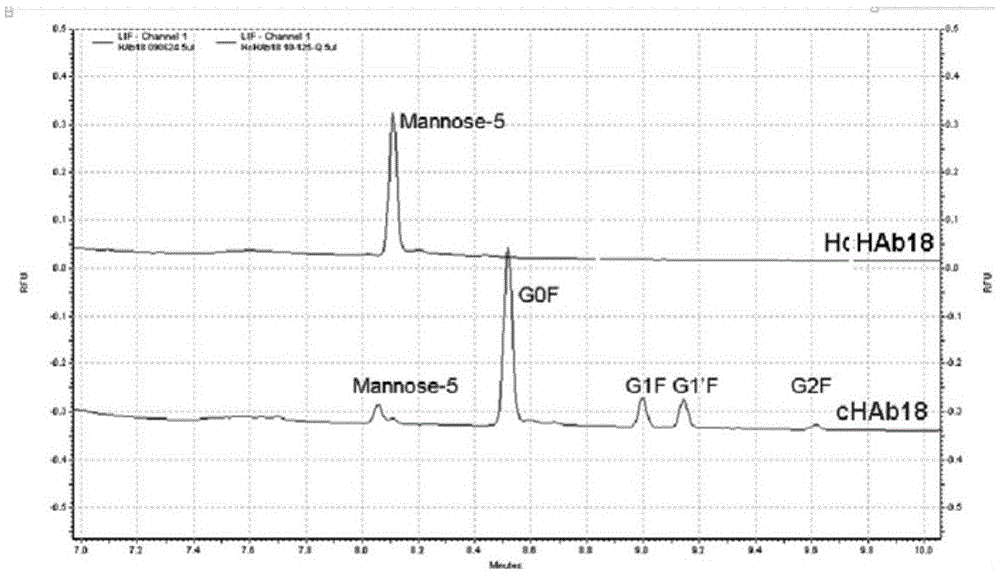
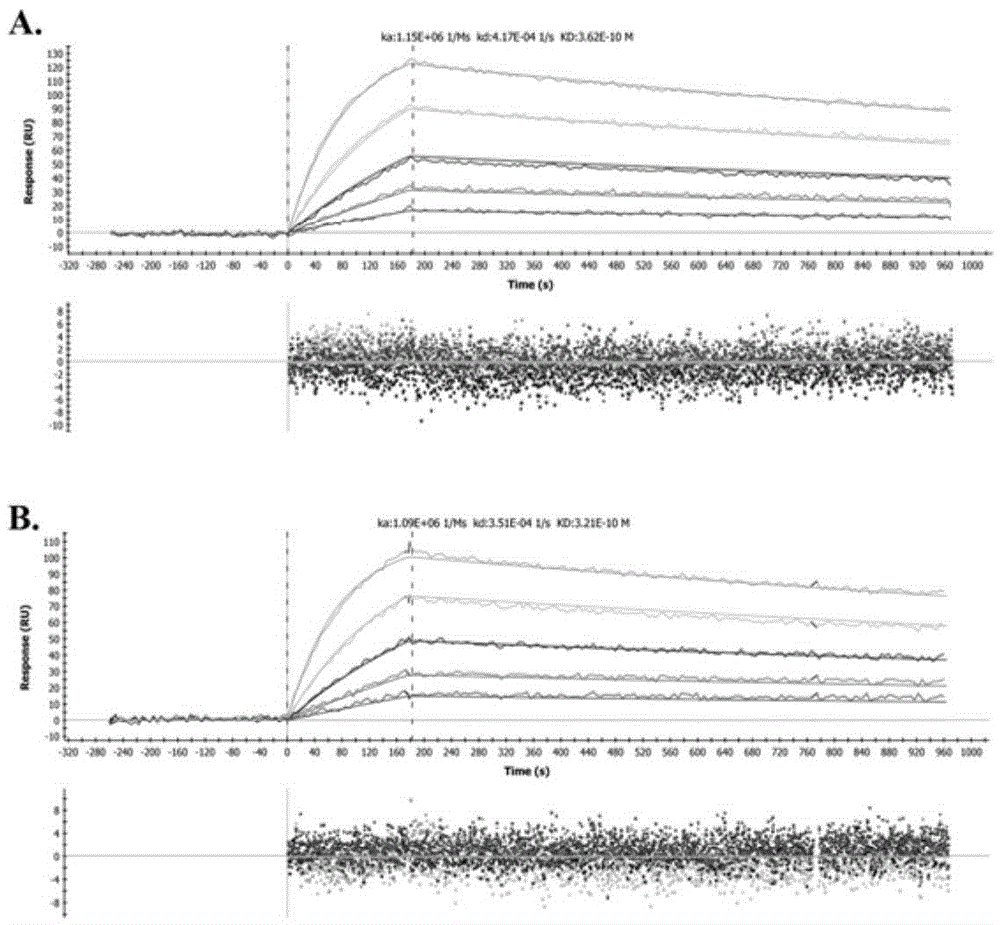
Humanized modified anti-CD147 chimeric antibody hchab18 and its application
A chimeric antibody and humanized technology, applied in the biological field, can solve problems such as short half-life, lack of immunoglobulin, and blocking antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Cloning and sequencing of the light and heavy chain variable region genes of the mouse-derived anti-CD147 monoclonal antibody
[0046] According to the instructions of the TrizolReagent kit (Invitrogen Company), 2 × 10 6 Total RNA of hybridoma cells secreting anti-human CD147 monoclonal antibody HAb18 (patent number: 02114471.0). After identifying the quality of total RNA by 1% agarose electrophoresis, use PrimeScript TM RTreagentKit (TaKaRa) reverse transcription kit was used for reverse transcription to obtain cDNA of total RNA. According to the sequence information of the light and heavy chain variable region genes of HAb18 monoclonal antibody HAb18 published in the patent "anti-human liver cancer monoclonal antibody HAb18 light and heavy chain variable region genes and its application" (patent number: 02114471.0), the corresponding primers were designed and synthesized. Increase the light and heavy chain variable region genes of HAb18 monoclonal antibod...
Embodiment 2
[0049] Example 2: Optimization of VL and VH genes and construction of chimeric antibodies
[0050] After obtaining the VL and VH sequencing results. First, the light and heavy chain genes were modified using the codon bias of hamsters to solve the low protein expression caused by rare codons in CHO cells during translation. The principle is based on solving the problem of transporting amino acids during amino acid synthesis The tRNA bias, that is, the use of codons corresponding to more tRNA content, accelerates protein synthesis. According to the codon utilization table of the hamster, the codon bias in the VL and VH nucleotide sequences obtained in Example 1 is optimized to obtain the light, light, Amino acid sequence of heavy chain variable region and its nucleotide coding sequence.
[0051] Secondly, the oligonucleotide fragments of antibody light and heavy chain genes were artificially synthesized, and NheI and BamH1 restriction sites were synthesized at both ends of th...
Embodiment 3
[0053] Example 3: Transfection of CHO cells and screening of recombinant clones
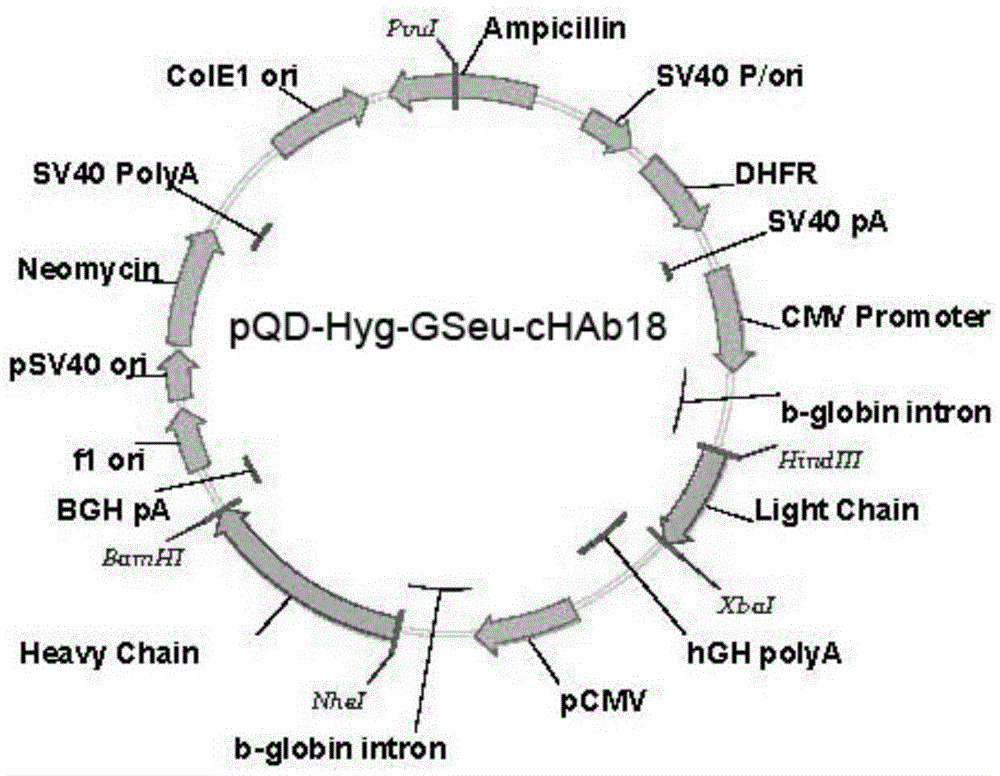
[0054] The expression vector pQD-Hyg-GSeu-cHAb18 containing the humanized antibody gene constructed above was used to transform Escherichia coli DH-5α strain, and inoculated on 100ml LB medium for amplification.
[0055] The expression vector pQD-Hyg-GSeu-cHAb18 plasmid DNA was extracted according to the instructions of the kit using an ultrapure plasmid DNA purification kit (Qiagen).
[0056] The pQD-Hyg-GSeu-cHAb18 plasmid DNA was transfected into fucosyltransferase-deficient CHO cells (MAGE1.5 cells) by electroporation (patent: 200980145664.9).
[0057] The transfected MAGE1.5 cells were pressure-selected with a final concentration of 500ug / ml Hygromycin B (Invitrogen, HyB for short), and then L-Methionine Sulfoximine (L-MethionineSulfoximine, MSX) (Sigma) continued to carry out cell selection and culture; and ClonePixFL (Genentix, Inc) was used to perform multiple clone selections on cells t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com