Long-acting recombinant GLP1-Fc-CD47 protein as well as preparation method and application thereof
An A-B-C-D, fusion protein technology, applied in the field of medicine, can solve the problem of low frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
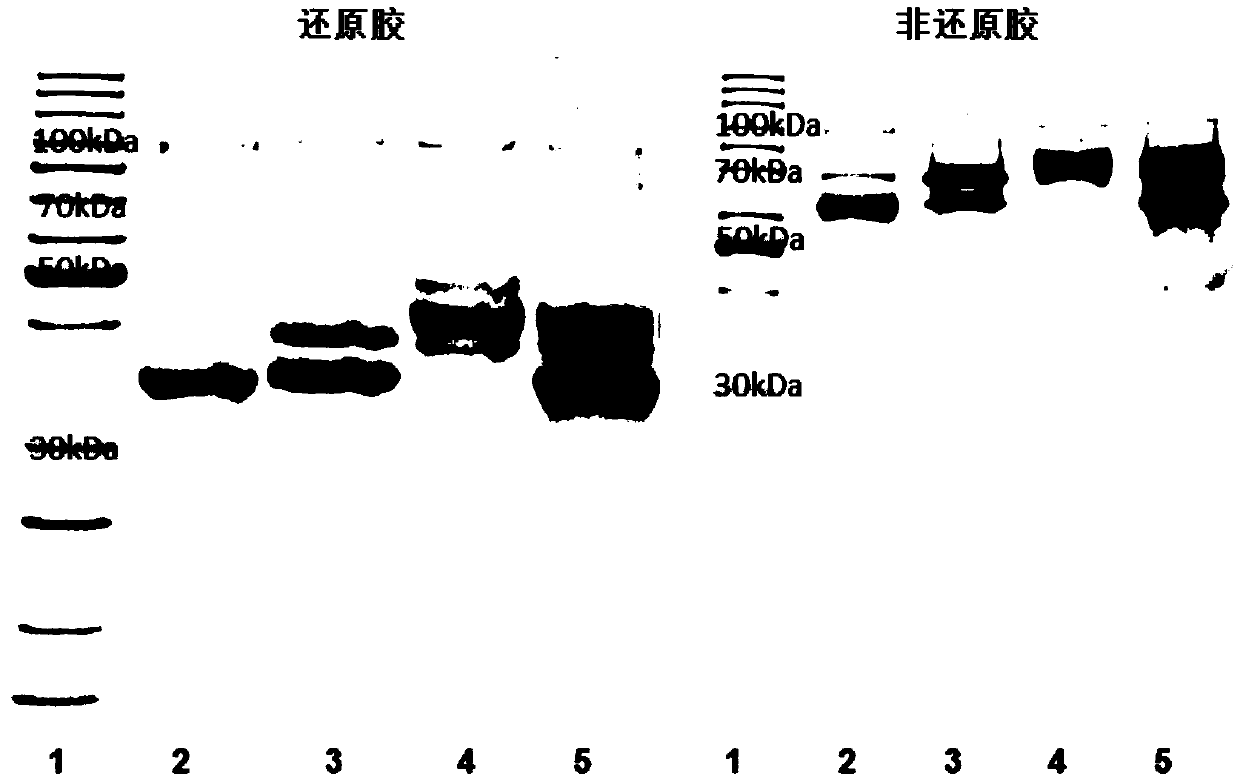
[0178] Example 1: Preparation of GLP-1-Fc recombinant protein
[0179] Mammalian cell expression vector (Bacmam) was selected as the vector, and 8 histidines (8His) were selected as the tag protein (Tag) in series with two maltose binding protein (MBP) and sumo protein. The DNA encoding the recombinant protein was artificially synthesized, and its optimized sequence was shown in SEQ ID NO.:16-20, and the gene fragment was cloned into Bacmam- 8His-2MBP-sumo.
[0180] The plasmid was transformed into DH10Bac competent cells, and after blue-white screening, bacmid DNA was extracted, transfected into insect cells to obtain the P0 generation virus, and the virus was further amplified in insect cells to the required volume. The amplified virus was transfected into mammalian cells for expression for 4 days.
[0181] Four days after the virus transfected the mammalian cells, the supernatant was collected, centrifuged at 2000rpm for 20min, and the supernatant was slowly loaded on the...
Embodiment 2
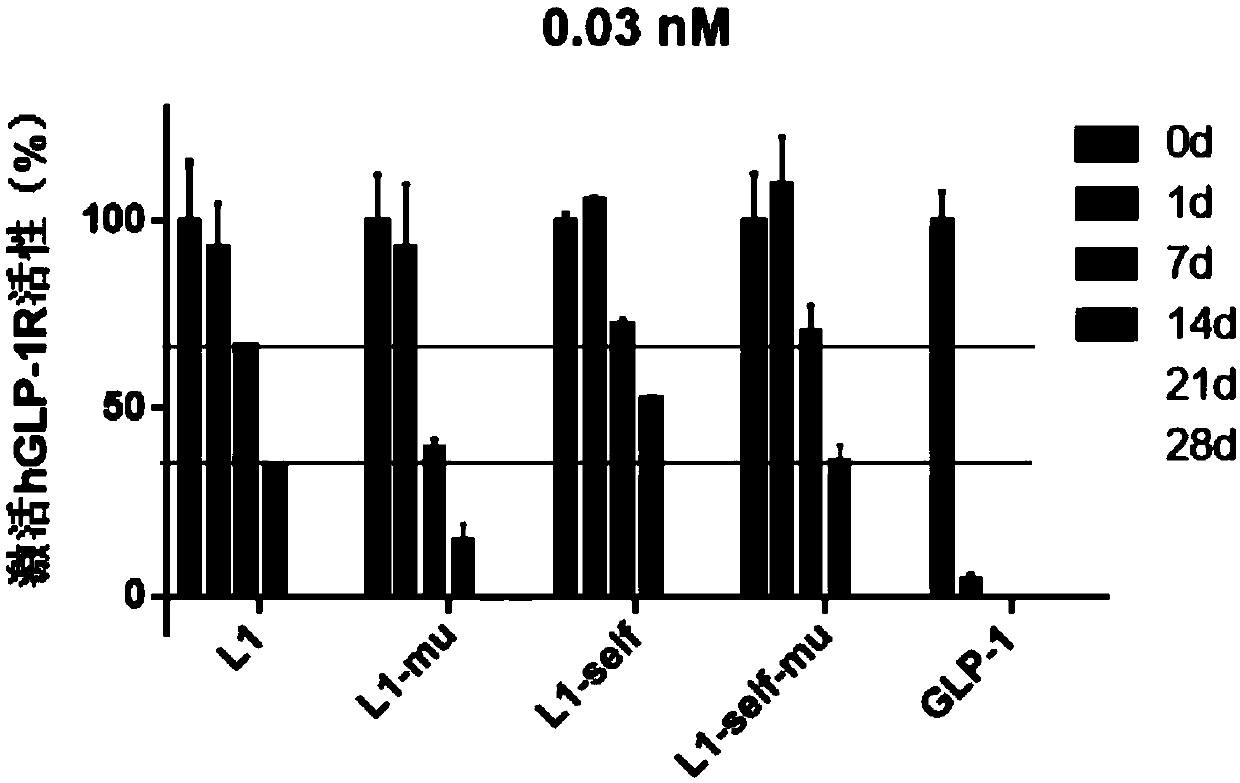
[0182] Example 2: Modification of the GLP-1 part of the GLP-1-Fc recombinant fusion protein and evaluation of cell activity
[0183] There are two main active forms of naturally occurring GLP-1: GLP-1(7-36)NH 2 And GLP-1(7-37) (SEQID NO.: 1), DPPIV enzyme acts between the 7th histidine (His) and the 8th alanine (Ala). A common strategy for prolonging GLP-1 drugs is to mutate the 8th alanine (Ala) to glycine (Gly) (SEQ ID NO.: 2) to resist degradation by DPPIV enzymes.
[0184] Studies have shown that after the 22nd glycine (Gly) of GLP-1 is mutated to glutamic acid (Glu) and the 36th arginine (Arg) is mutated to glycine (Gly), the activity of GLP-1 increases, and GLP-1 The risk of the fusion protein causing an immune response in the body is reduced (US 2007 / 0036806 A1). The GLP-1 portion of the marketed drug dulaglutide introduces mutations at positions 8, 22, and 36, respectively (SEQ ID NO.: 3).
[0185] In this embodiment, the 34th lysine (Lys) of GLP-1 is further mutate...
Embodiment 3
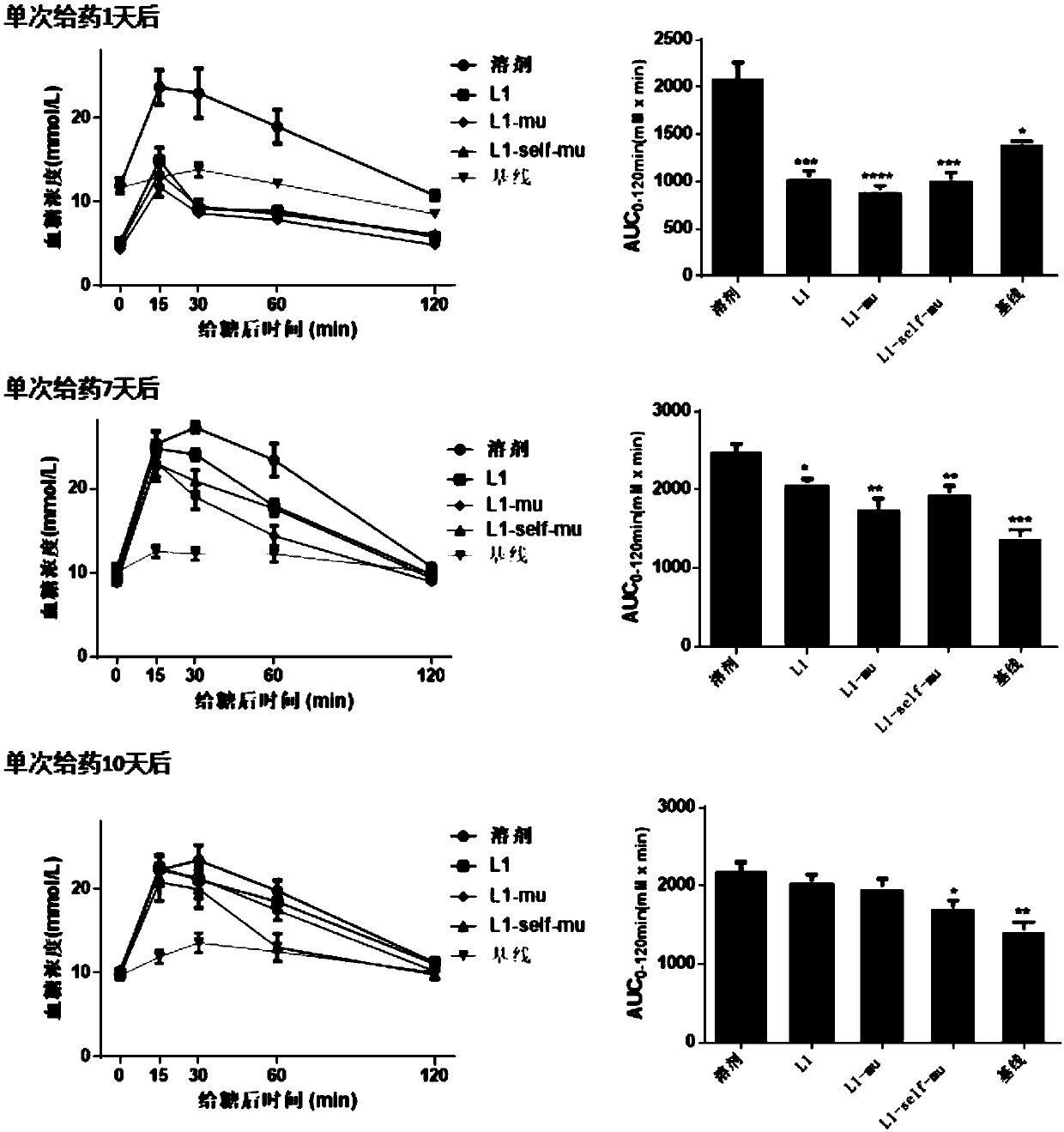
[0189] Example 3: Introduction of CD47 signal into GLP-1-Fc recombinant fusion protein
[0190] The Fc part of the recombinant protein GLP-1-Fc in the present invention is from human IgG4, including the hinge region, CH2 and CH3 domains. Although the ADCC effect induced by IgG4 is weak, it still has potential cytotoxicity.
[0191] The present invention introduces two mutations F234A and L235A in the Fc part of natural human IgG4, which can further effectively reduce the ADCC effect. In addition, the present invention also introduces the amino acid mutation S228P, which can effectively reduce the formation of GLP1-Fc monomers and increase the homogeneity and stability of the recombinant fusion protein.
[0192] Rodriguez and other researchers designed the smallest polypeptide (self) by computer to simulate the function of human CD47 to reduce macrophage clearance (self sequence is shown in SEQ ID NO.: 10). The self polypeptide was introduced into the N-terminus and C-terminu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com