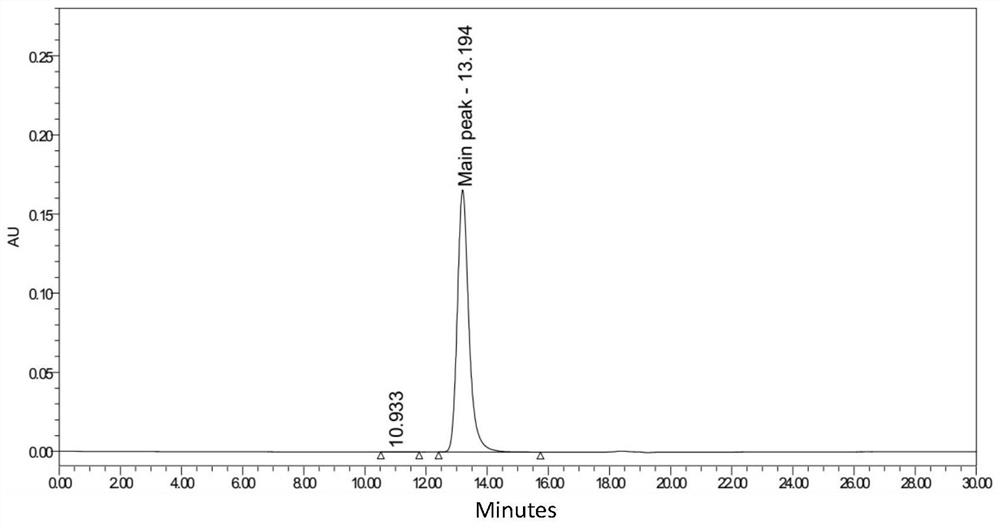
[0110] Example 3 Monoclonal Antibody Affinity Determination
[0111] Biacore affinity determination was carried out with GE 8K, and HBS-EP+ was used as the experimental buffer at 25°C. The ligand was diluted to 5 μg / mL and immobilized on the Protein A chip at a flow rate of 10 μL / min for 20 seconds. The analyte CD38 was diluted to 0, 0.111, 0.333, 1, 3, 9 (×3), 27, 81nM with a flow rate of 30 μL / min, combined for 300 s, dissociated for 300 s, and regenerated with 10 mM Glycine-HCl (pH 1.5) at a flow rate of 100 μL / min min, time 30s, repeat once. Determination of affinity data is shown in Table 4 below:
[0112] Table 4 Affinity data
[0113] Antibody affinity (human) RB0021 PC (Daratumumab) 8.86E-08 RB0021-S1 1.17E-09 RB0021-S2 1.17E-09 RB0021-S3 1.04E-09 RB0021-S4 1.04E-09
[0114] It can be known from the above table 4 that the monoclonal antibody of the present invention can specifically recognize human CD38 protein, and its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com