Placenta-like chondroitin sulfate A immunization composition and application
An immune composition and placenta technology, applied in the directions of drug combinations, vaccines, contraceptive vaccine components, etc., can solve the problems of inability to produce antibody titers, affect the normal function of the body, affect the contraceptive effect, etc., and achieve increased contraceptive effect, rich content, The effect of structural stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
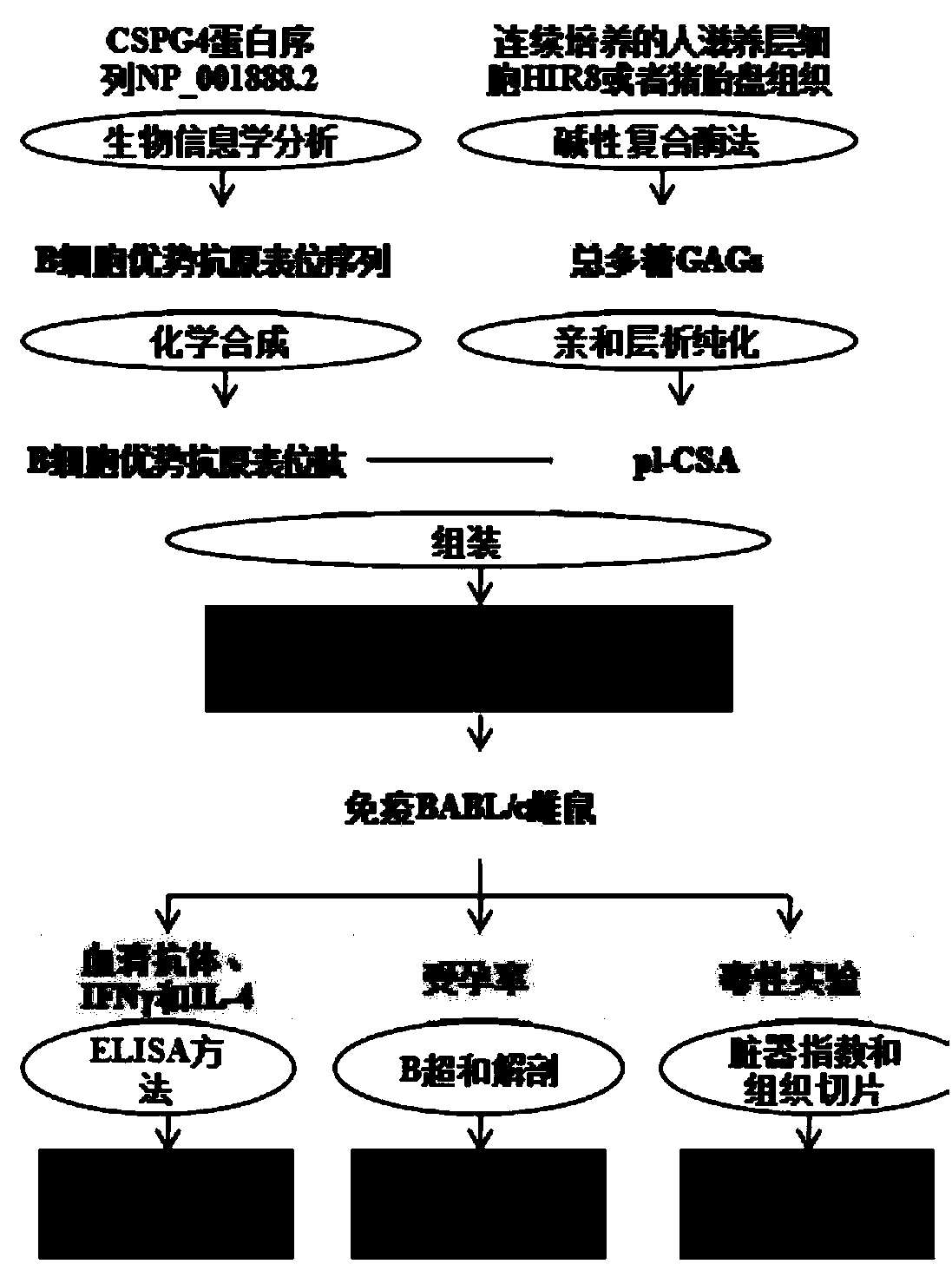
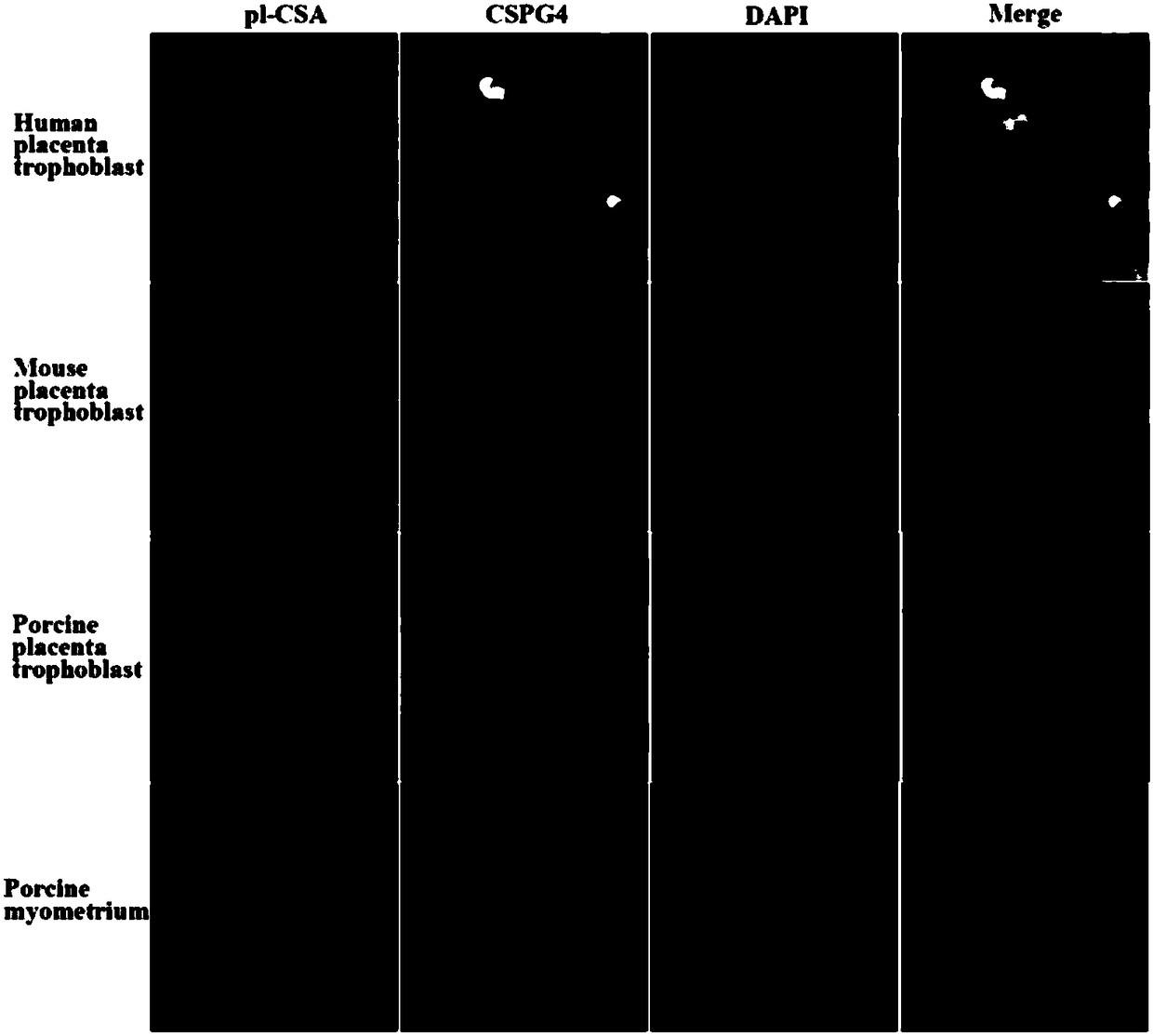
[0067] Expression and localization of embodiment 1pl-CSA and its core protein CSPG4
[0068] This part was performed by immunofluorescence. First, collect human delivery placenta, mouse placenta of 15 days of pregnancy and pigs' placenta of 70 days of pregnancy, fully fixed with 4% paraformaldehyde; 2 o 2 (10min) to inactivate endogenous peroxidase; pH6.0 citrate buffer in 95°C water bath for 10min for antigen retrieval; rabbit anti-CSPG4 antibody (1:200) and self-made rVAR2-His-tag (1 :100) reagent and incubated overnight at 4°C; PBST (containing 0.1% Triton X-100) was soaked 3 times, 5min each time; FITC-labeled goat anti-rabbit secondary antibody (1:1000) and Dylight650-labeled anti-6×His monoclonal antibody Cloned antibody (1:1000), incubate at room temperature for 40min; soak in PBST 3 times, 5min each; The slides were sealed with mounting medium, observed and photographed under a fluorescent confocal microscope. For experimental results, see image 3 . From image...
Embodiment 2
[0069] The preparation of embodiment 2 immune agent
[0070] Preparation and purification of placenta-like chondroitin sulfate A:
[0071] (1) Broken pig placenta or semi-mechanical separation of continuously cultured trophoblast cell line to obtain tissue cell slurry;
[0072] (2) The tissue cell slurry obtained in step (1) is mixed with the lysate (V cell slurry: V lysate=1:10), the lysate contains RNase, DNase I, trypsin and protease K, and the lysed tissue is obtained cell plasma;
[0073] (3) inactivating the enzyme in the lysate of the lysed tissue cell serum obtained in step (2); adding 1 / 4 volume of Sevag solution to remove protein to obtain crude pl-CSA;
[0074] (4) Add a BamHI restriction site to the 5' end of the erythrocyte surface antigen VAR2CSA gene sequence SEQ ID No.1 of Plasmodium infection, and add a Sal I restriction site to the 3' end; combine the gene fragment SEQ ID No.1 with pET28a (+) The prokaryotic expression vector was connected, transformed int...
Embodiment 3
[0082] Immunogenicity and contraceptive effect evaluation of embodiment 3pl-CSA
[0083] Table 2. pl-CSA vaccine immunization groups
[0084]
[0085]
[0086] ① Immune grouping: 6-week-old female BALB / c mice were randomly divided into 4 groups, with PBS as negative control, 20 mice in each group (Table 2), and 40 10-week-old BALB / c male mice were fed.
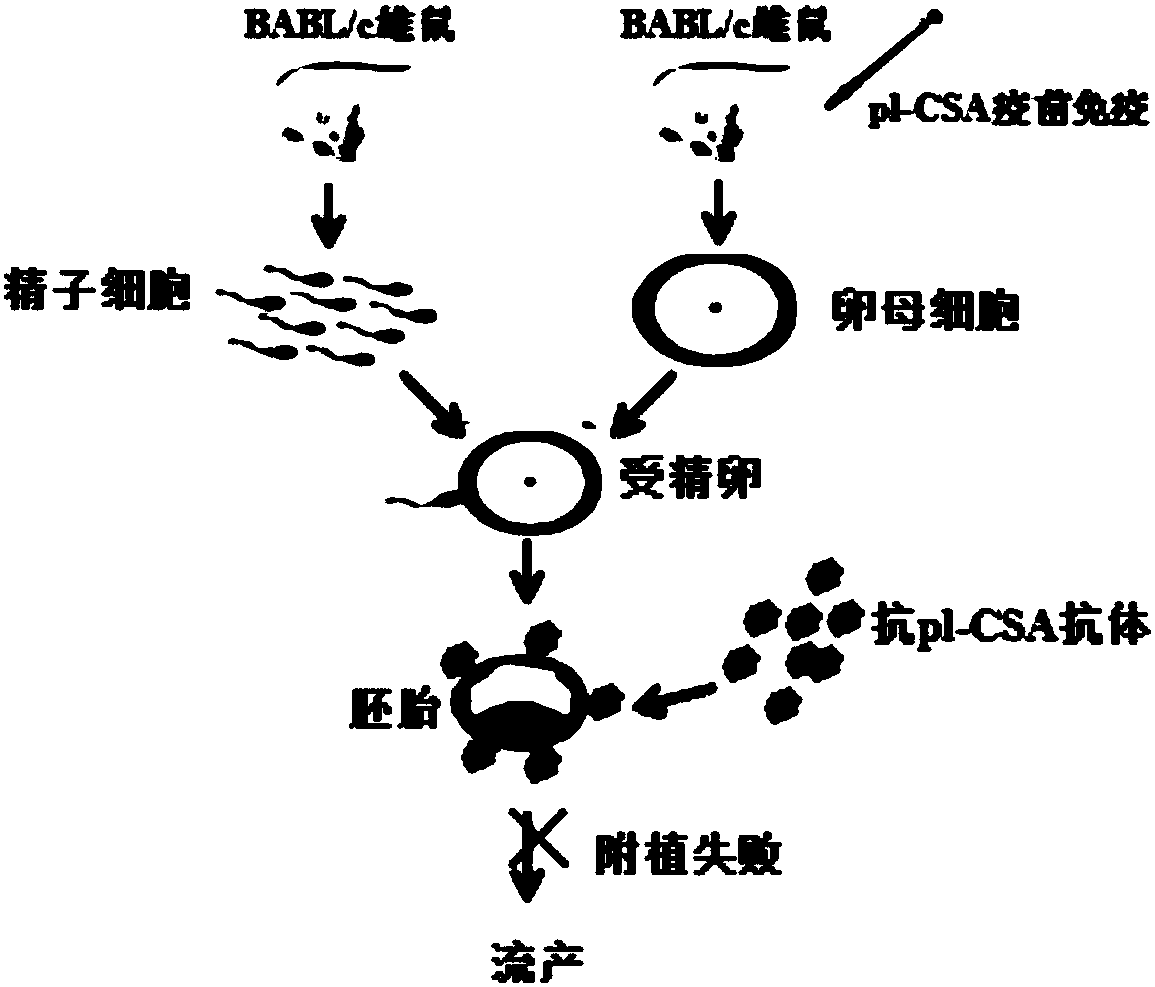
[0087] ②Immunization procedure: Female mice in each group had free access to food and water, 2 mice per cage, and the time for inoculation of pl-CSA and pl-CSA+CBZ-1 vaccines was 0, 1, and 2 weeks, respectively (set to 0 at the time of purchase). Weeks), a total of 3 times of immunization, the first time using Freund's complete adjuvant to mix with the immunogen, the second and third times using Freund's incomplete adjuvant to mix with the immunogen. From the 4th week, the female mice of each group were caged with the male mice, and B-ultrasound was used to monitor whether they were pregnant on the 9th day after the vag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com