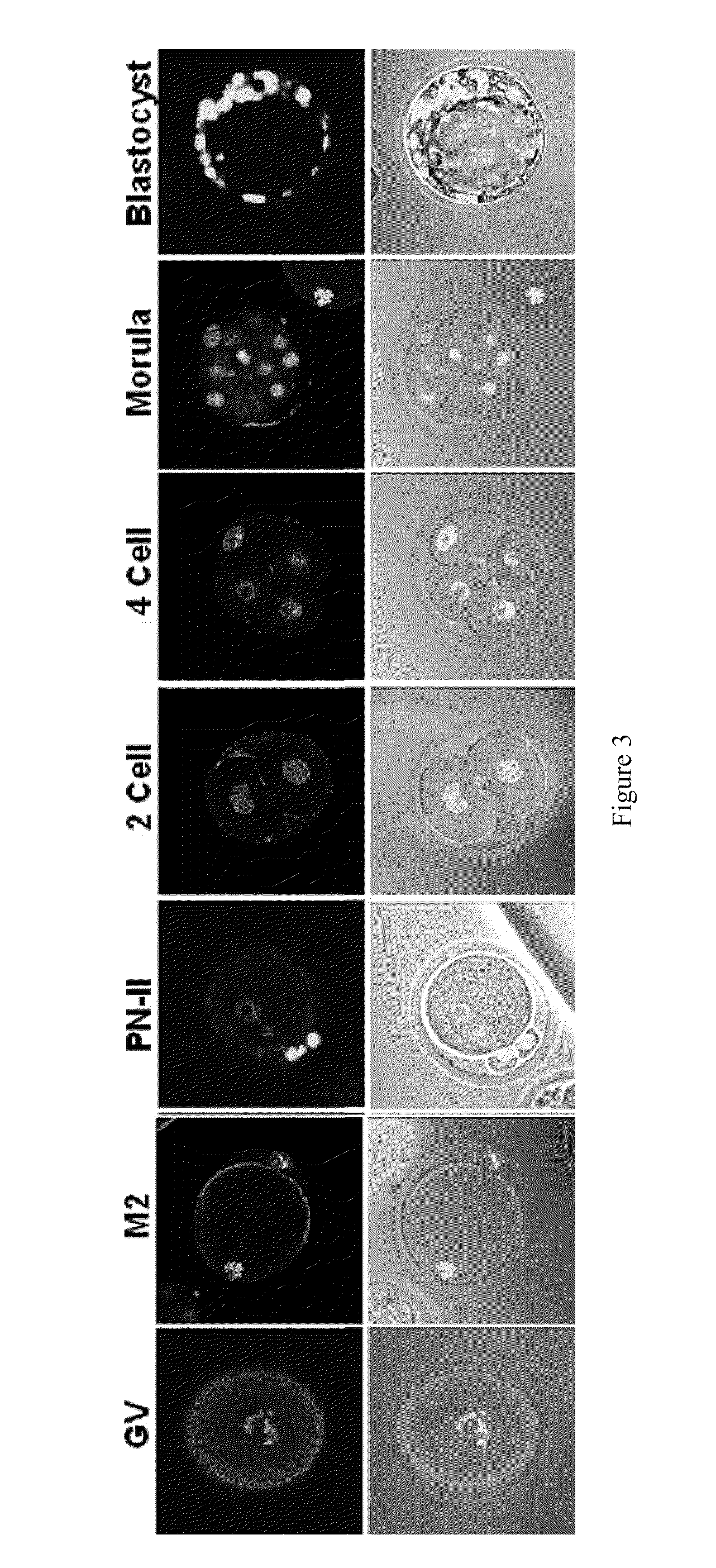
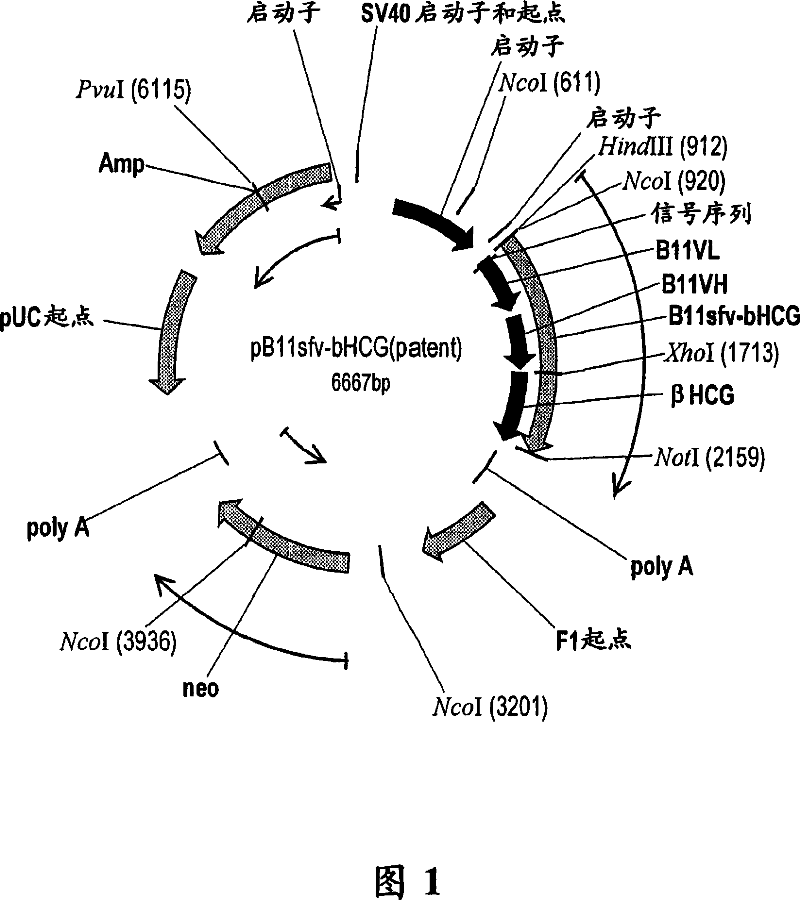
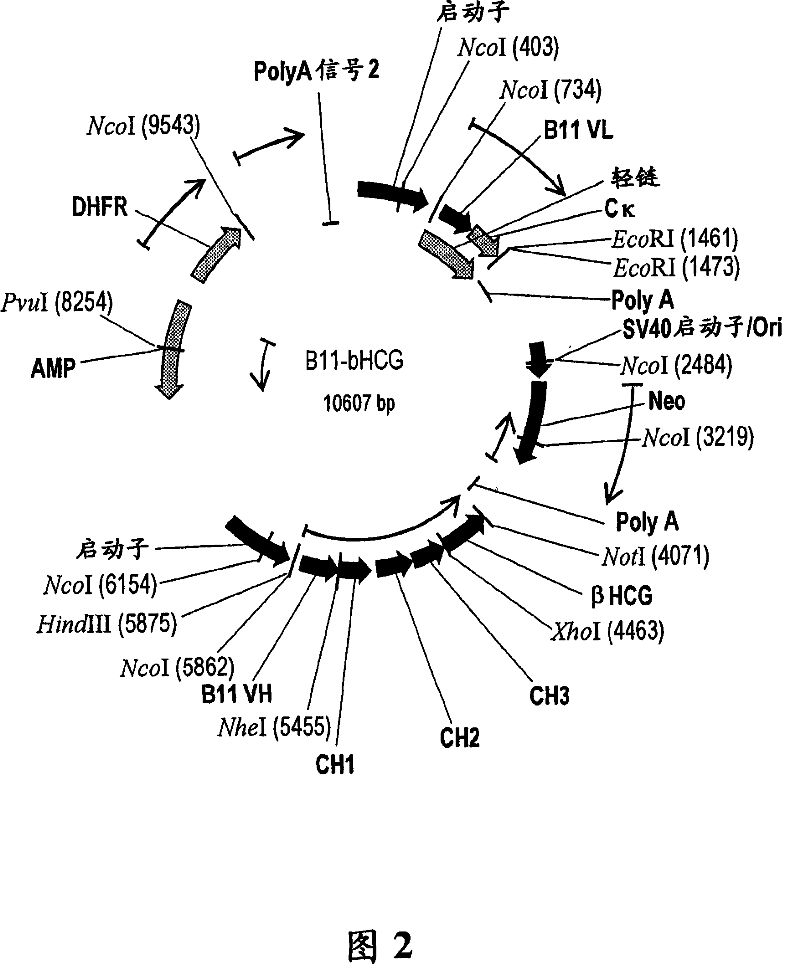
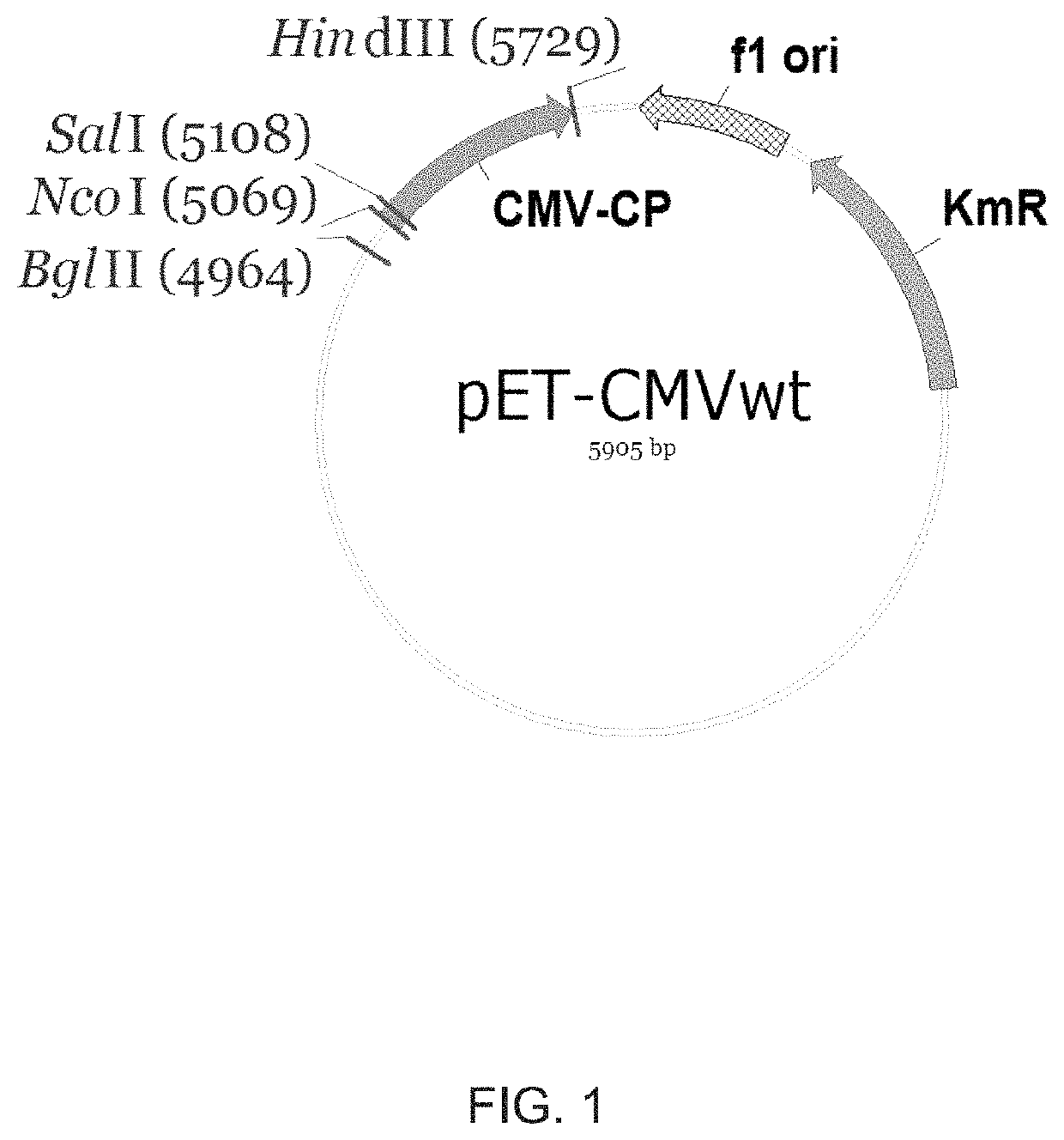
Patents
Literature
65results about "Contraceptive vaccin ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nanoparticle vaccines
InactiveUS7285289B2Simple and inexpensive to synthesizeEnhance immune responseUltrasonic/sonic/infrasonic diagnosticsPowder deliveryLipid formationAntigen
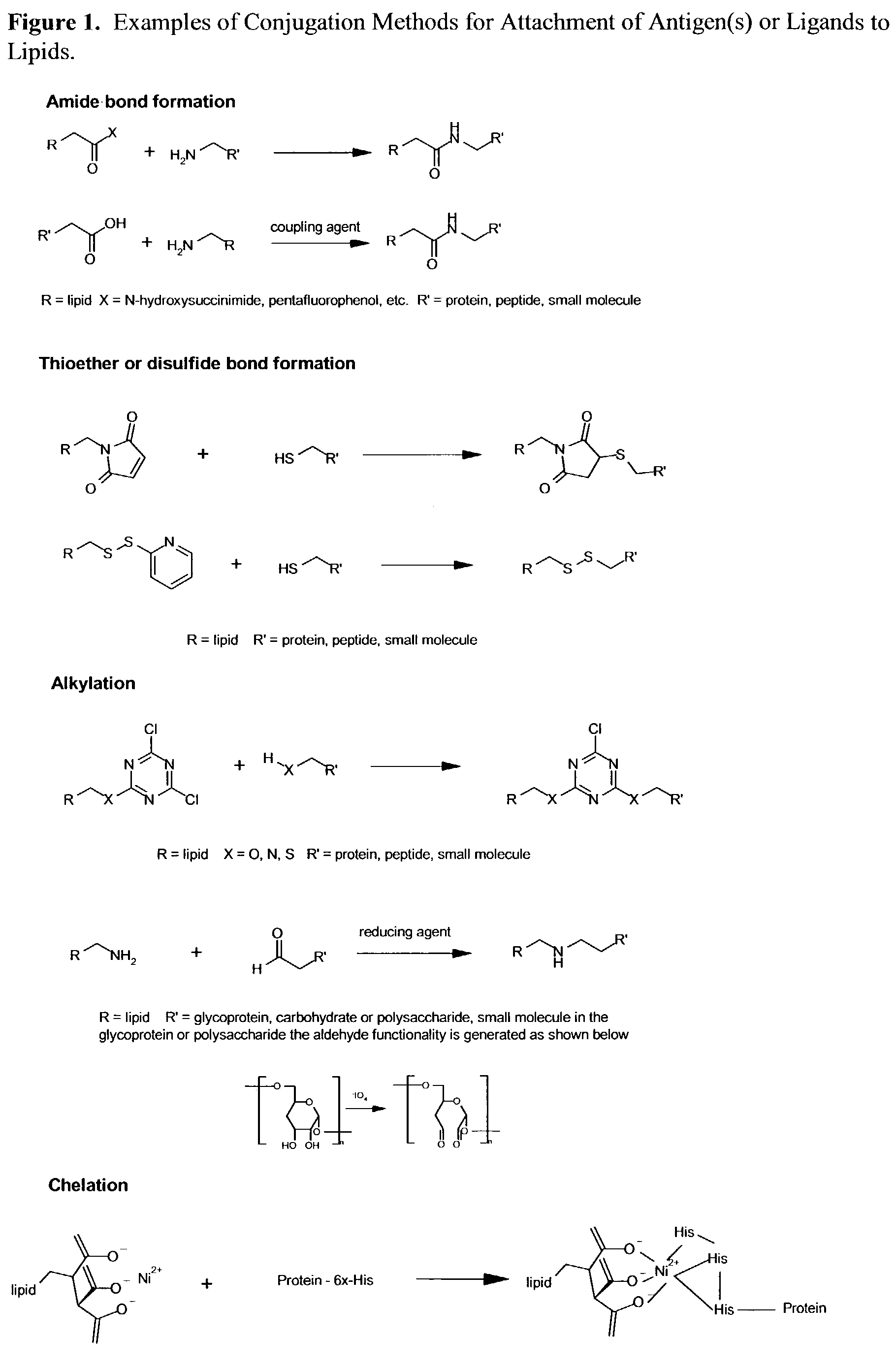
The present invention relates to nanoparticle vaccines comprised of a carrier, particularly polymerized lipids, having multiple copies of an antigen or combinations of different antigens displayed on the carrier. Such antigen-displaying nanoparticles may also display a targeting molecule on its surface in order to direct it to a specific site or cell type to optimize a desired immune response. The present invention also relates to encapsulating an antigen or combinations of different antigens within such nanoparticles, with or without a targeting molecule displayed on its surface. The antigens used in this invention are effective to produce an immune response against a variety of pathological conditions.
Owner:NANOVALENT PHARMA
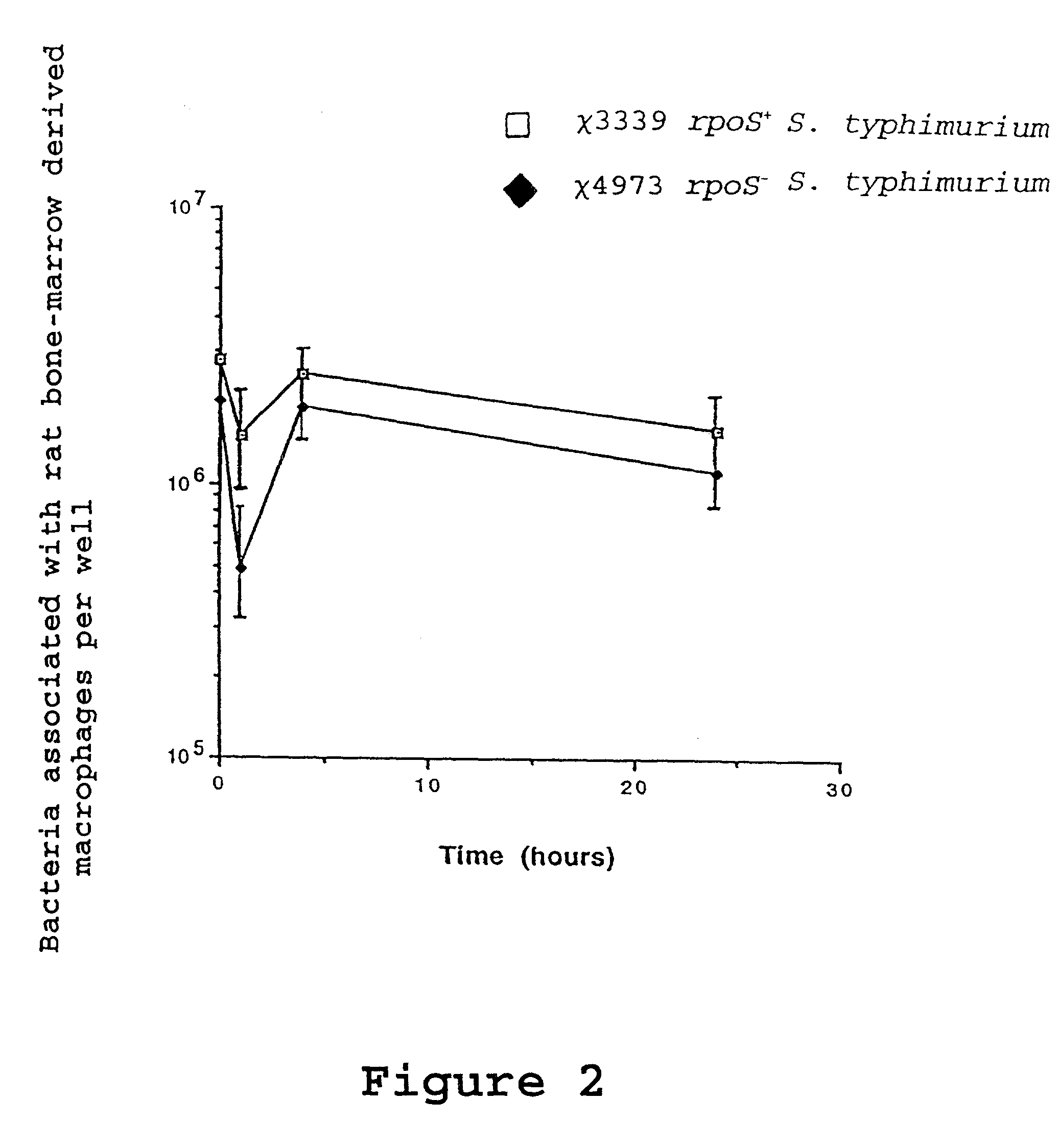
Recombinant vaccines comprising immunogenic attenuated bacteria having RpoS positive phenotype
InactiveUS7083794B2Improve balanceImproving immunogenicityAntibacterial agentsBiocideSalmonella entericaSalmonella serotype typhi
Attenuated immunogenic bacteria having an RpoS+ phenotype, in particular, Salmonella enterica serotype Typhi having an RpoS+ phenotype and methods therefor are disclosed. The Salmonella have in addition to an RpoS+ phenotype, an inactivating mutation in one or more genes which render the microbe attenuated, and a recombinant gene capable of expressing a desired protein. The Salmonella are attenuated and have high immunogenicity so that they can be used in vaccines and as delivery vehicles for genes and gene products. Also disclosed are methods for preparing the vaccine delivery vehicles.
Owner:WASHINGTON UNIV IN SAINT LOUIS
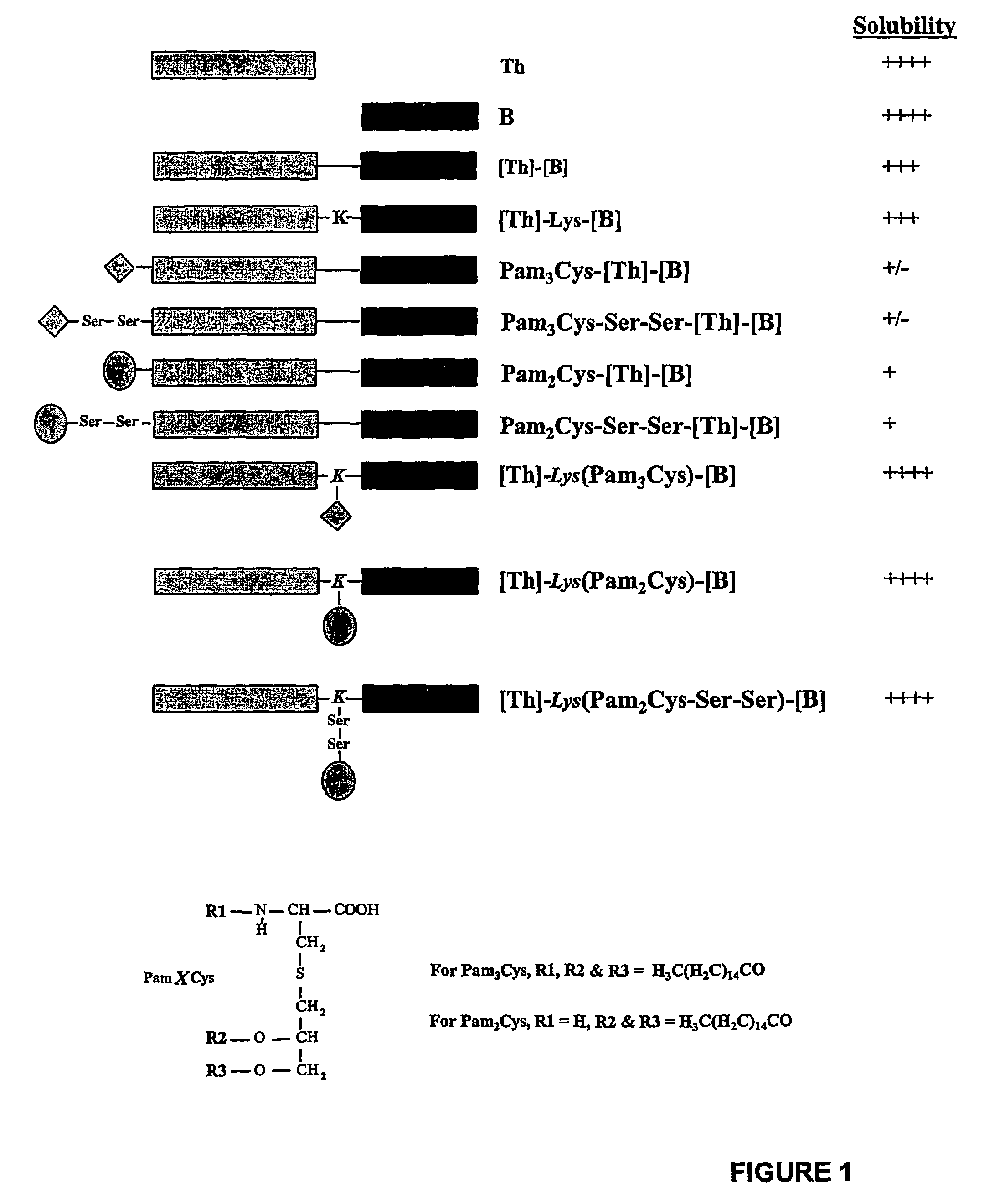
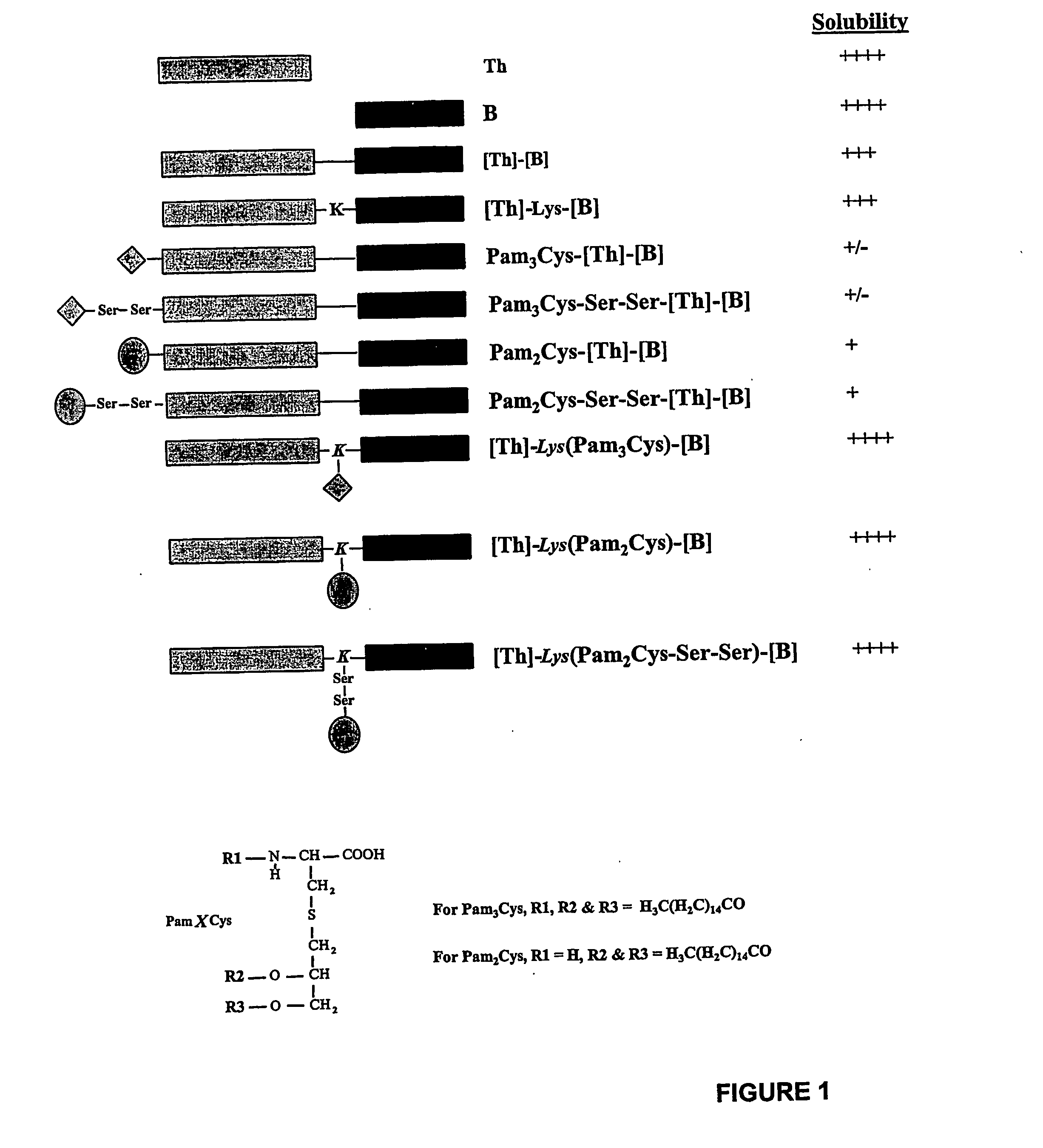
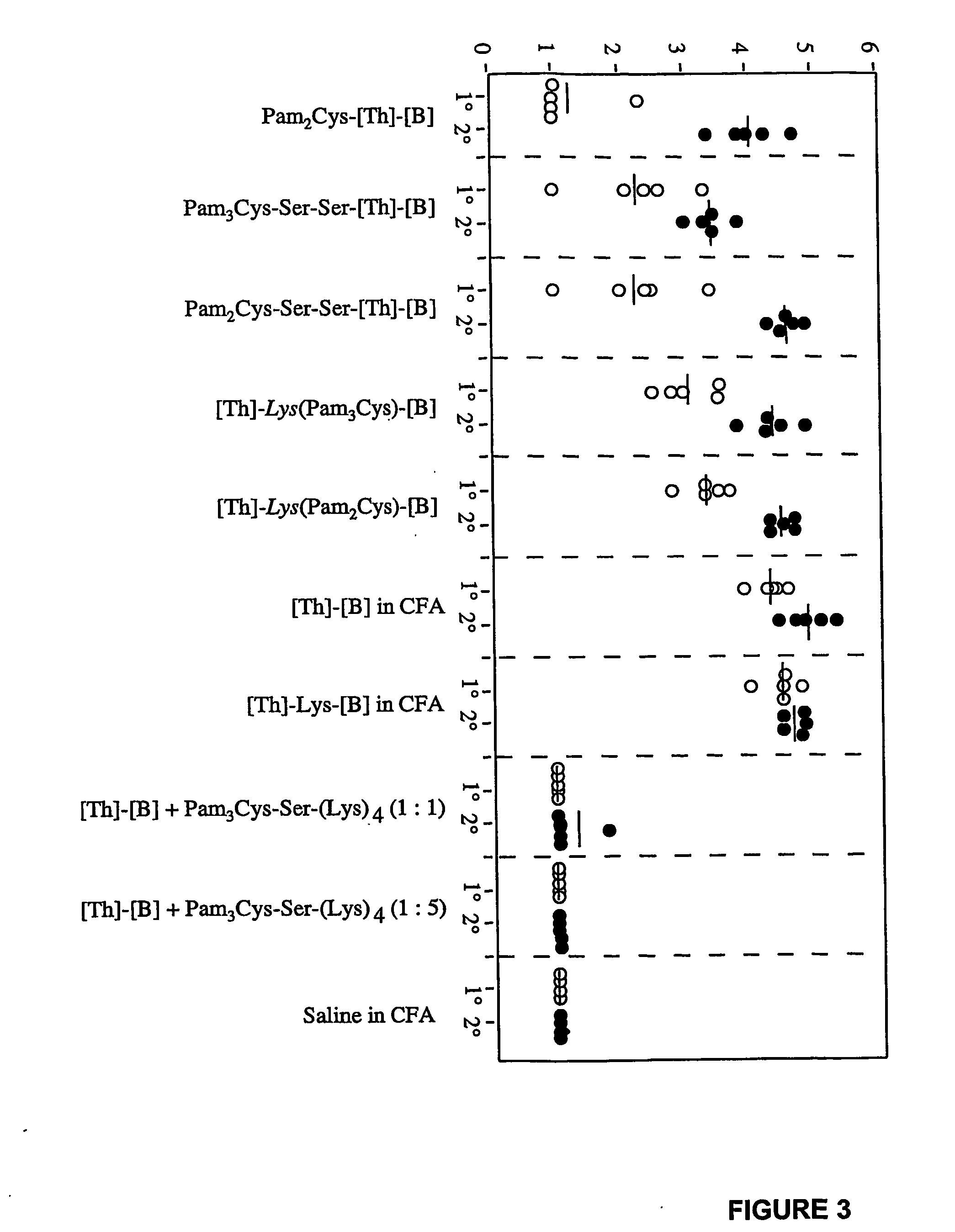
Immunogenic lipopeptides comprising T-helper and B-cell epitopes
InactiveUS7569225B2Increase profitEasy to specifyAntibacterial agentsBiocideSynthetic ImmunogensVaccination
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
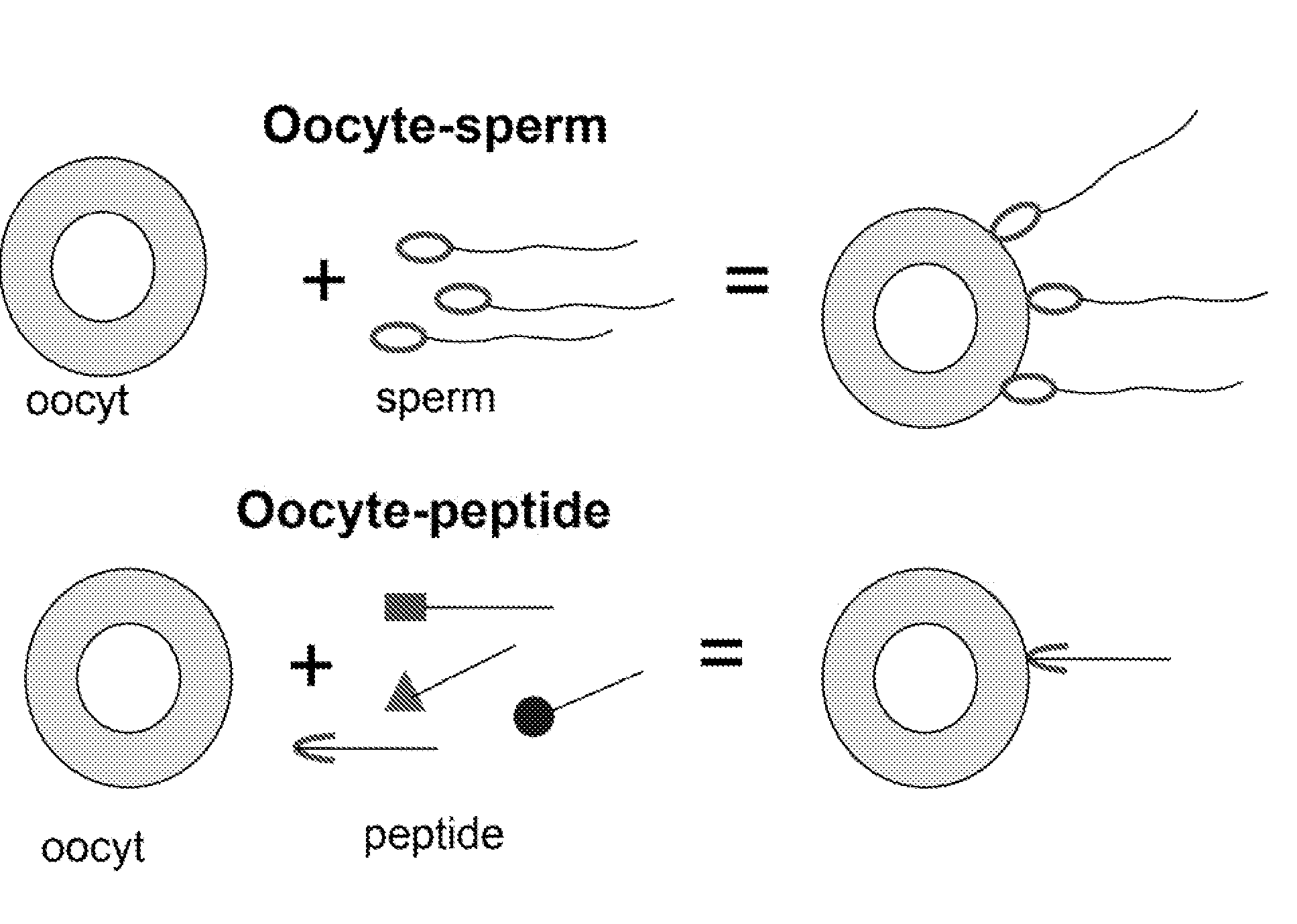
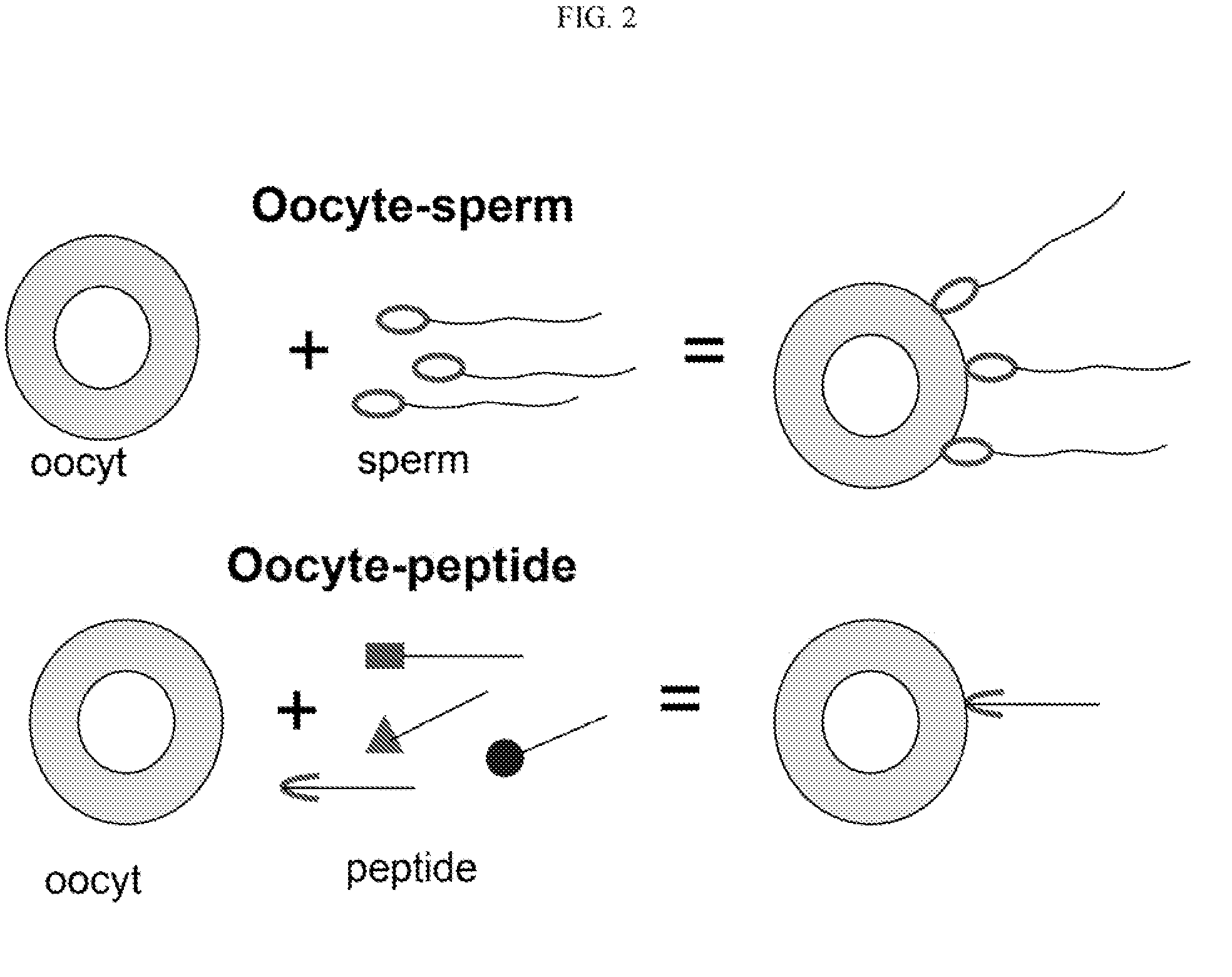
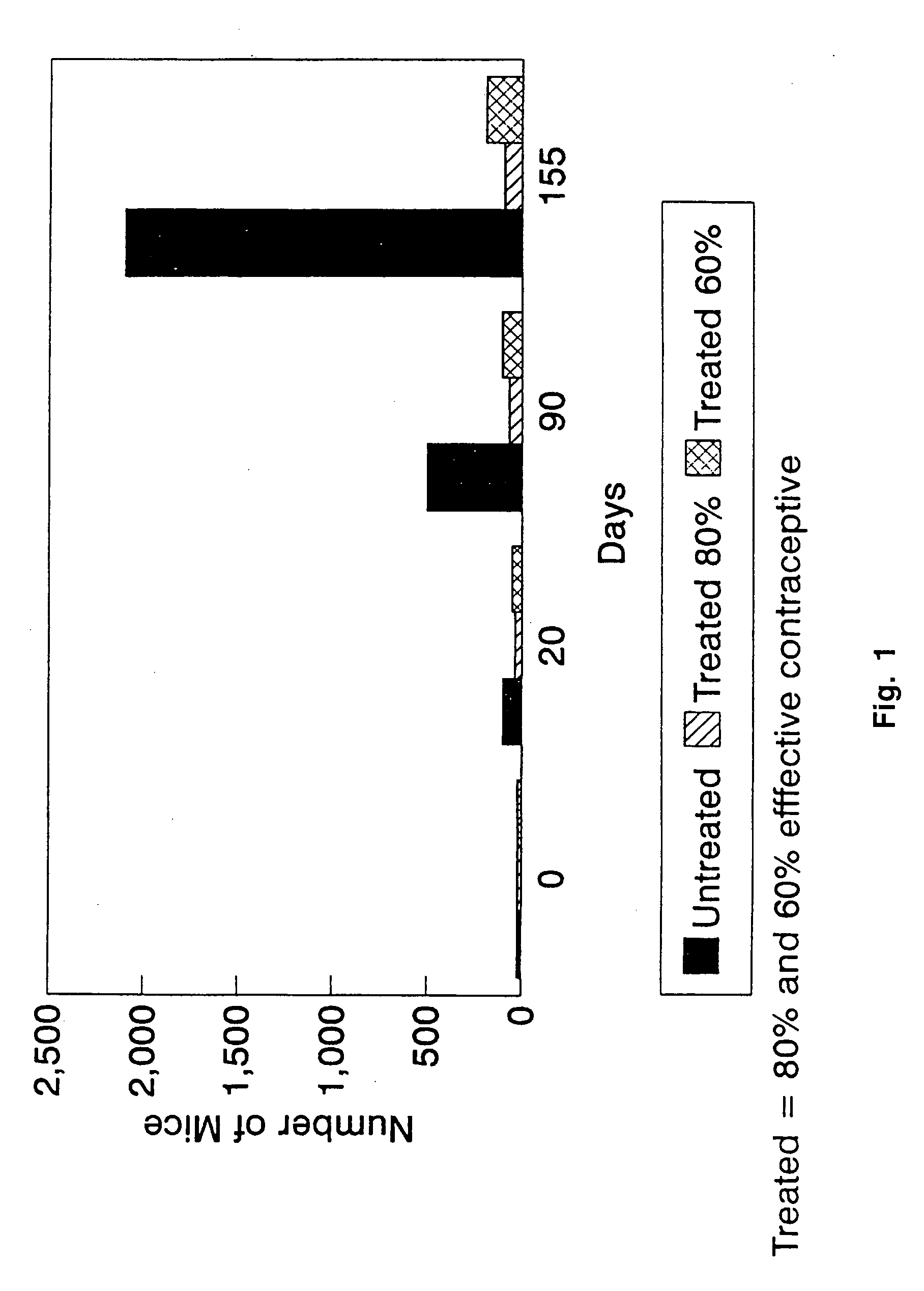
Method to prevent fertilization in mammals by administering a single dose of zona pellucida derived antigens, liposome and adjuvant
InactiveUSRE37224E1Extended maintenance periodReduce deliveryPeptide/protein ingredientsSnake antigen ingredientsAntigenAdjuvant
A vaccine for the immunocontraception of mammals is described. The vaccine consists of zona pellucida antigens and an adjuvant encapsulated in a liposome delivery system. The liposome delivery system allows for the slow release of antigen resulting in a prolonged immune response. In particular, after a single injection of the vaccine, levels of anti-zona pellucida antibodies were detected for up to 22 months in seals. Thus, the vaccine according to the present invention is effective after a single dose and is therefore very useful in immunocontraceptive protocols.
Owner:IMMUNOVACCINE TECH INC
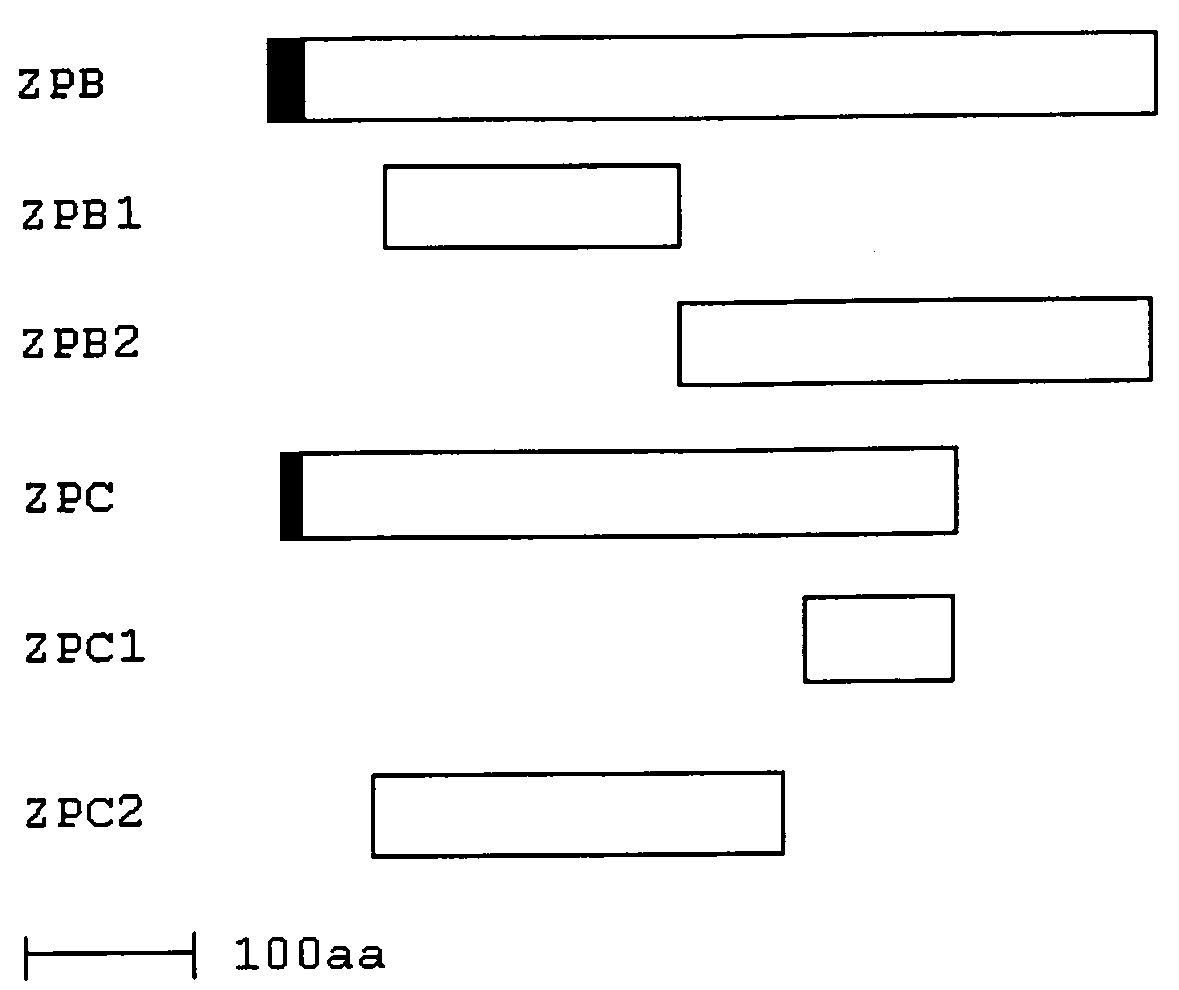
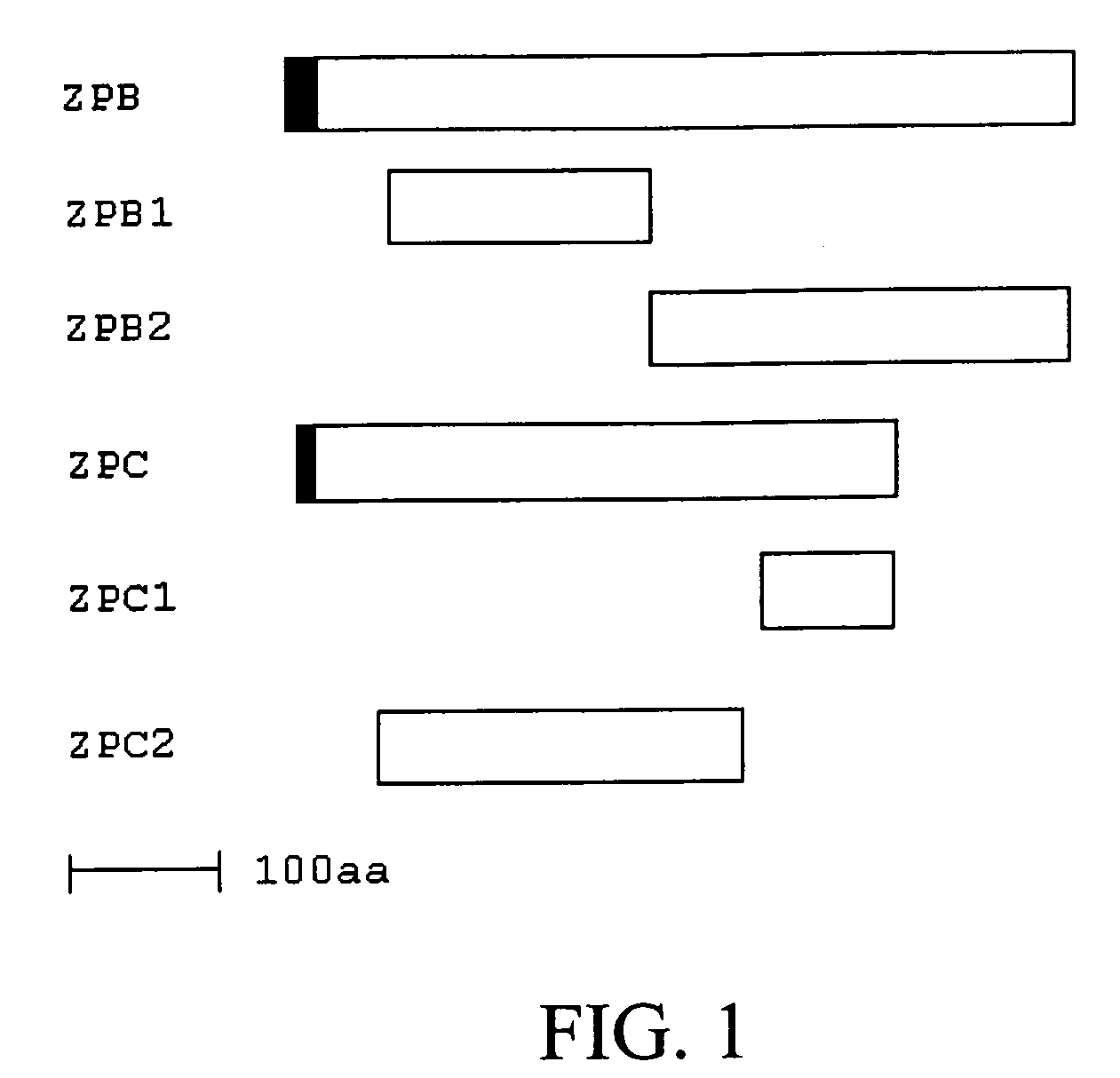
Methods, Compositions, and Sequences of ZP-Binding Peptides for Immunocontraception of Dogs and Other Animals
InactiveUS20090280137A1Easy to producePeptide/protein ingredientsLibrary screeningBiotechnologyBinding peptide
Disclosed are methods, compositions, and zona pellucida binding peptides and polypeptides for use in immunocontraception of canines and other animals. The disclosed compositions may include pharmaceutical compositions.
Owner:AUBURN UNIV
Compositions and methods for regulating sas1r
InactiveUS20100183617A1Preserve ovarian reserveCompound screeningApoptosis detectionSperm proteinEmbryo
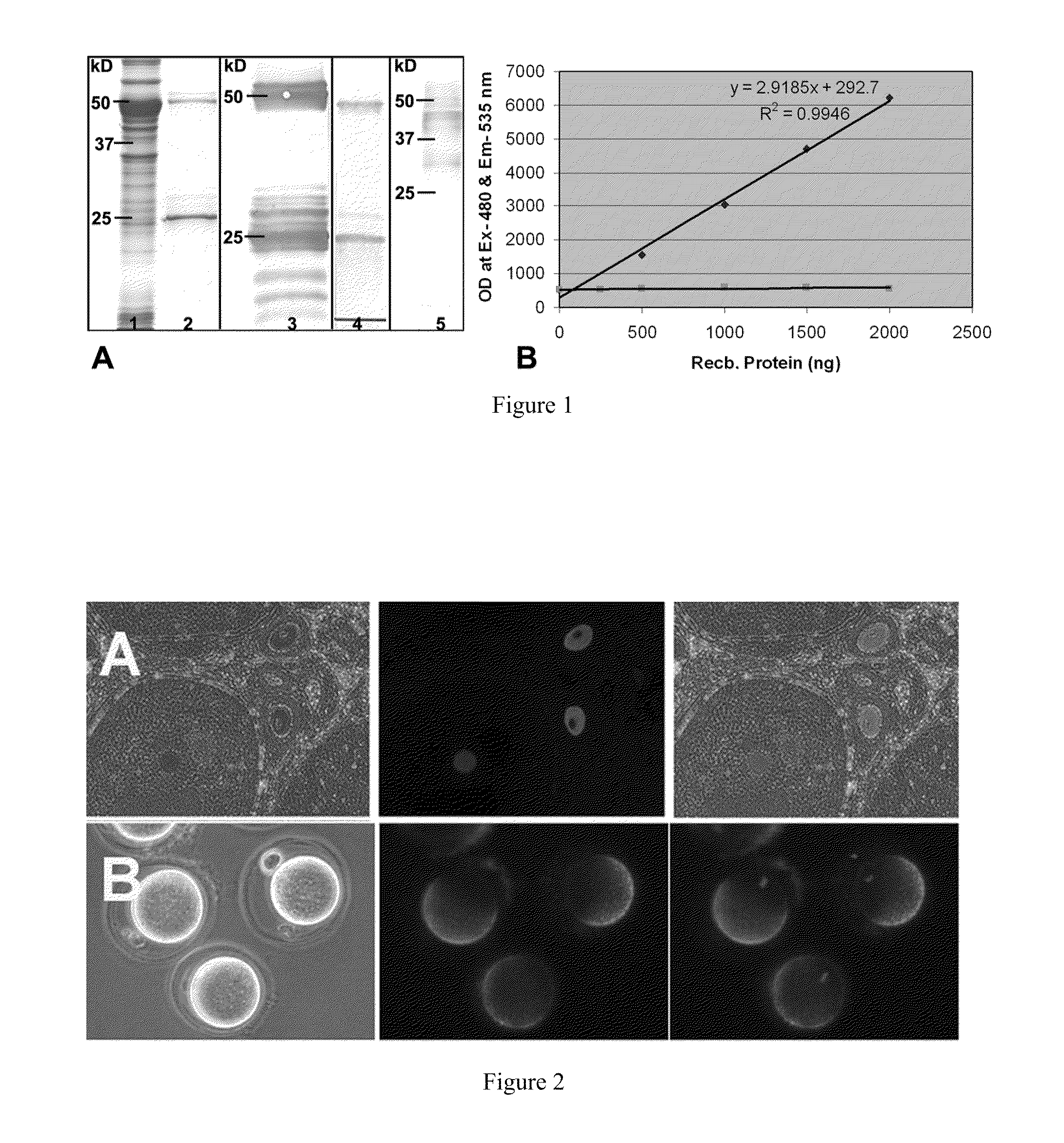
The present invention provides compositions and methods useful for regulating fertilization and for use as a contraceptive based on the discovery herein of an oocyte specific protein, SAS1R (Sperm Acrosomal SLLP1 Receptor), which is a sperm protein receptor. Six SAS1R variants, including the full length SAS1R, were identified. mSLLP1 and SAS1R co-localized to oocytes and to acrosomes of acrosome-reacted sperm. Interactions between mSLLP1 and SAS1R were demonstrated by far-western analysis, in a yeast two-hybrid system under stringent selection conditions, and by immunoprecipitation of SAS1R by anti-mSLLP1 as well as the converse. Purified recombinant SAS1R was found to have protease activity, to inhibit fertilization in-vitro, and to induce an immune response in females. Together, the results suggest SAS1R is a proteolytically active, oocyte and early embryo specific oolemmal metalloprotease receptor for the sperm intra-acrosomal ligand SLLP1 and is a target for regulating fertilization and as a contraceptive.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Novel immunogenic lipopeptides comprising t-helper and b-cell epitopes
InactiveUS20070066534A1Promote maturityEnhance antigen presentationAntibacterial agentsBiocideSynthetic ImmunogensDiamino acid
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Process for preparing conjugate vaccines and the conjugate vaccines
InactiveUS7166708B2Organic active ingredientsHydrolysed protein ingredientsConjugate vaccineDelivery vehicle
This invention relates to a process for preparing a hapten-protein-polysaccharide conjugate and a hapten-protein conjugate by reacting a protein with a hapten to produce a hapten-protein conjugate, followed by reacting the hapten-protein conjugate with a polysaccharide to provide a conjugate mixture including the hapten-protein conjugate and a hapten-protein-polysaccharide conjugate. This invention also includes the process described above with the addition of a pharmaceutically acceptable medium or delivery vehicle into the conjugate mixture. The invention further includes the process described above where the hapten is luteinizing hormone releasing hormone peptides derived from E coil, or malaria derived peptides.
Owner:BIOSYNEXUS INC
Vaccines with enhanced immune response and methods for their preparation
InactiveUS20090074853A1Extended durationEnhance immune responseBacterial antigen ingredientsProtozoa antigen ingredientsEpitopeAdjuvant
The present invention is concerned with vaccines and their preparation. An effective long-term immune response, especially in mammals, can be produced using a vaccine comprising an antigen encapsulated in liposomes, a suitable adjuvant and a carrier comprising a continuous phase of a hydrophobic substance. The vaccine is particularly effective in eliciting the production of antibodies that recognize epitopes of native proteins.
Owner:IMMUNOVACCINE TECH INC
VLP-antigen conjugates and their uses as vaccines
InactiveUS7959928B2Minimizing and avoiding T cell responseStrong immune responseViral antigen ingredientsAntipyreticDiseaseVirus-like particle
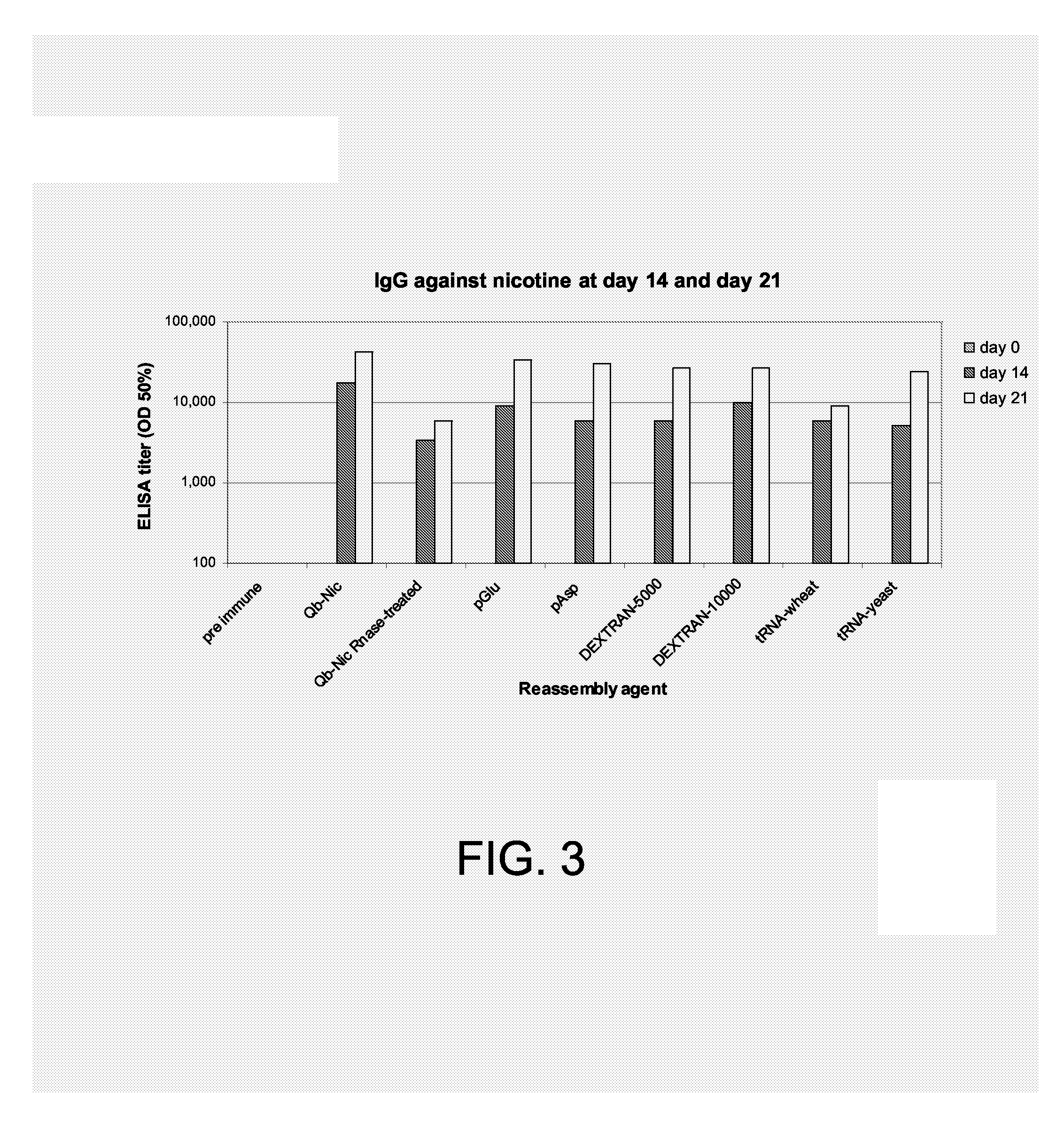
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising a virus-like particle (VLP) of an RNA-bacteriophage and at least one antigen, wherein the VLP is recombinantly produced in a host, and wherein the amount of host RNA with secondary structure comprised by the VLP is at most 20% of the amount of host RNA with secondary structure originally comprised by the VLP; and wherein the VLP and the at least one antigen are linked with one another. The invention also provides methods for producing the compositions of the invention. The compositions of the invention are useful in the production of vaccines for the treatment of diseases, disorders and conditions. Furthermore, the compositions of the invention are particularly useful to efficiently induce strong antibody responses against the antigen within the indicated context while lowering or eliminating unwanted T cell responses.
Owner:CYTOS BIOTECHNOLOGY AG
Carrier conjugates of gnrh-peptides
InactiveUS20090028886A1Lower Level RequirementsStrong responseVaccinesContraceptive vaccin ingredientsDiseaseVirus-like particle
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising a virus like particle (VLP) and at least one GnRH peptide or fragment or variant thereof linked thereto.The invention also provides a process for producing the composition. The compositions of the invention are useful in the production of vaccines for the treatment of GnRH-related diseases and conditions and to efficiently induce immune responses, in particular antibody responses. Furthermore, the compositions of the invention are particularly useful to efficiently induce self-specific immune responses within the indicated context.
Owner:CYTOS BIOTECHNOLOGY AG
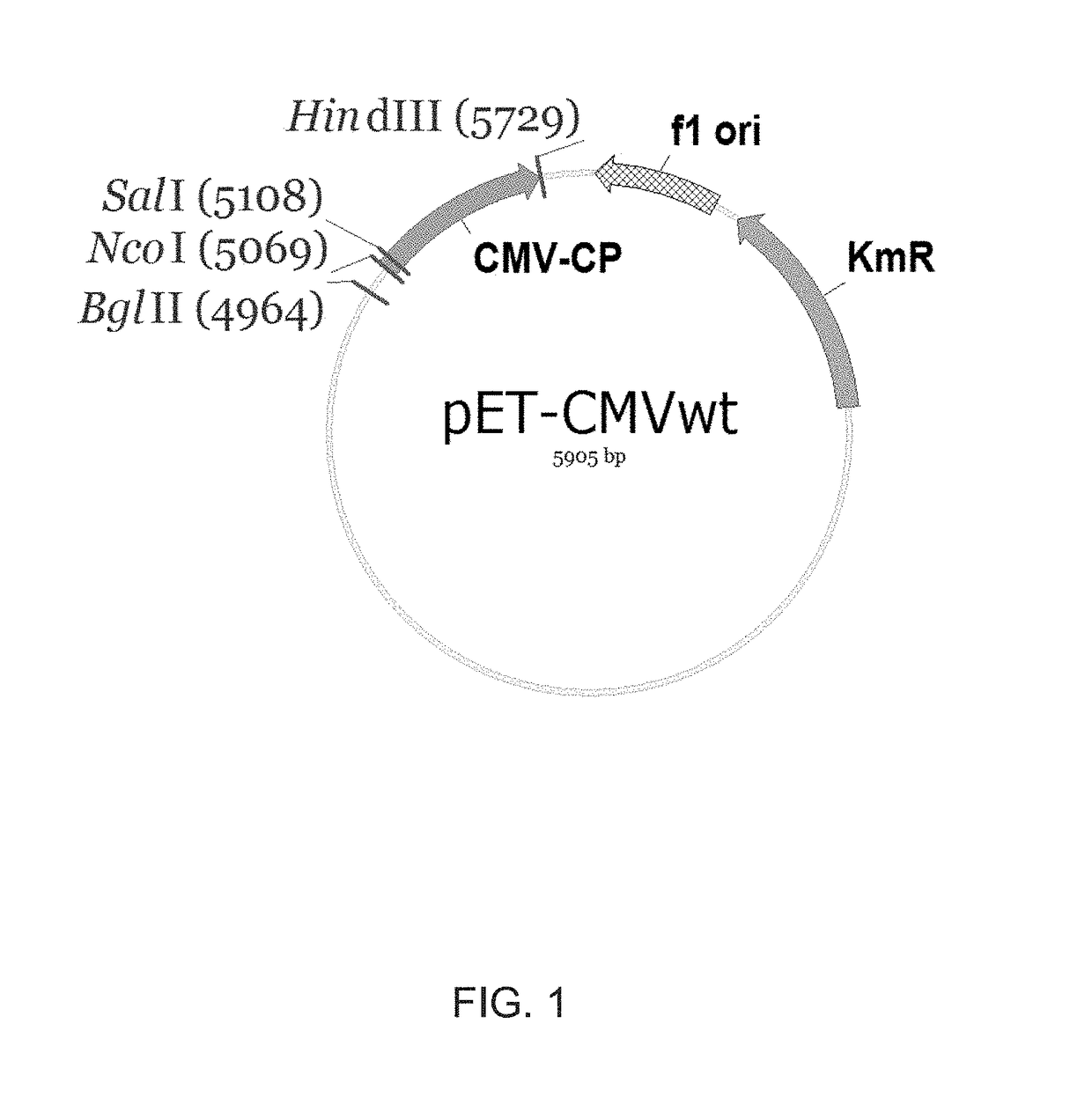
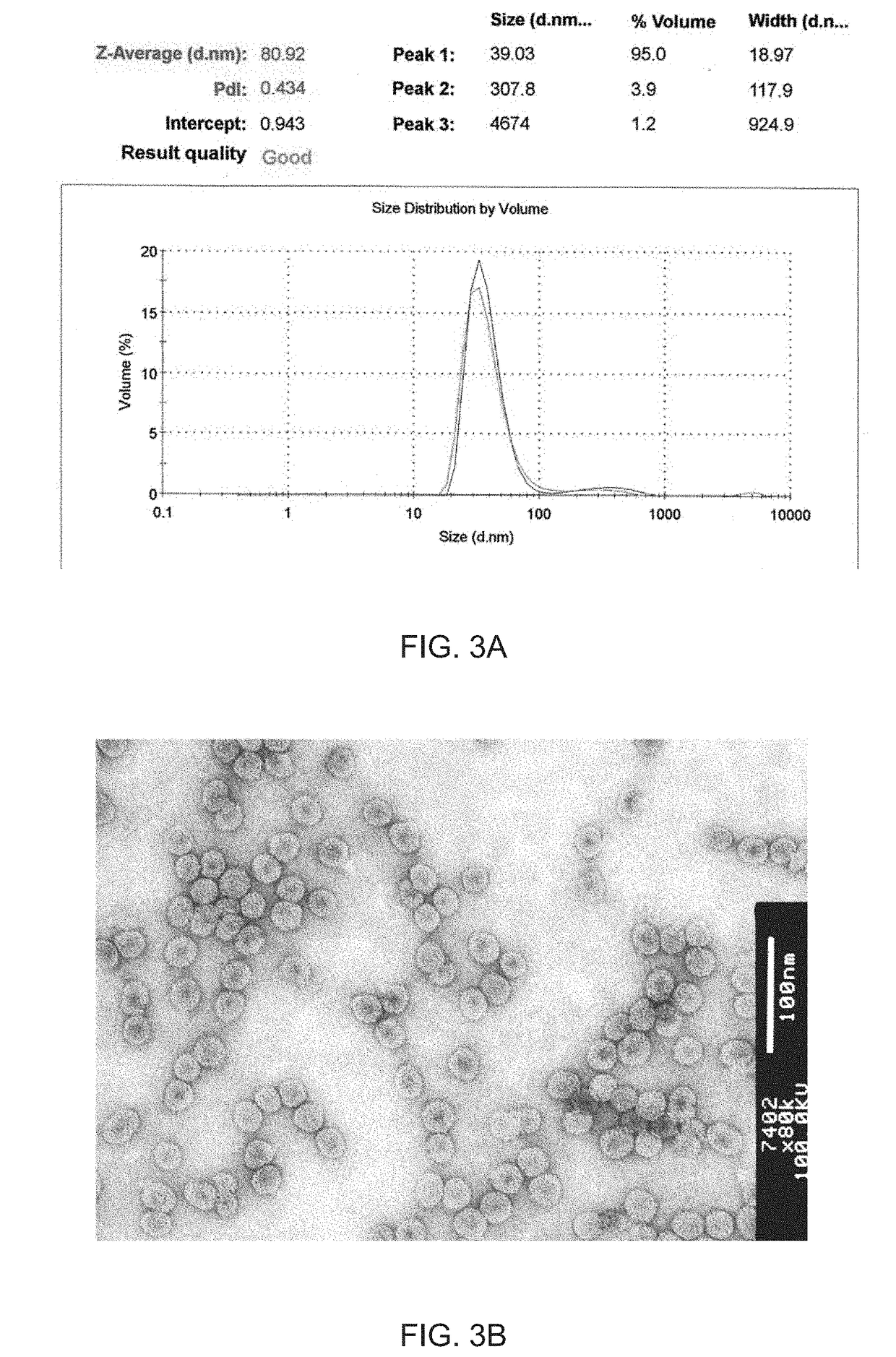
Modified virus-like particles of cmv
ActiveUS20170312371A1Hinder self-assemblyImproving immunogenicitySsRNA viruses negative-senseNervous disorderEpitopeVirus-like particle
The present invention relates to virus-like particles of plant virus Cucumber Mosaic Virus (CMV), and in particular to modified VLPs of CMV comprising Th cell epitopes, in particular universal Th cell epitopes. Furthermore, these modified VLPs serve as, preferably, vaccine platform, for generating immune responses, in particular antibody responses, against antigens linked to said modified VLPs. The presence of the Th cell epitopes, in particular universal Th cell epitopes, led to a further increase in the generated immune response.
Owner:SAIBA AG
Heat shock fusion-based vaccine system
InactiveUS20060121051A1Lower Level RequirementsIncrease ratingsPeptide/protein ingredientsTumor necrosis factorEpitopeHeat shock
Disclosed are self-epitope-containing heat shock fusion proteins, DNA constructs encoding such fusion proteins, and methods of use. More specifically, disclosed are ubiquitin fusion proteins comprising ubiquitin fused to a plurality of identical or non-identical self-epitopes at specified locations. Immunization of an animal with these ubiquitin fusion proteins elicits an immune response to self-antigens present on endogenous proteins. Generation of an immune response to a specified self-antigen is a mechanism to decrease the levels of the endogenous protein below base-line.
Owner:WELLSTAT BIOCATALYSIS
Process for preparing conjugate vaccines including free protein and the conjugate vaccines, immunogens, and immunogenic reagents produced by this process
InactiveUS20050074460A1Organic active ingredientsHydrolysed protein ingredientsConjugate vaccineFree protein
A process for preparing a protein-polysaccharide conjugate includes reacting a protein with a polysaccharide to produce a mixture including a protein-polysaccharide conjugate and free protein. At least one unreacted reagent or low molecular weight component is removed from this mixture, without removing all of the free protein, to provide a purified mixture that contains the protein-polysaccharide conjugate and free protein. This purified mixture can be used as a conjugate vaccine, immunogen, or immunological reagent. Keeping the free protein in the purified mixture with the conjugate saves time and money in the conjugate production process. In another aspect of the invention, the purified mixture of the protein-polysaccharide conjugate and free protein is reacted with a hapten to produce a conjugate mixture including a hapten-protein conjugate and a hapten-protein-polysaccharide conjugate. Alternatively, the hapten-protein conjugate can be prepared first, this conjugate then reacting with a polysaccharide reagent to produce the conjugate mixture. This conjugate mixture can be treated further to remove the free hapten. The conjugate mixture, including the hapten-protein-polysaccharide conjugate and the hapten-protein conjugate, also can be used as a conjugate vaccine, immunogen, or immunological reagent.
Owner:BIOSYNEXUS INC
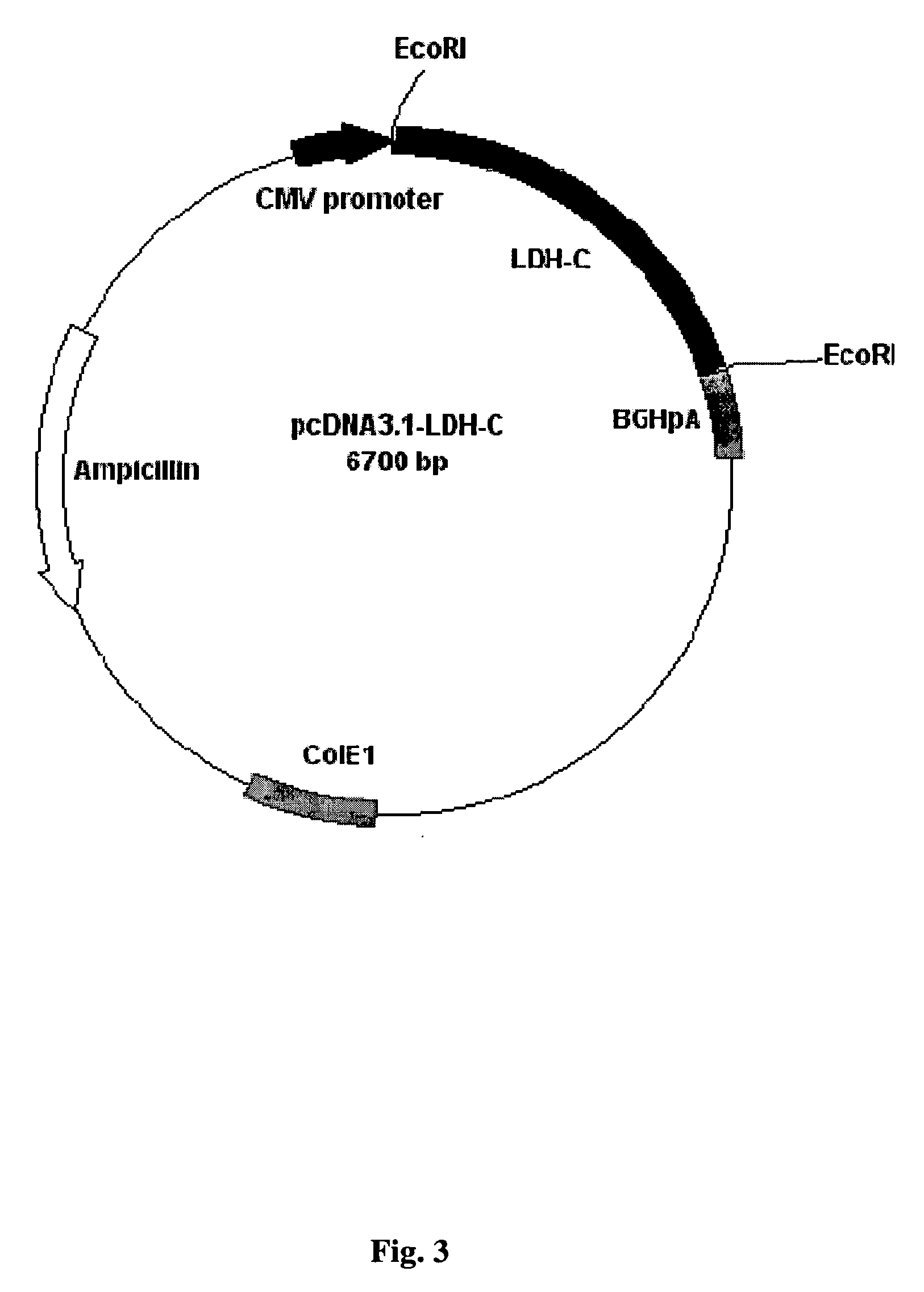
DNA immunocontraceptive vaccines and uses thereof
InactiveUS20050191318A1Increased toxicityEliminate useBacterial antigen ingredientsBacteriaPopulation sizeSperm specific protein
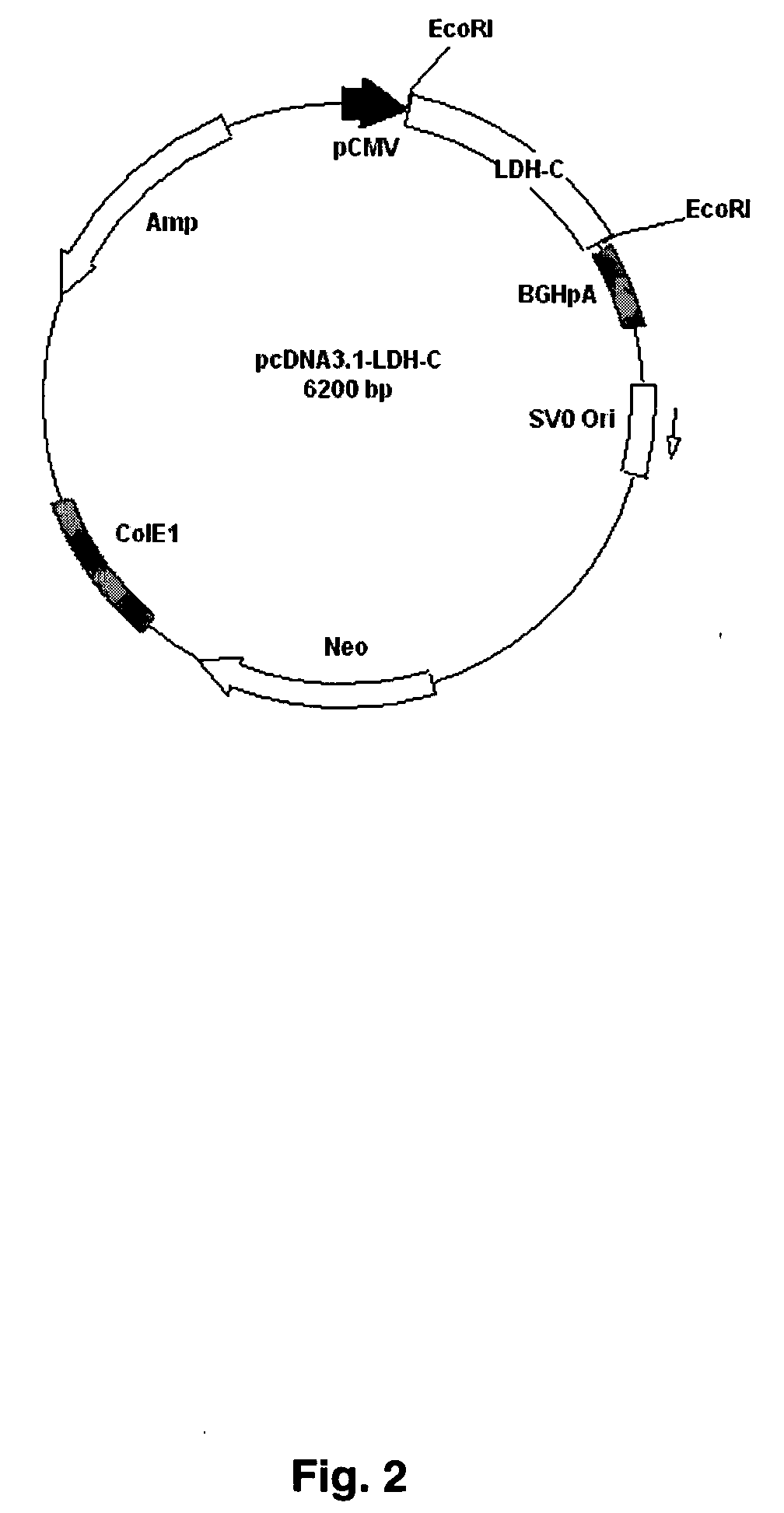
The present invention provides an immunocontraceptive vaccine comprising a recombinant bacterial host that has been modified to produce egg- or sperm-specific polypeptide such as the sperm-specific protein lactate dehydrogenase-C (LDH-C). When animals such as rodents eat these bacteria, their immune systems produce antibodies that attack their sperms. Not only would the males have less viable sperm, the females would also have antibodies to sperm entering their reproductive systems. Immunocontraception is an attractive method for reducing the population size of animals with high fecundity, and sterilizing animals using such immunocontraceptives can reduce targeted animal populations to acceptable levels in an efficient, cost-effective, humane and, importantly, a species-specific manner.
Owner:RES DEVMENT FOUND
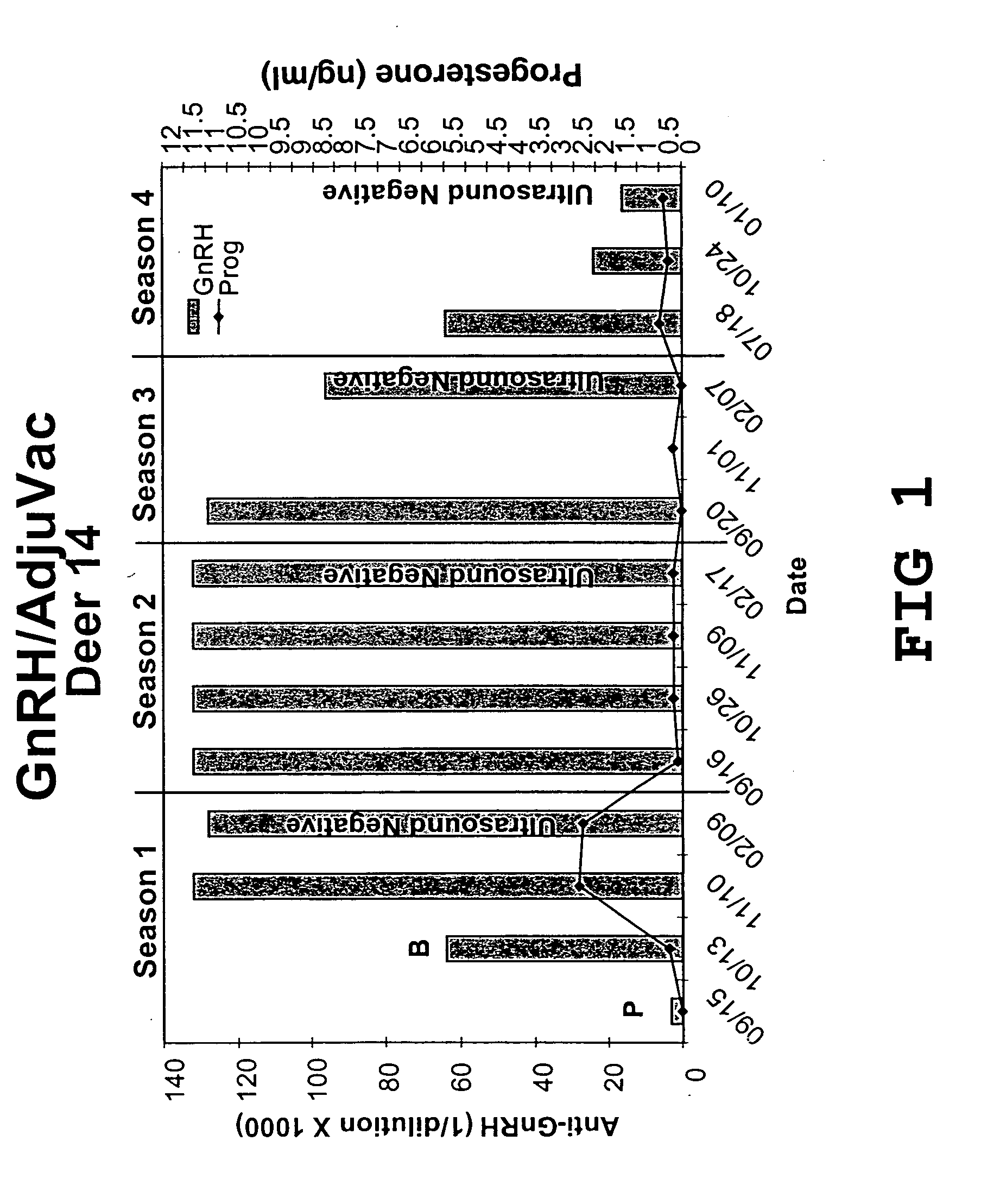
Pharmaceutical composition using gonadotropin - releasing hormone (GNRH) combined variants as immunogen
InactiveUS20110250196A1Faster and more potent immunological responseVigorous immunocastration actionPeptide/protein ingredientsDigestive systemHuman tumorHuman fertility
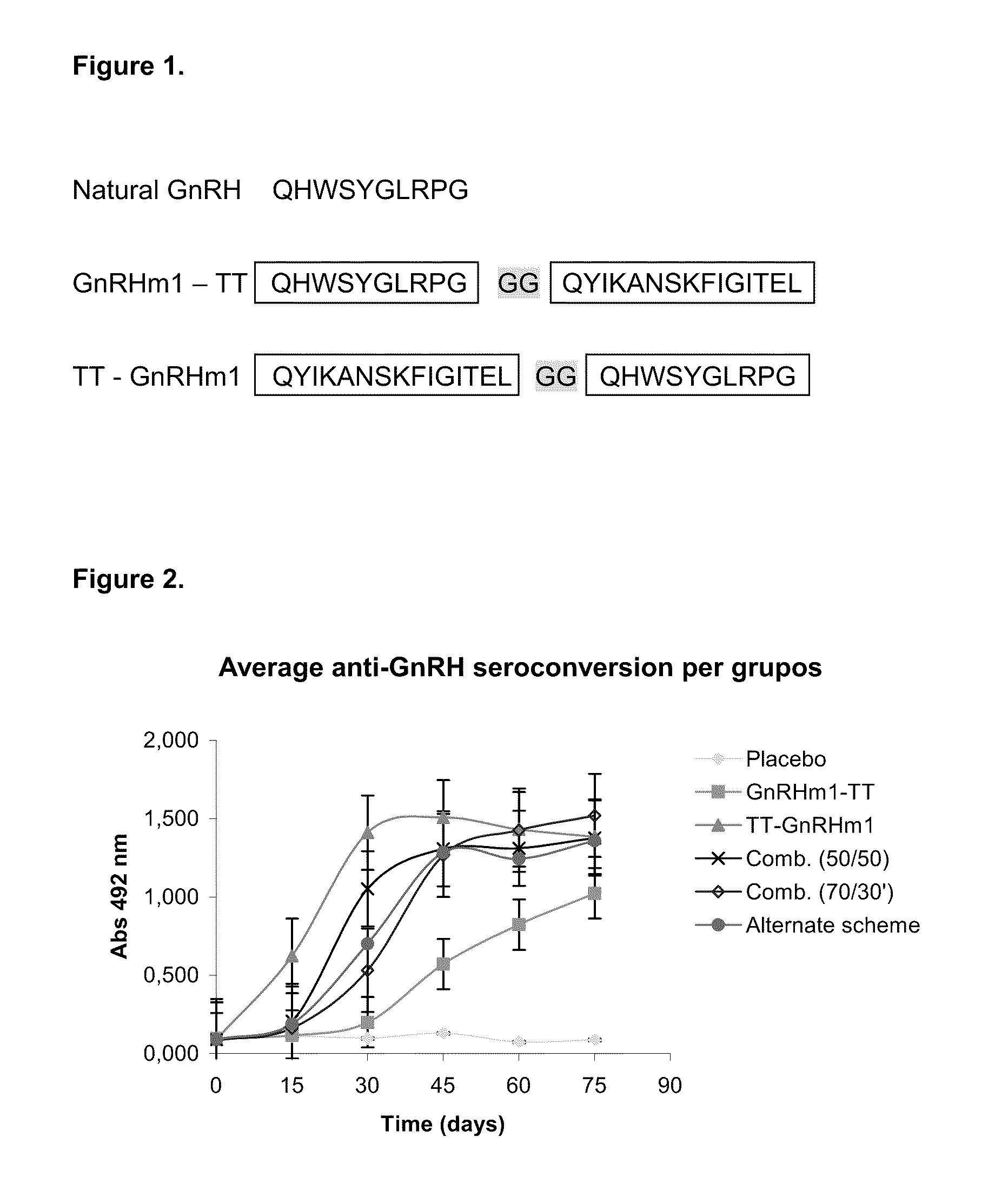
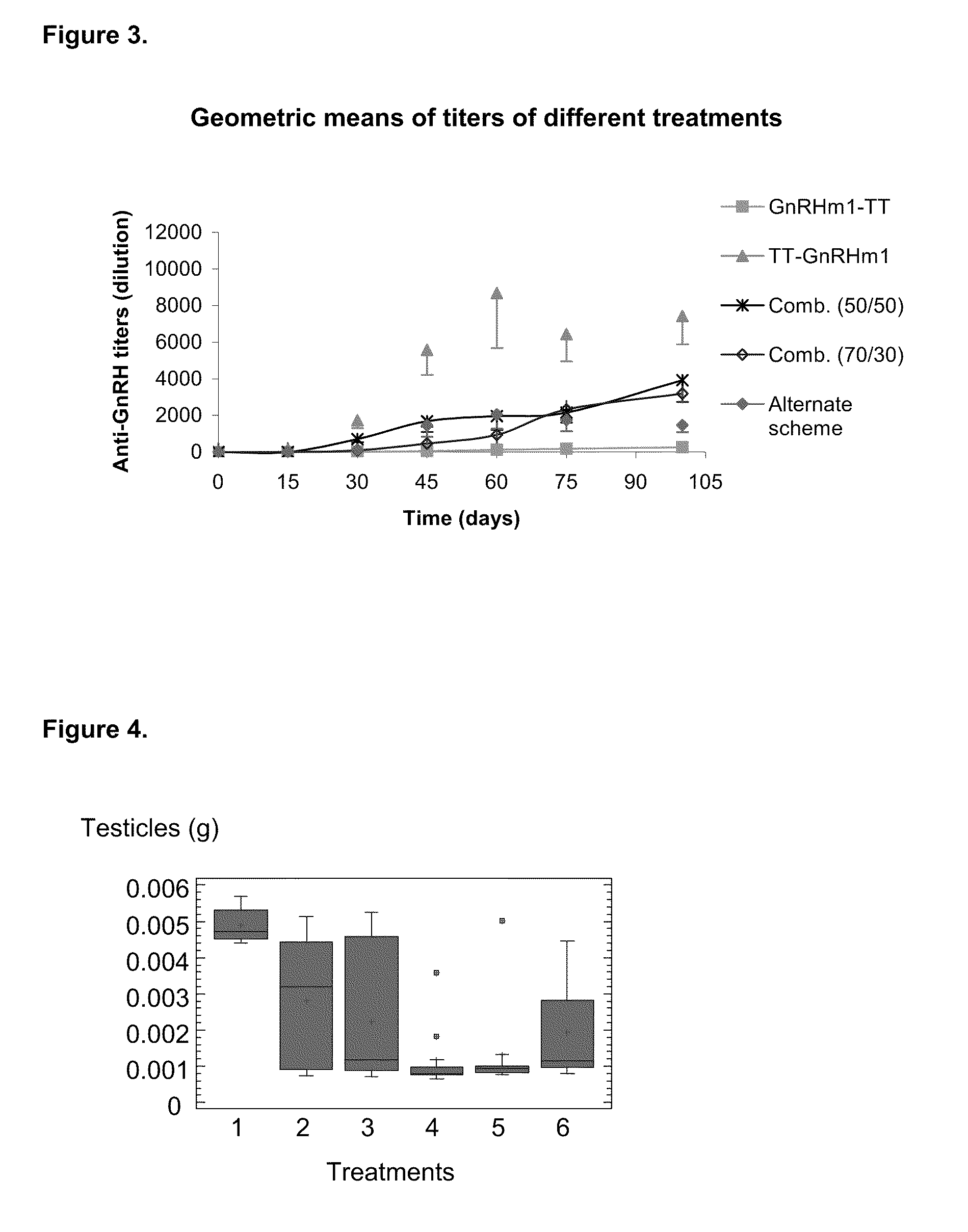
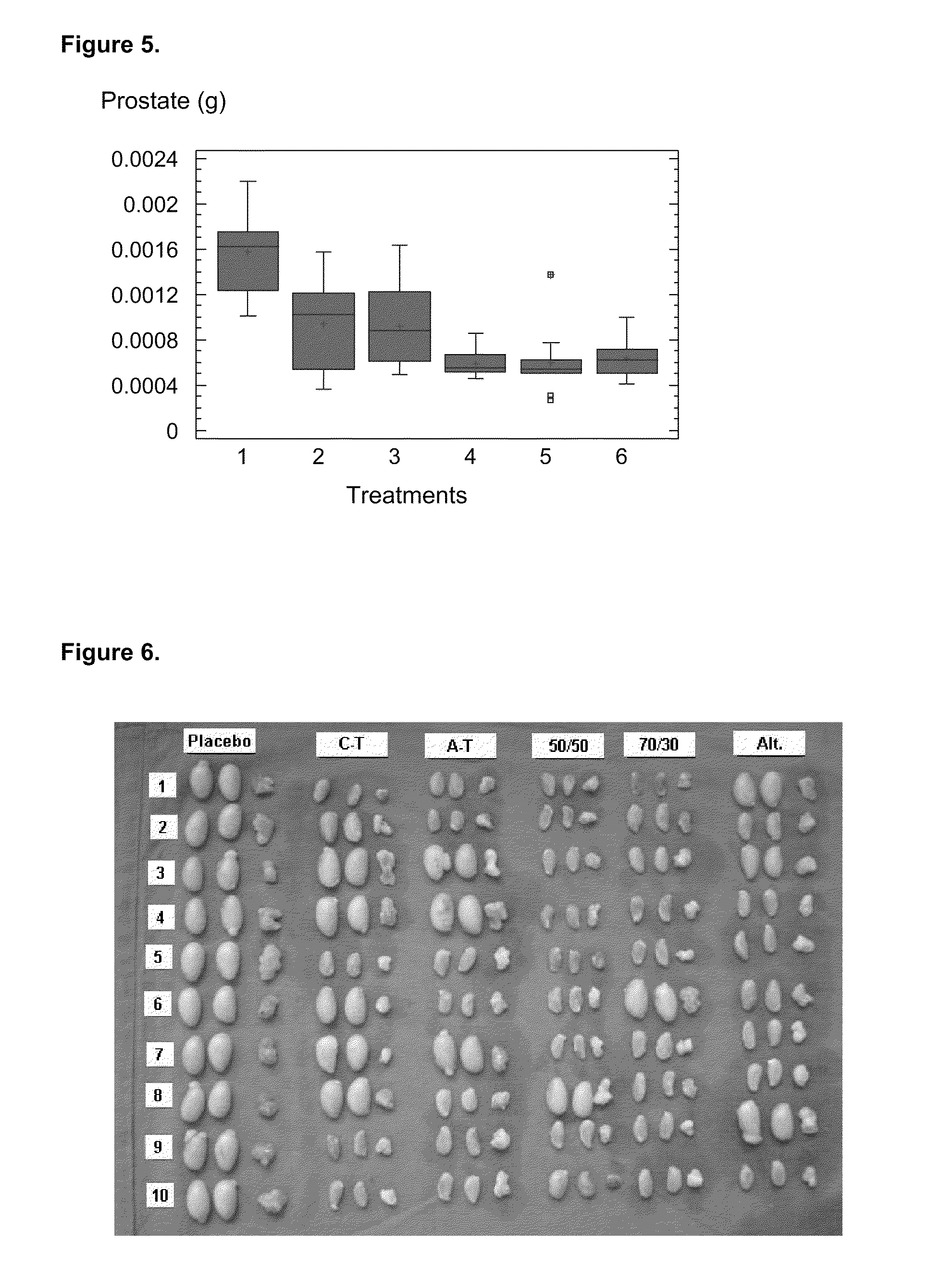
A pharmaceutical composition using natural gonadotropin-releasing hormone (GnRH), and / or some of its mimetic peptides, indistinctly bound by its amino or carboxyl extremes to a carrier molecule; in one case by its carboxyl extreme and in the other case by the amino terminal extreme, thus eliciting a faster and more potent immunological response against the endogenous GnRH hormone. This finally leads to the ablation of the GnRH and consequently of the rest of the involved hormones in the stream GnRH / LH-FSH / Testosterone-(estrogens). An advantage of this formulation consists on facilitating the exposition to the immune system of a greater number of epitopes of the GnRH or its mimetics, minimizing thus the steric hindrance produced by the carriers. This invention has a direct application in the castration of pets and animals of economic interest, in the control of human fertility as well as in the treatment of hormone-sensitive tumors, such as that of the prostate, the breast, ovary, the endometry, testicles, hypophysis, salivary glands and other kinds of human tumors.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Viral vaccine composition, process and methods of use
InactiveUS20090226489A1Regain healthAvoid infectionSsRNA viruses negative-senseBacterial antigen ingredientsInfected cellInduced infections
A composition for treating or preventing virus-induced infections is described, along with a process of producing the composition and methods of the composition's use. The composition comprises viral pathogen-infected cell or tissue, or malignantly or immunologically aberrant cells or tissues which has been reduced and / or denatured. The preferred composition is administered across a mucosal surface of an animal suffering or about suffer from infection. The composition is administered as preventive or therapeutic vaccine.
Owner:IMMUNITOR USA
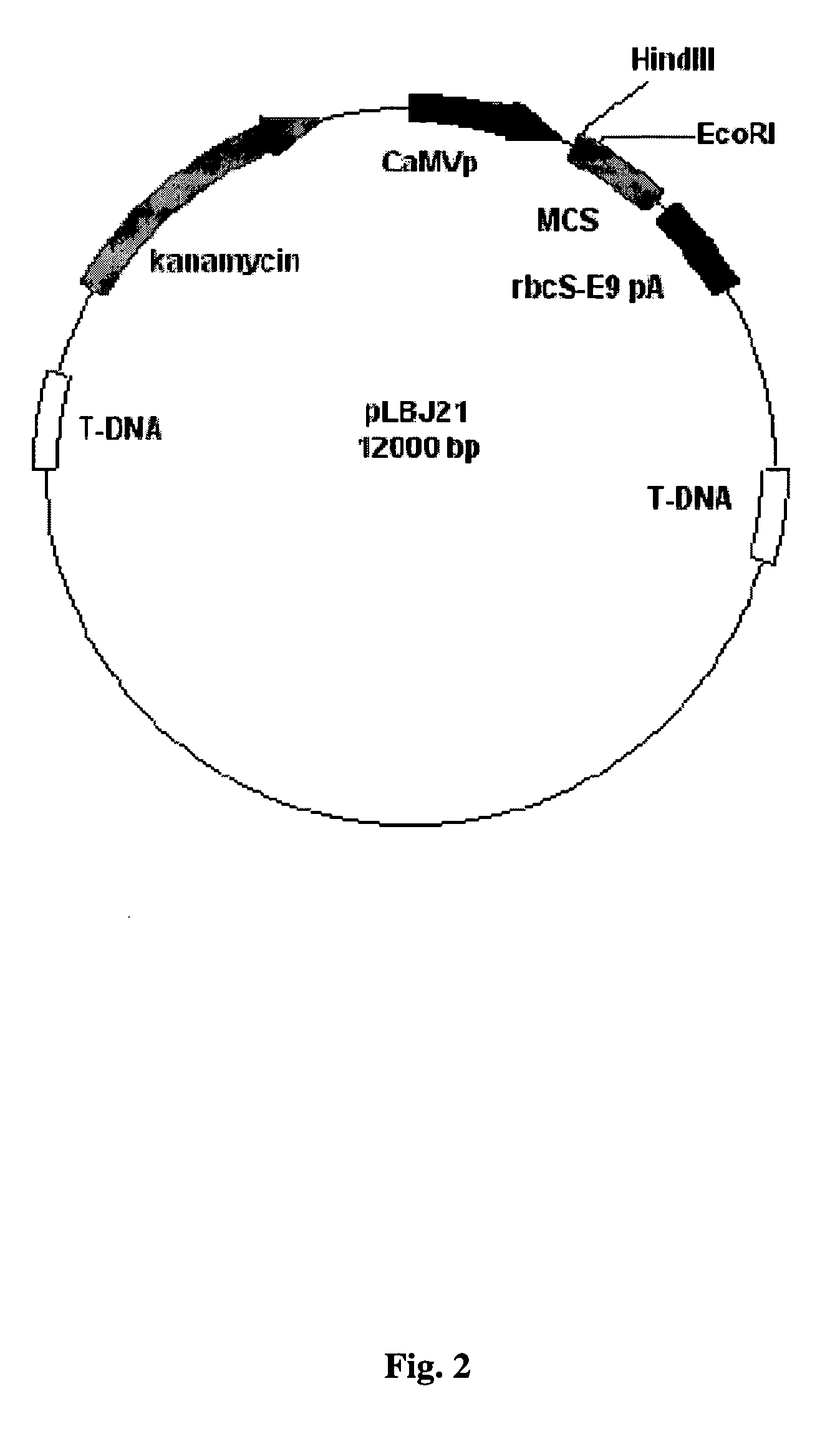
Animal immunocontraceptives expressed in plants and uses thereof
InactiveUS20050009188A1Effective and humaneSmall sizeVaccinesContraceptive vaccin ingredientsAnimal useEngineered genetic
The present invention provides immunocontraceptive vaccines comprising a genetically engineered plant that has been modified to produce the sperm-specific protein lactate dehydrogenase-C (LDH-C). When animals such as rodents eat this plant, their immune systems produce antibodies that attack their sperms. Not only would the males have less viable sperm, the females would also have antibodies to sperm entering their reproductive systems. Immunocontraception is an attractive method for reducing the population size of animals with high fecundity, and sterilizing animals using such immunocontraceptives can reduce targeted animal populations to acceptable levels in an efficient, cost-effective, humane and, importantly, a species-specific manner.
Owner:RES DEVMENT FOUND
Viral vaccine composition, process and methods of use
InactiveUS7838006B2Minimize changesSsRNA viruses negative-senseBacterial antigen ingredientsInfected cellInduced infections
A composition for treating or preventing virus-induced infections is described, along with a process of producing the composition and methods of the composition's use. The composition comprises viral pathogen-infected cell or tissue, or malignantly or immunologically aberrant cells or tissues which has been reduced and / or denatured. The preferred composition is administered across a mucosal surface of an animal suffering or about suffer from infection. The composition is administered as preventive or therapeutic vaccine.
Owner:IMMUNITOR USA
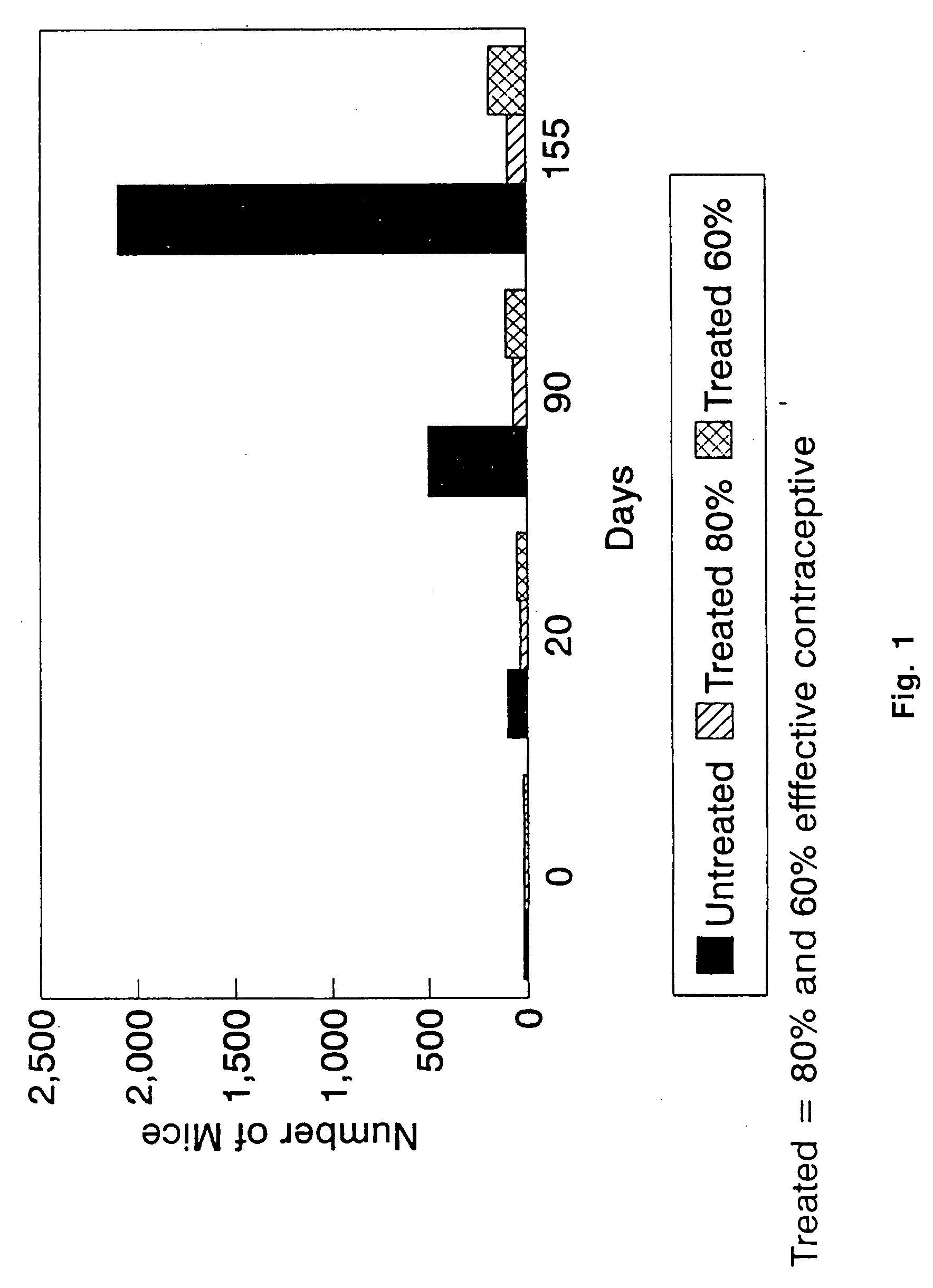
Saponin adjuvant compositions and methods relating thereto
InactiveUS9149520B2High activityLow reactogenicityVaccinesContraceptive vaccin ingredientsAdjuvantImmunostimulating Complexes
An adjuvant composition which comprises an anionic macromolecule component particularly an ionic polysaccharide such as DEAE-dextran, and a saponin component, particularly an immunostimulating complex component. Immunogenic compositions comprising an immunogen and this adjuvant composition are also disclosed together with methods of use thereof.
Owner:ZOETIS SERVICE LLC
Vaccine compositions and adjuvant
InactiveUS20060013821A1Enhance immune responseIncrease heightBacterial antigen ingredientsSnake antigen ingredientsAdjuvantMycobacterium
The immune response of an animal to a target immunogen may be enhanced by use of a novel adjuvant which includes low concentrations of killed cells of Mycobacterium avium subspecies avium in combination with mineral oil. The adjuvant may be used in vaccine compositions for the immunization of an animal against any target immunogen, and is particularly preferred for use with immunocontraceptive vaccines such as GnRH and PZP immunocontraceptive vaccines.
Owner:MILLER LOWELL +1
Vlp-Antigen Conjugates and Their Uses as Vaccines
InactiveUS20080095738A1Minimizing and avoiding unwanted and other side effectStrong immune responseViral antigen ingredientsAntipyreticDiseaseVirus-like particle
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising a virus-like particle (VLP) of an RNA-bacteriophage and at least one antigen, wherein the VLP is recombinantly produced in a host, and wherein the amount of host RNA with secondary structure comprised by the VLP is at most 20% of the amount of host RNA with secondary structure originally comprised by the VLP; and wherein the VLP and the at least one antigen are linked with one another. The invention also provides methods for producing the compositions of the invention. The compositions of the invention are useful in the production of vaccines for the treatment of diseases, disorders and conditions. Furthermore, the compositions of the invention are particularly useful to efficiently induce strong antibody responses against the antigen within the indicated context while lowering or eliminating unwanted T cell responses.
Owner:CYTOS BIOTECHNOLOGY AG
Chimeric peptide immunogens
InactiveUS20050106137A1Suppresses antibody responseSuppressing antibody responseAntibody mimetics/scaffoldsVaccinesTetanus toxoidsMalaria
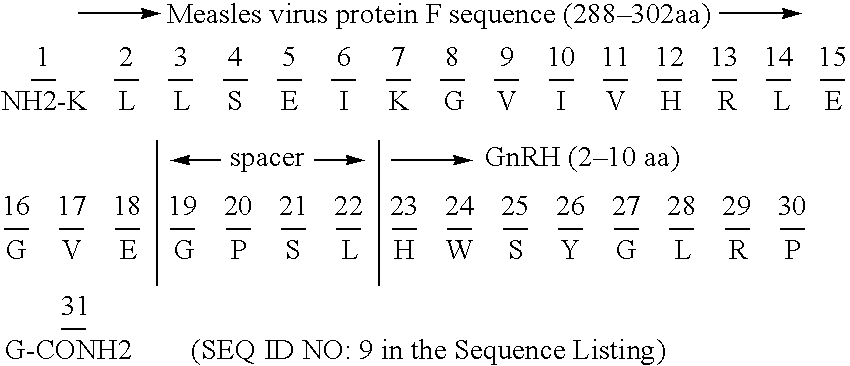
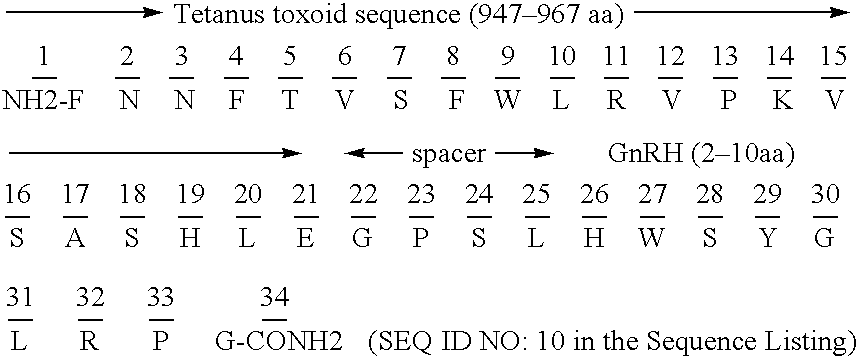
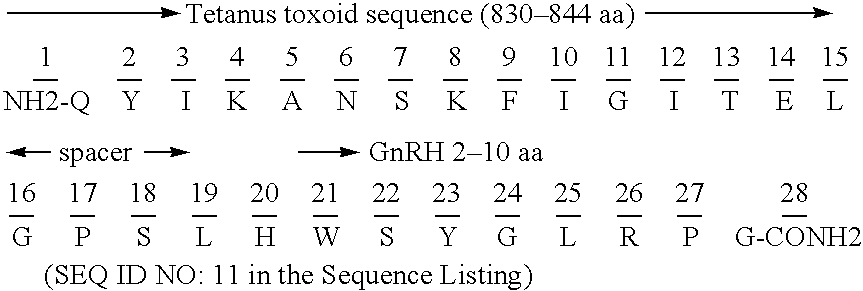
Chimeric peptide epitopes can serve as effective immunogens against hormones and other small peptides or proteins. Immunogenic peptides are selected from promiscuous helper T-lymphocyte epitopes and synthesized together with self antigenic peptide sequences optionally fused through a spacer moiety. Examples of the chimeric peptide immunogens of the invention include peptide sequences which may be from any antigen, such as a gonadotropin releasing hormone (GnRH), linked with an immunogenic peptide sequence such as a promiscuous helper T-lymphocyte epitope of measles virus protein F, tetanus toxoid, or malaria protein CSP. Compositions of the chimeric immunogen are found effective in eliciting high and specific anti-GnRH antibody titers. This invention also relates to compositions and methods for synergistically enhancing or suppressing an immune response to a target antigen.
Owner:APHTON
Vaccine conjugate including a human chorionic gonadotropin beta subunit antigen linked to an anti-mannose receptor (mr) antibody
InactiveCN101039700AAntibacterial agentsAntibody mimetics/scaffoldsAntigenHuman Chorionic Gonadotropin Beta Subunit
The present invention provides novel antibody vaccine conjugates and methods of using the same to induce a cytotoxic T cell (CTL) response. In a particular, embodiment, the vaccine conjugate includes a human chorionic gonadotropin beta subunit (betahCG) antigen linked to an anti-mannose receptor (MR) antibody.
Owner:CELLDEX THERAPEUTICS INC
Modified virus-like particles of CMV
ActiveUS10532107B2Hinder self-assemblyImproving immunogenicitySsRNA viruses negative-senseSsRNA viruses positive-senseEpitopeVirus-like particle
The present invention relates to virus-like particles of plant virus Cucumber Mosaic Virus (CMV), and in particular to modified VLPs of CMV comprising Th cell epitopes, in particular universal Th cell epitopes. Furthermore, these modified VLPs serve as, preferably, vaccine platform, for generating immune responses, in particular antibody responses, against antigens linked to said modified VLPs. The presence of the Th cell epitopes, in particular universal Th cell epitopes, led to a further increase in the generated immune response.
Owner:SAIBA AG
Chorionic gonadotropin DNA vaccines and methods
InactiveUS20060121010A1Elicit immune responseInhibit progressOrganic active ingredientsBiocideEpitopeChorionic gonadotropins
The invention relates to immunotherapy of a mammalian subject by exposing the immune response cells of the subject to a nucleic acid construct encoding at least one hCG immunogenic epitope or precursor thereof such that the nucleic acid construct is taken up and processed by the immune response cells. The invention further relates to compositions comprising such hCG-encoding nucleic acid constructs.
Owner:AVI BIOPHARMA
Modified coiled coil type proteins having improved properties
ActiveCN104981541AImproving immunogenicityAntibacterial agentsSsRNA viruses negative-senseArginineCarrier protein
The present application is related to a modified protein comprising a protein having a coiled coil domain and a peptide having the sequence such as shown in SEQ ID NO 1: ZXBBBBZ that is linked to the coiled coil domain wherein: Z is any amino acid or is absent; X is any amino acid; B is an arginine (R) or a lysine (K). Said modified protein is in particular an antigen or a carrier protein, associated to an antigen. This modified protein has an increased affinity for negatively charged polymers such as nucleic acids or heparin, and shows an increased immunogenicity.
Owner:OSIVAKOS CORP
Placenta-like chondroitin sulfate A immunization composition and application
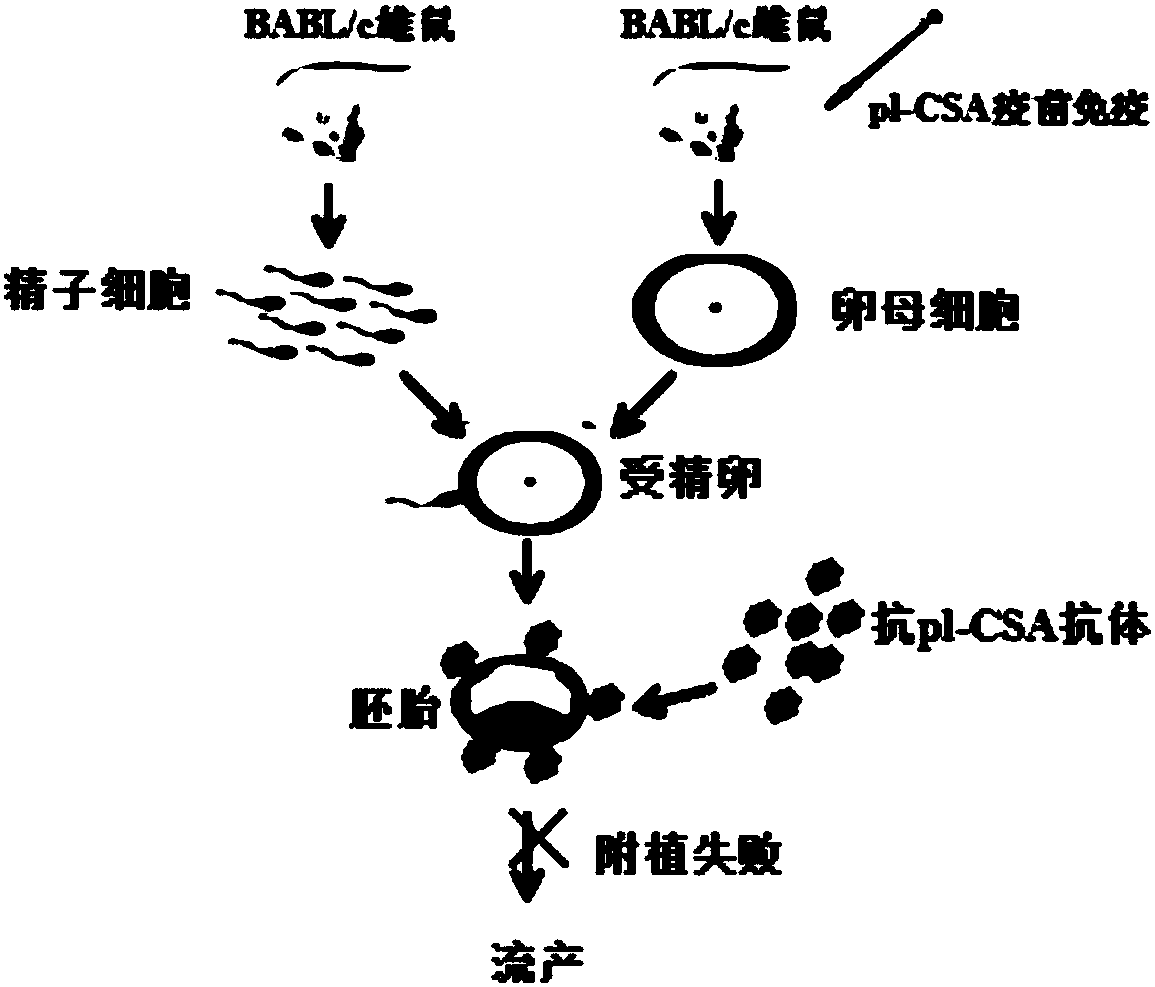
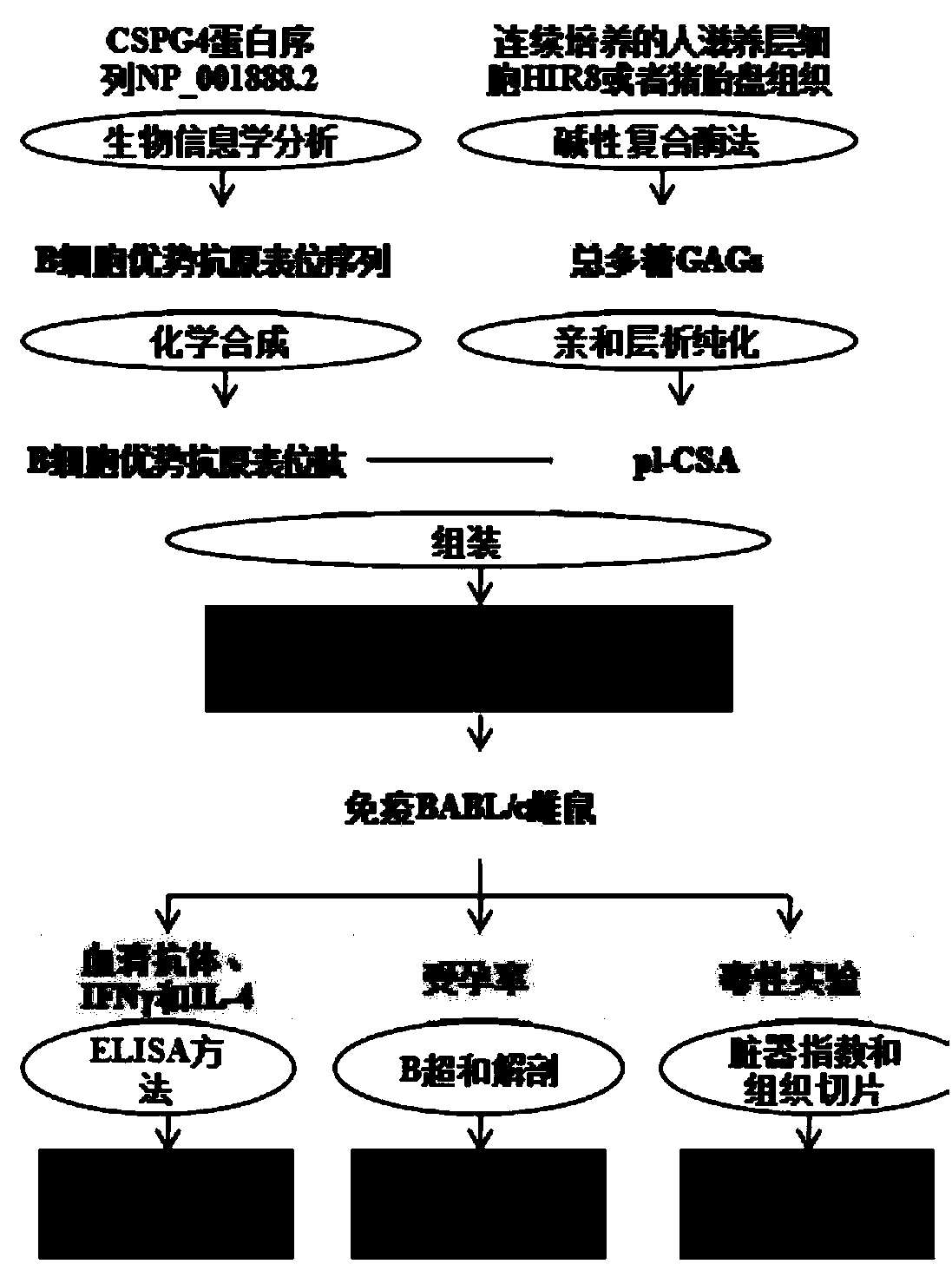
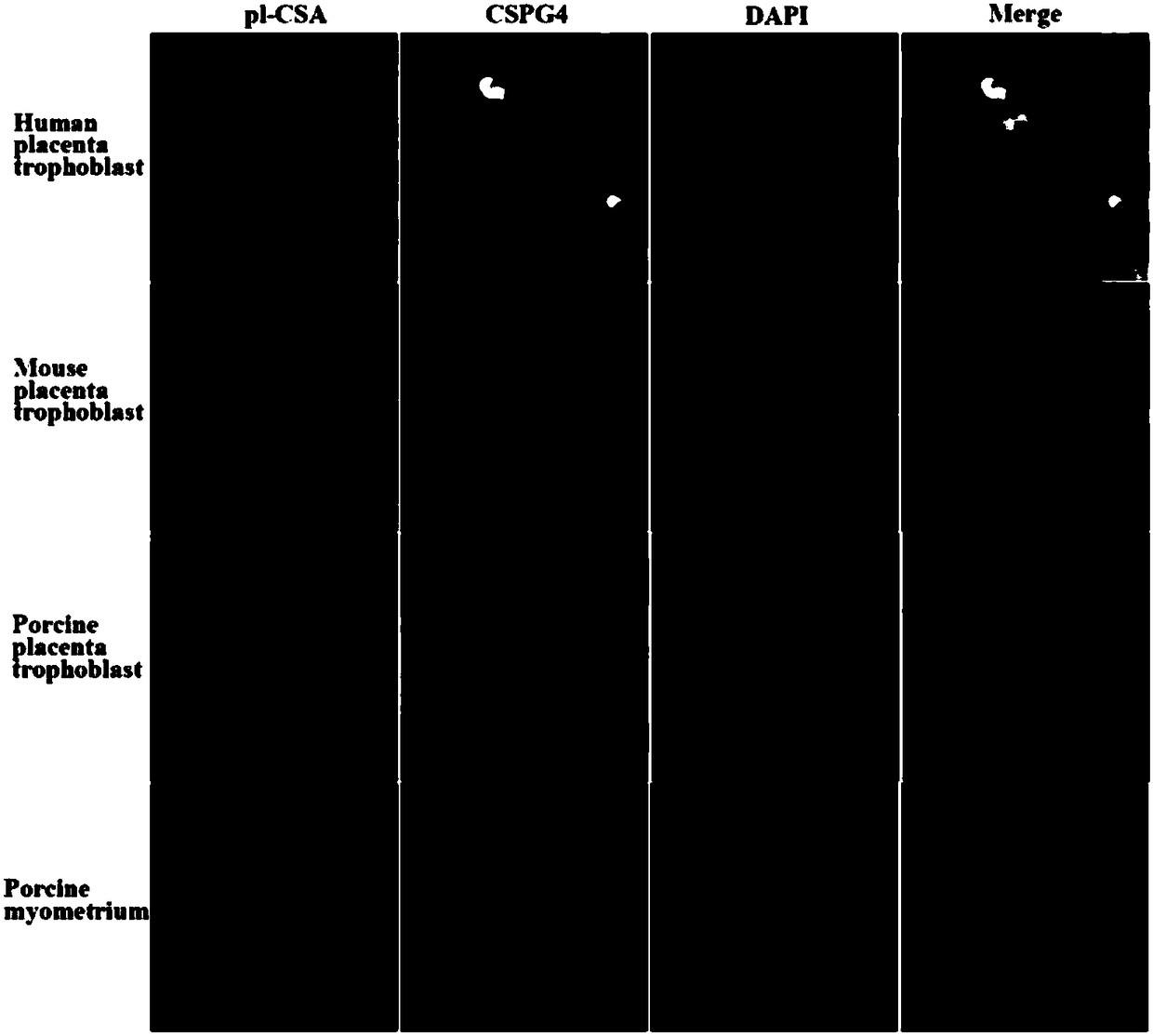
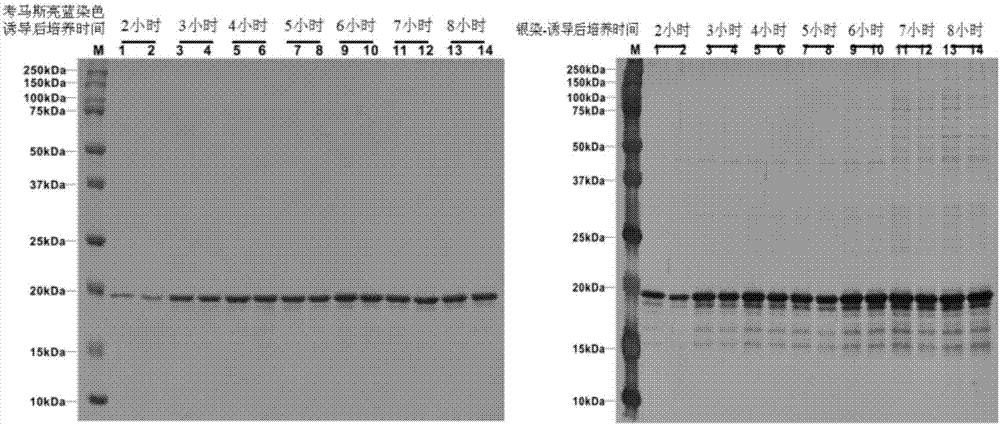
ActiveCN108567973AIncreased sensitivityImprove effectivenessContraceptive vaccin ingredientsWhole-cell/virus/DNA/RNA ingredientsB cellCytotrophoblast
The invention relates to a placenta-like chondroitin sulfate A immunization composition and application, and particularly discloses a contraceptive immunization composition. The placenta-like chondroitin sulfate A immunization composition comprises at least one type of placenta trophoblast cell specific expression polysaccharides and / or protein antigens and B cell dominant antigen epitopes of placenta-like chondroitin sulfate A core proteins CSPG4. The embryo or placenta trophoblast cell specific expression polysaccharides are selected from placenta-like chondroitin sulfate A. The placenta-like chondroitin sulfate A immunization composition and the application have the advantages that the opinion that implantation execution critical cytotrophoblast cells are targeted by contraceptive vaccine for early implantation which is a placentation and embryogenesis critical and sensitive period is proposed for the first time, and accordingly the contraception sensitivity and effectiveness can beimproved.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Chicken luteinizing hormone releasing hormone recombinant antigen castrating vaccine and preparation method therefor
InactiveCN107880135AHigh purityAvoid the hassle of purificationPolypeptide with localisation/targeting motifVaccinesEscherichia coliEnzyme digestion
The invention provides a chicken luteinizing hormone releasing hormone recombinant antigen castrating vaccine and a preparation method therefor. The method comprises the steps: (1) designing a cLHRH gene; (2) subjecting a designed gene sequence to synthesis, annealing, enzyme digestion and connecting, and then, transferring the gene sequence into an Escherichia coli competent cell, so as to obtaina recombinant plasmid; (3) preparing an original bacterial solution; (4) carrying out lactose induction; (5) carrying out low-temperature osmotic shock separation and purification; (6) carrying out hydroformylation by adopting 10*hydroformylation brine; and (7) carrying out vaccine preparation. The preparation method is simple, the cost is low, the whole preparation process is free of any substance which is potentially toxic and harmful to animals or is difficult to degrade, and the prepared vaccine has a good castrating effect and is convenient to use and good in stability.
Owner:SICHUAN HUAPAI BIO PHARMA
Truncated LHRH formulations
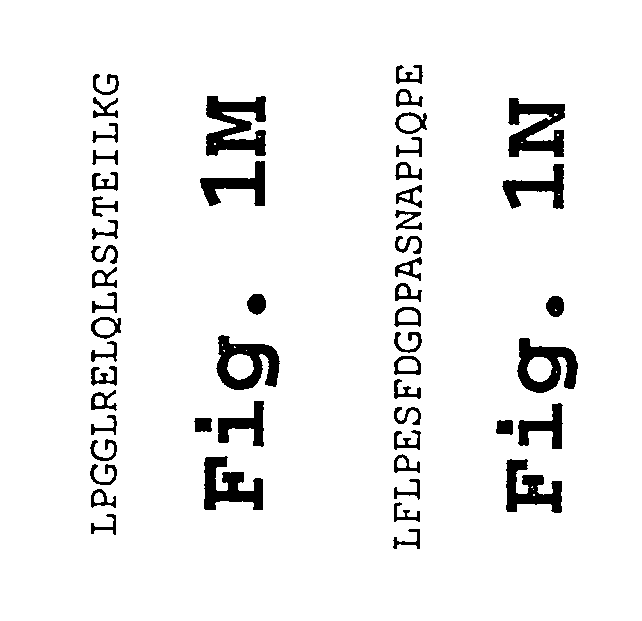
The present invention provides a peptide useful for raising an antiLHRH response in an animal. The peptide comprises a first and second region, the first region consisting of a sequence of less than 60 amino acids which comprises at least one T helper cell epitope and the second region consisting of the sequence SYGLRPG.
Owner:昆士兰医学研究所理事会(QIMR)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com