Compositions and methods for regulating sas1r
a technology of sas1r and sas1b, which is applied in the field of compositions and methods for regulating sas1r, can solve the problems of less success in identifying oolemmal receptors for sperm ligands, and achieve the effect of modulating fertilization and fertility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0272]The present invention provides compositions and methods to prevent or inhibit SAS1R function or activity, including the use of SAS1R proteins and fragments and homologs thereof. The present invention further provides compositions and methods utilizing SAS1R, and fragments and homologs thereof, to elicit an immune response against SAS1R. In one aspect, administration of SAS1R or antigenic fragments or homologs thereof results in inhibiting SAS1R. In one aspect, such an immunogenic response and resulting inhibition of SAS1R results in a decrease in fertility.
[0273]The following are useful mammalian SAS1R sequences:
mouse SAS1R Variant 1, 435 residues(SEQ ID NO: 19)MGIMGSLWPWILTMLSLLGLSMGAPSASRCSGVCSTSVPEGFTPEGSPVFQDKDIPAINQGLISEETPESSFLVEGDIIRPSPFRLLSVTNNKWPKGVGGFVEIPFLLSPKYDELSRRVIMDAFAEFERFTCIRFVAYHGQRDFVSILPMAGCFSGVGRSGGMQVVSLAPTCLRKGRGIVLHELMHVLGFWHEHSRADRDRYIQVNWNEILPGFEINFIKSRSTNMLVPYDYSSVMHYGRFAFSWRGQPTIIPLWTSSVHIGQRWNLSTSDITRVCRLYNCSRSVPDSHGRGFEAQSDGSSLTPASISRLQRLLEALSEES...
example 1
[0404]Materials & Methods:
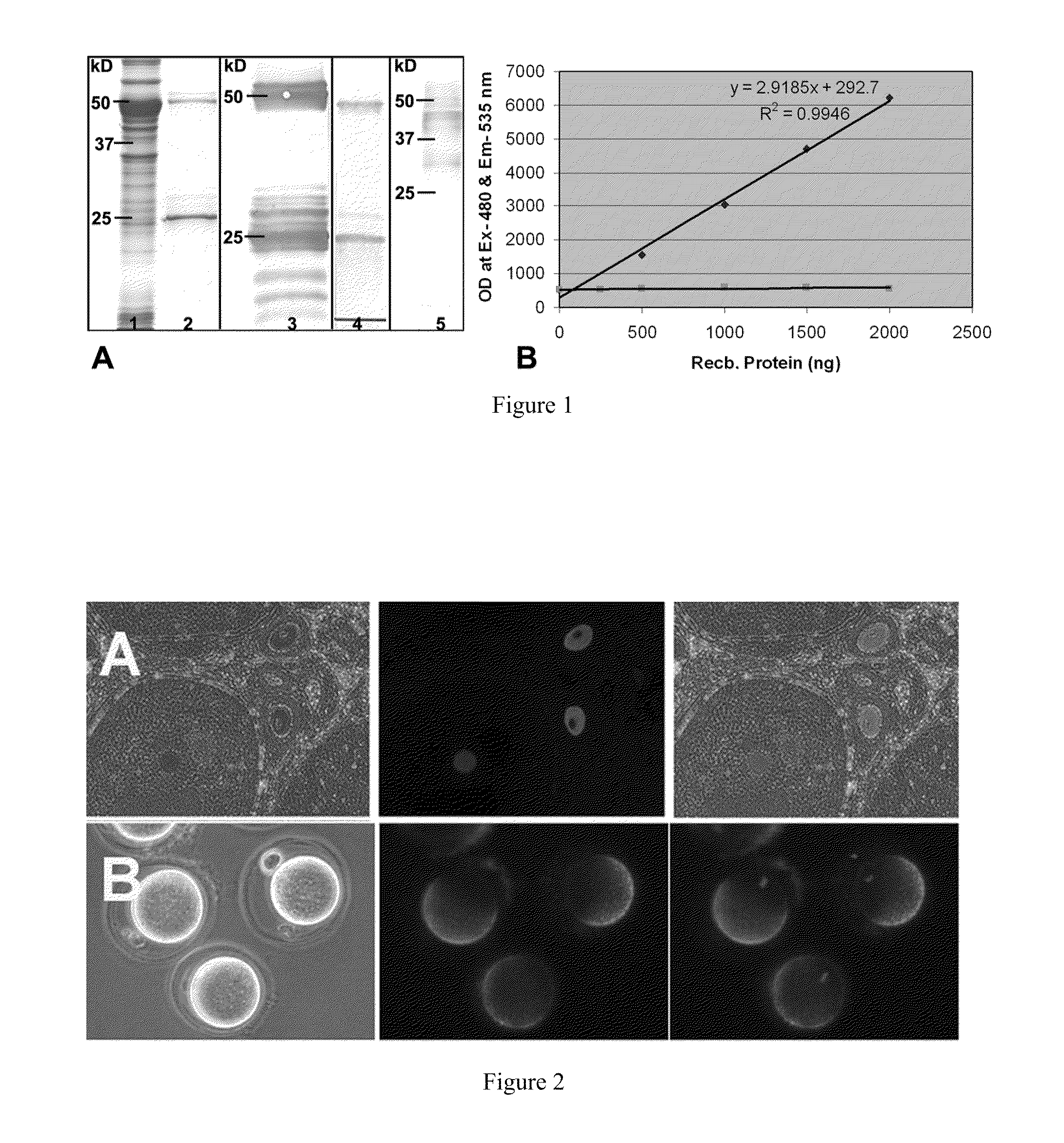
[0405]Identification of SAS1R by SPR: Using purified soluble rSLLP1 as bait, SAS1R was initially identified as a SLLP1 binding partner using mouse oocyte protein lysates.
[0406]Purification of mouse SAS1R and SLLP1: Mature SAS1R (without signal sequence, 414 a.a.) was cloned by PCR from mouse ovary cDNA, expressed in E. coli and purified by affinity column chromatography. SLLP1 was purified as described earlier (2).
[0407]Antibody production and Western analyses: Antibody to purified rSAS1R was raised in guinea pigs. Specificity of the antibody was tested against rSAS1R and mouse oocyte extracts following SDS-PAGE and western blotting using HRP-2nd antibody and TMB substrate.
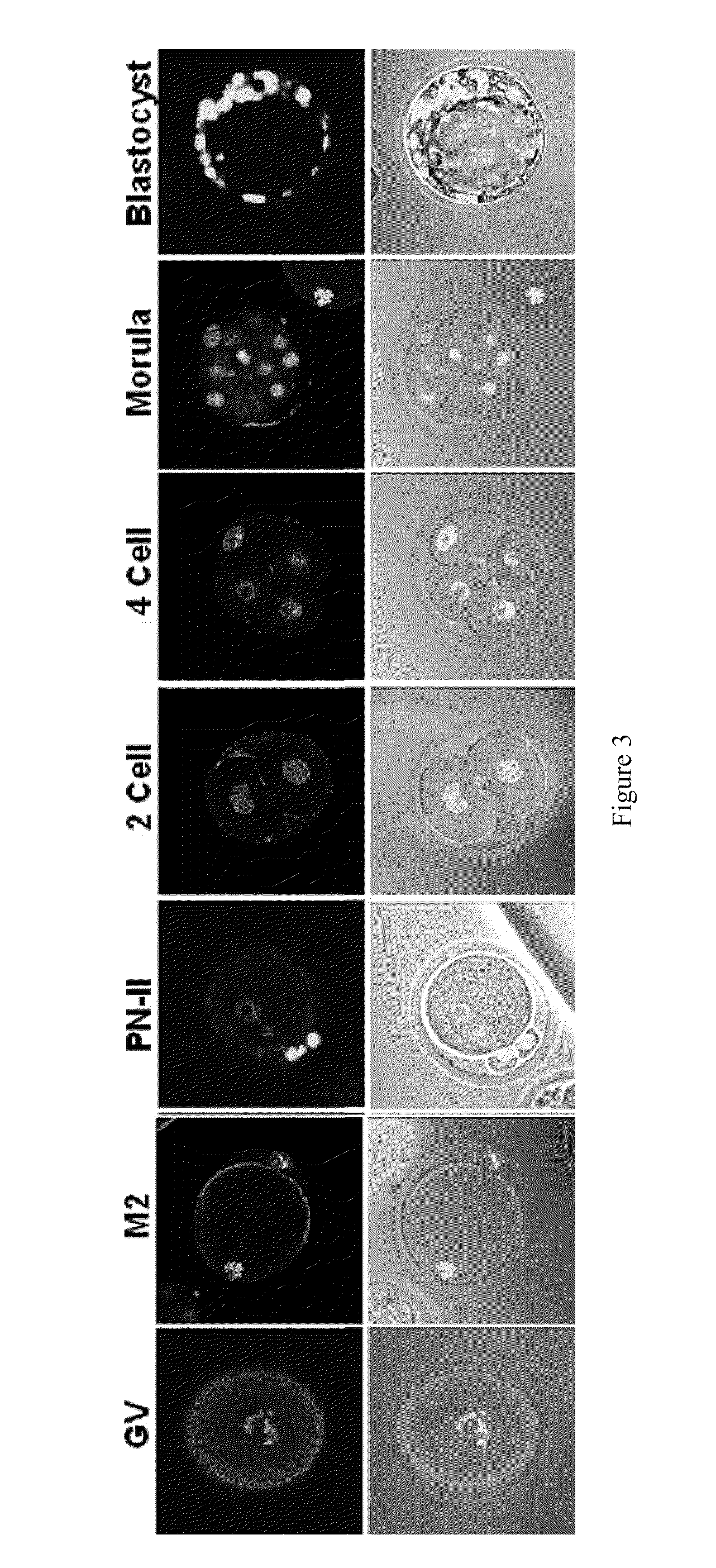
[0408]IF localization of SAS1R in mouse ovary: Fixed ovary sections were probed with anti-SAS1R antibody followed by Cy3-2nd antibody and imaged with UV-microscopy.
[0409]IF microscopy of oocytes, early embryos and sperm: Zona intact-, zona free oocytes, early embryos and sperm were collec...
example 2
SAS1R as a Target for Contraception
[0475]Effect of SAS1R antibody on mouse in-vitro fertilization. Antibodies and sera directed SAS1R were prepared. Cumulus intact mouse oocytes were incubated with either preimmune or immune sera at 1 / 10 & 1 / 20 (N=4) or 1 / 80 (N=2) dilutions for 45 min followed by insemination with capacitated sperm. The number of two cell embryos was scored as fertilized eggs. The statistical significance between the preimmune and immune sera was calculated by t test assuming equal variances and are presented in FIG. 16. It can be seen that an inhibitor of SAS1R, such as an antibody directed against SAS1R, is an effective inhibitor of fertilization.
[0476]SAS1R is an Effective Immunogen. Next, it was shown that the SAS1R protein is an effective immunogen in females. As an oocyte specific, sperm oolemmal receptor, SAS1R was hypothesized herein to be a candidate contraceptive vaccinogen and immunogen. Immunogenicity of recombinant mouse SAS1R was tested in female mice ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| mole fraction | aaaaa | aaaaa |
| mole fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com