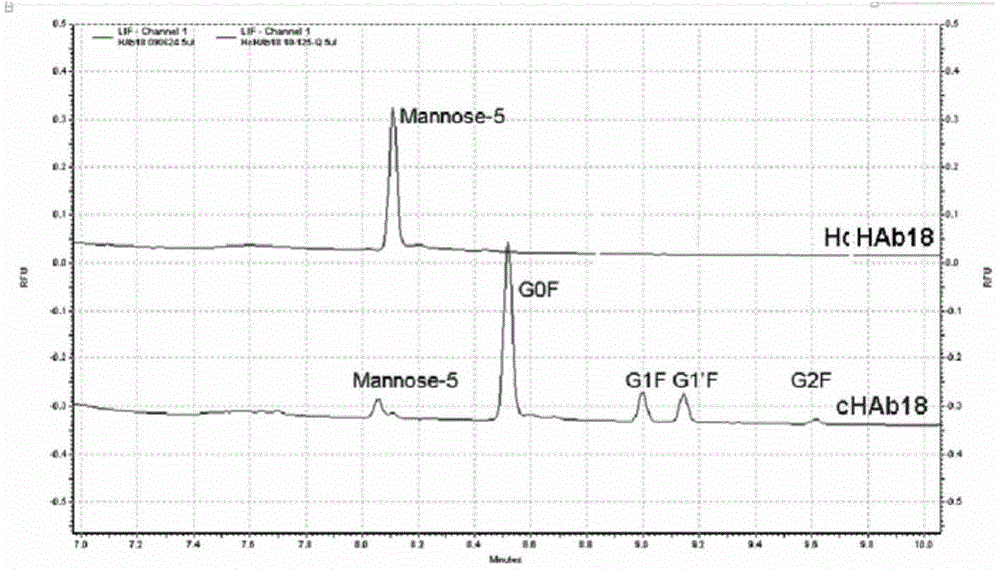
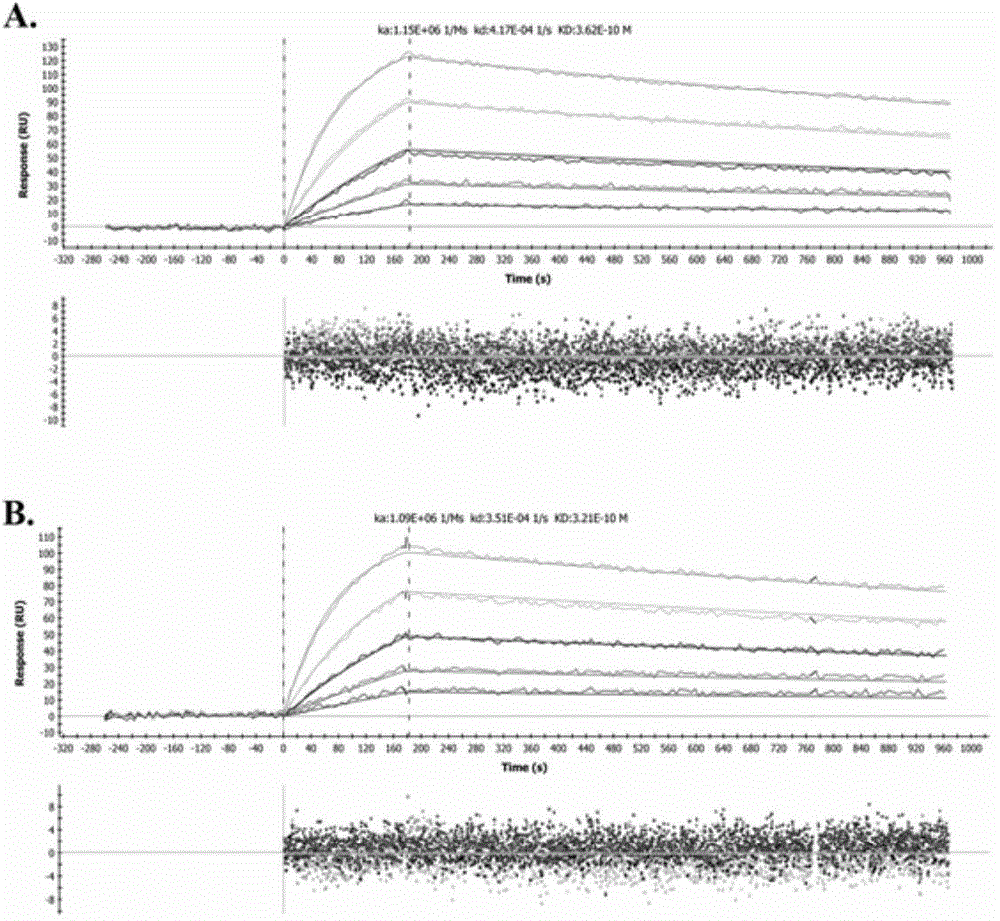
Humanization modified anti-CD147 chimeric antibody HcHAb18 and application thereof
A chimeric antibody, humanized technology, applied in the biological field, can solve the problems of blocking antibodies, short half-life, allergic and toxic reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Cloning and sequencing of the light and heavy chain variable region genes of the mouse-derived anti-CD147 monoclonal antibody
[0046] According to the instructions of the Trizol Reagent kit (Invitrogen Company), 2 × 10 6 Total RNA of hybridoma cells secreting anti-human CD147 monoclonal antibody HAb18 (patent number: 02114471.0). After identifying the quality of total RNA by 1% agarose electrophoresis, use PrimeScript TM RT reagent Kit (TaKaRa) reverse transcription kit was used for reverse transcription to obtain cDNA of total RNA. According to the sequence information of the light and heavy chain variable region genes of HAb18 monoclonal antibody HAb18 published in the patent "anti-human liver cancer monoclonal antibody HAb18 light and heavy chain variable region genes and its application" (patent number: 02114471.0), the corresponding primers were designed and synthesized. Increase the light and heavy chain variable region genes of HAb18 monoclonal ant...
Embodiment 2
[0049] Example 2: Optimization of VL and VH genes and construction of chimeric antibodies
[0050] After obtaining the VL and VH sequencing results. First, the light and heavy chain genes were modified using the codon bias of hamsters to solve the low protein expression caused by rare codons in CHO cells during translation. The principle is based on solving the problem of transporting amino acids during amino acid synthesis The tRNA bias, that is, the use of codons corresponding to more tRNA content, accelerates protein synthesis. According to the codon utilization table of the hamster, the codon bias in the VL and VH nucleotide sequences obtained in Example 1 was optimized to obtain the monoclonal as shown in SEQ ID NO:1 to SEQ ID NO:8 The amino acid sequence of the light and heavy chain variable regions of the antibody and its nucleotide coding sequence.
[0051] Secondly, the oligonucleotide fragments of antibody light and heavy chain genes were artificially synthesized, ...
Embodiment 3
[0053] Example 3: Transfection of CHO cells and screening of recombinant clones
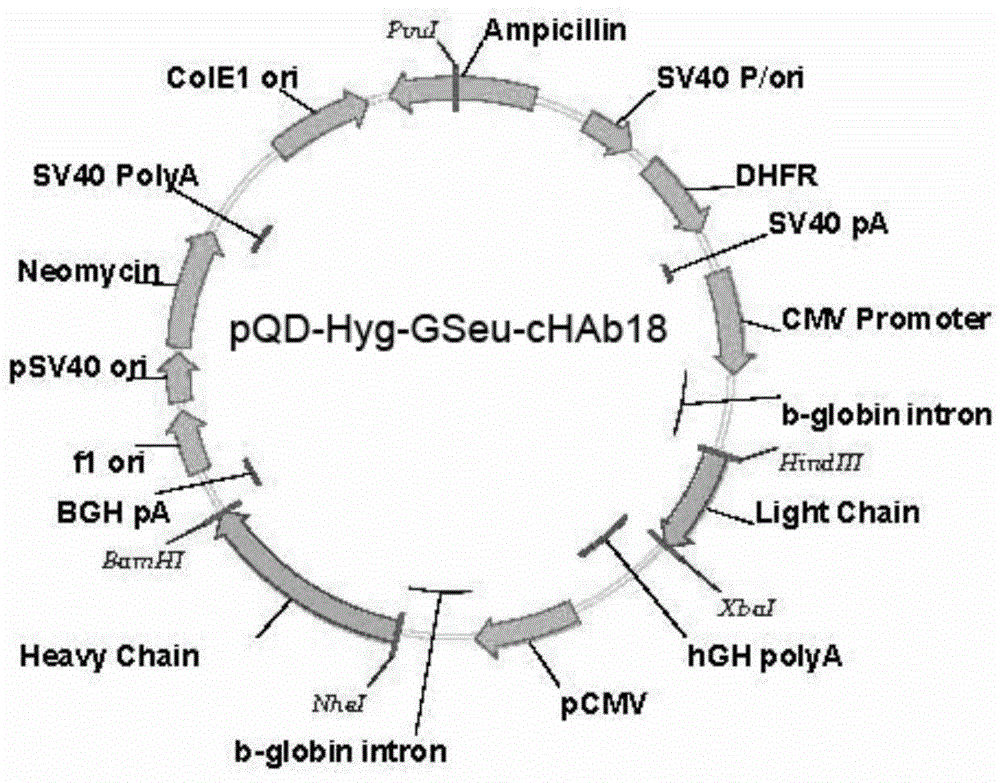
[0054] The expression vector pQD-Hyg-GSeu-cHAb18 containing the humanized antibody gene constructed above was used to transform Escherichia coli DH-5α strain, and inoculated on 100ml LB medium for amplification.
[0055] The expression vector pQD-Hyg-GSeu-cHAb18 plasmid DNA was extracted according to the instructions of the kit using an ultrapure plasmid DNA purification kit (Qiagen).
[0056] The pQD-Hyg-GSeu-cHAb18 plasmid DNA was transfected into fucosyltransferase-deficient CHO cells (MAGE1.5 cells) by electroporation (patent: 200980145664.9).
[0057] The transfected MAGE1.5 cells were pressure-selected with a final concentration of 500ug / ml Hygromycin B (Invitrogen, HyB for short), and then L-Methionine Sulfoximine (L-Methionine Sulfoximine) with a final concentration of 25uM was used. , MSX) (Sigma) continued to perform cell selection and culture; and ClonePix FL (Genentix, Inc) was used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com