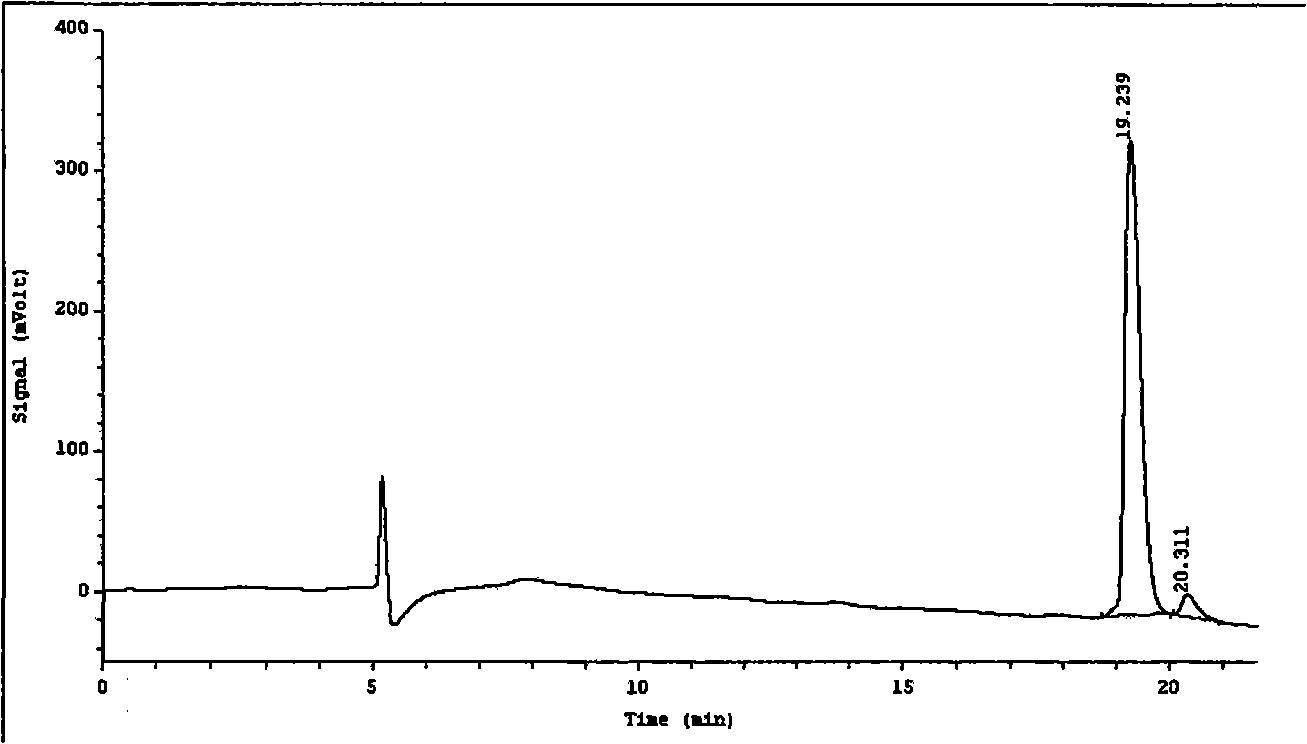
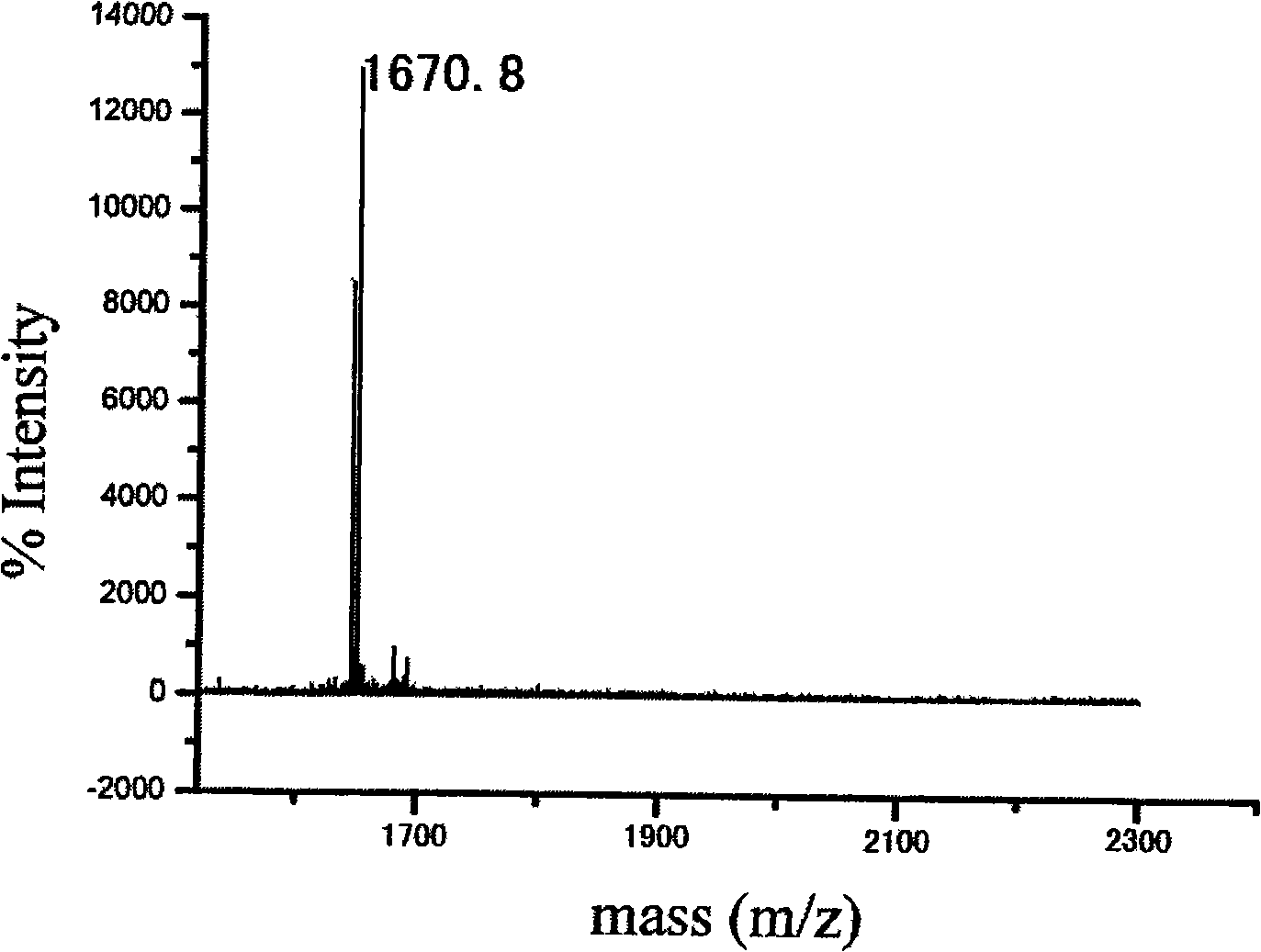
Patents
Literature
78 results about "Measles virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Measles morbillivirus, formerly called measles virus (MeV), is a single-stranded, negative-sense, enveloped, non-segmented RNA virus of the genus Morbillivirus within the family Paramyxoviridae. It is the cause of measles. Humans are the natural hosts of the virus; no animal reservoirs are known to exist.
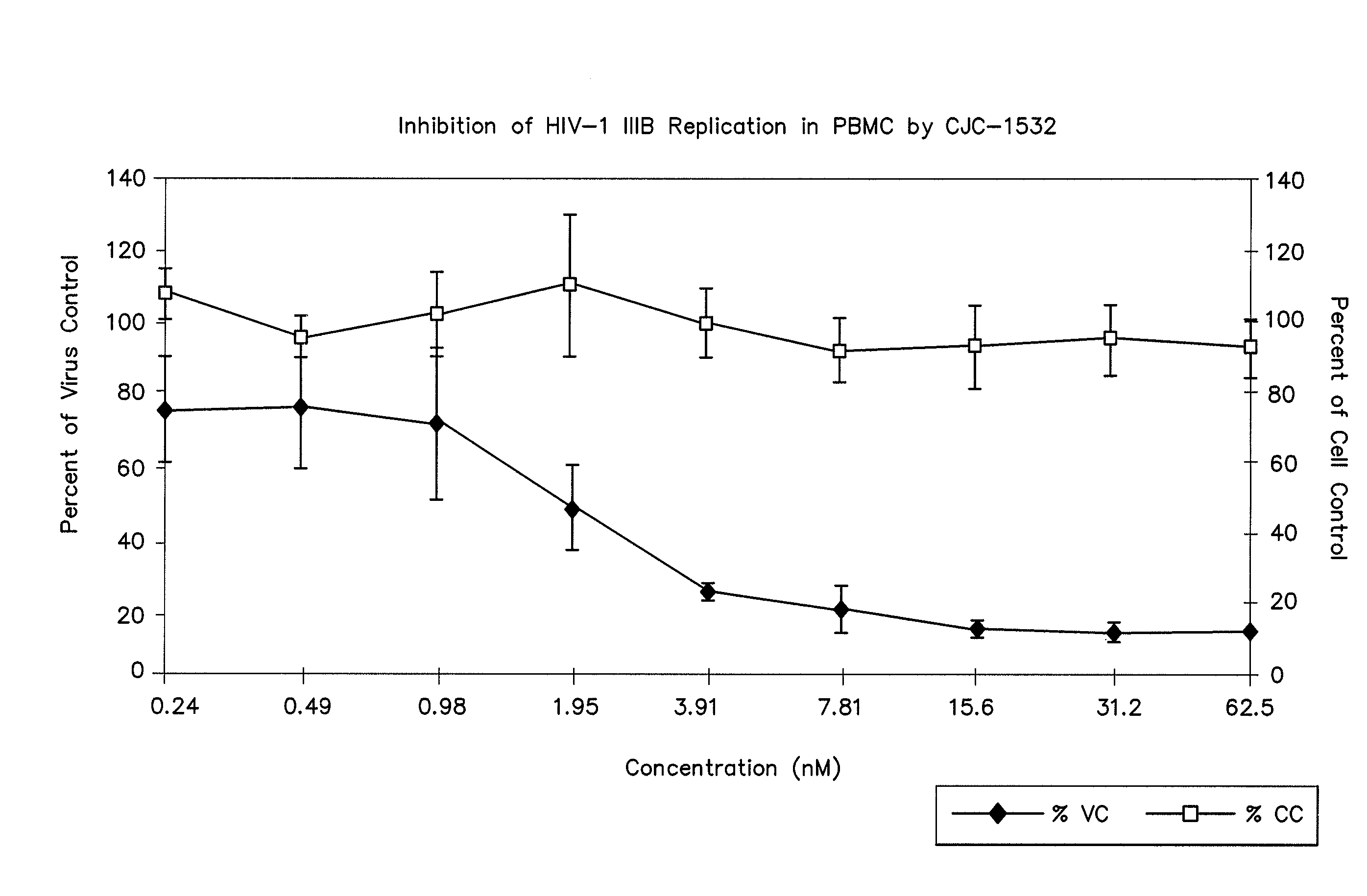
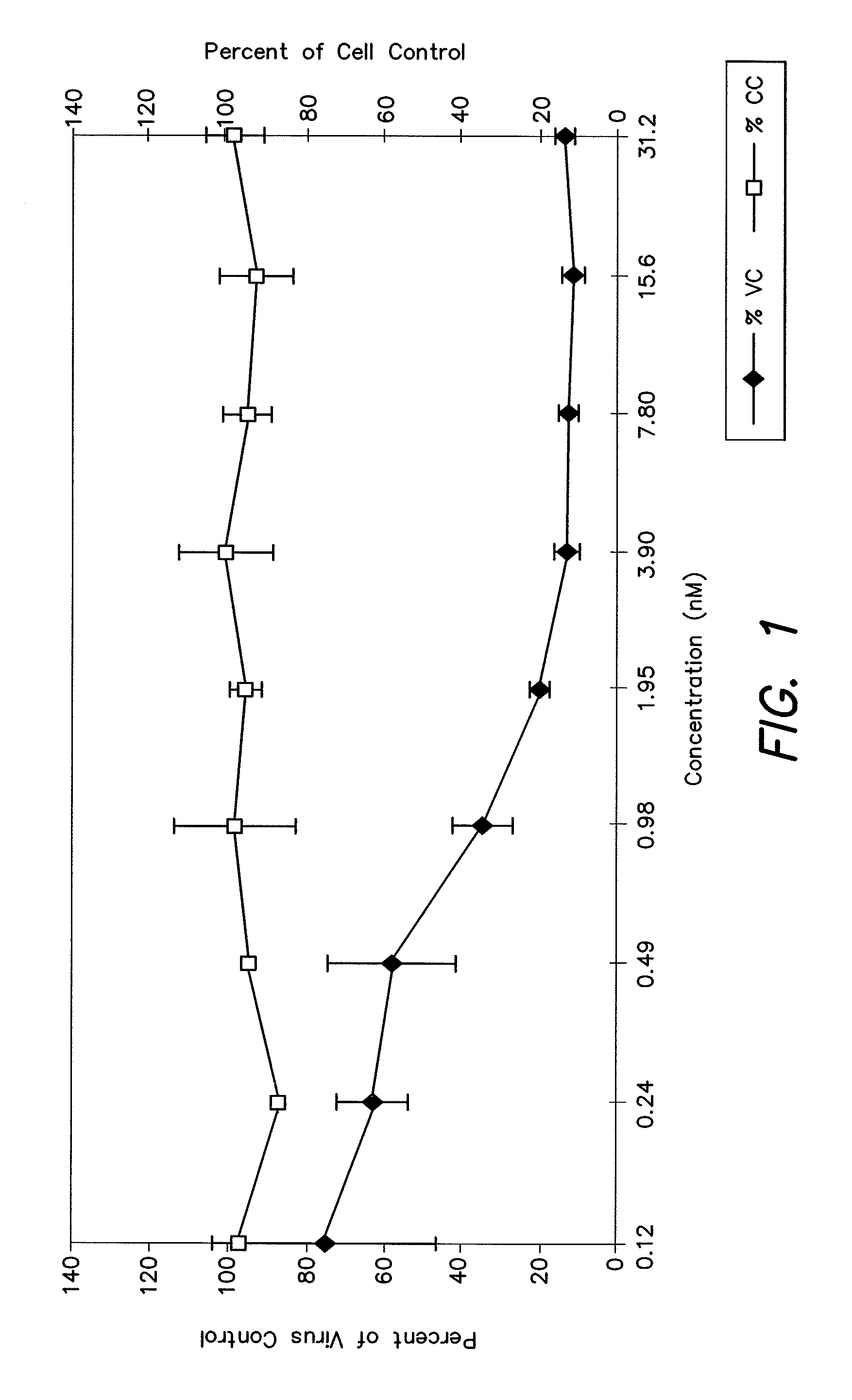
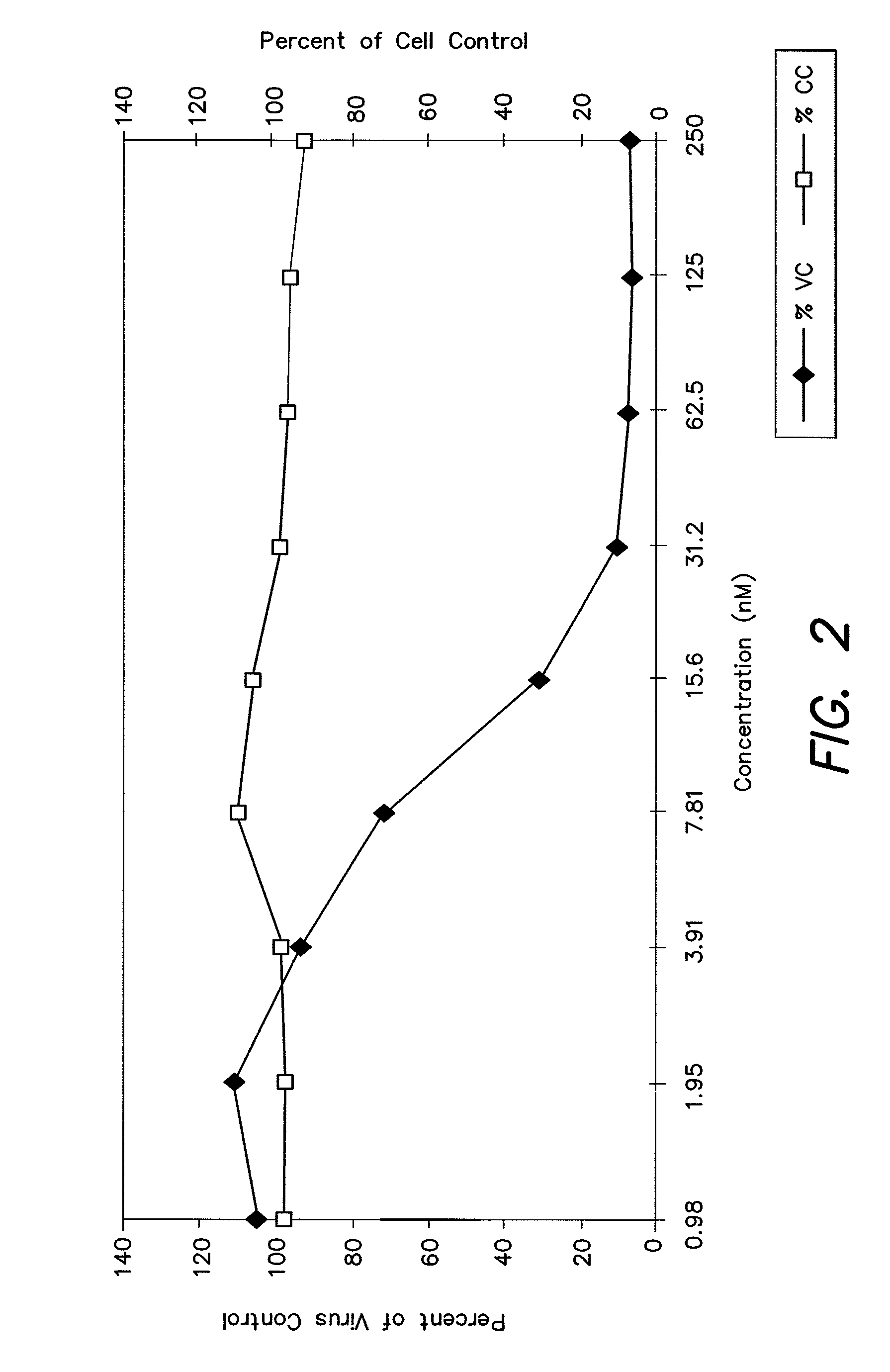
Measles virus peptides with antifusogenic and antiviral activities
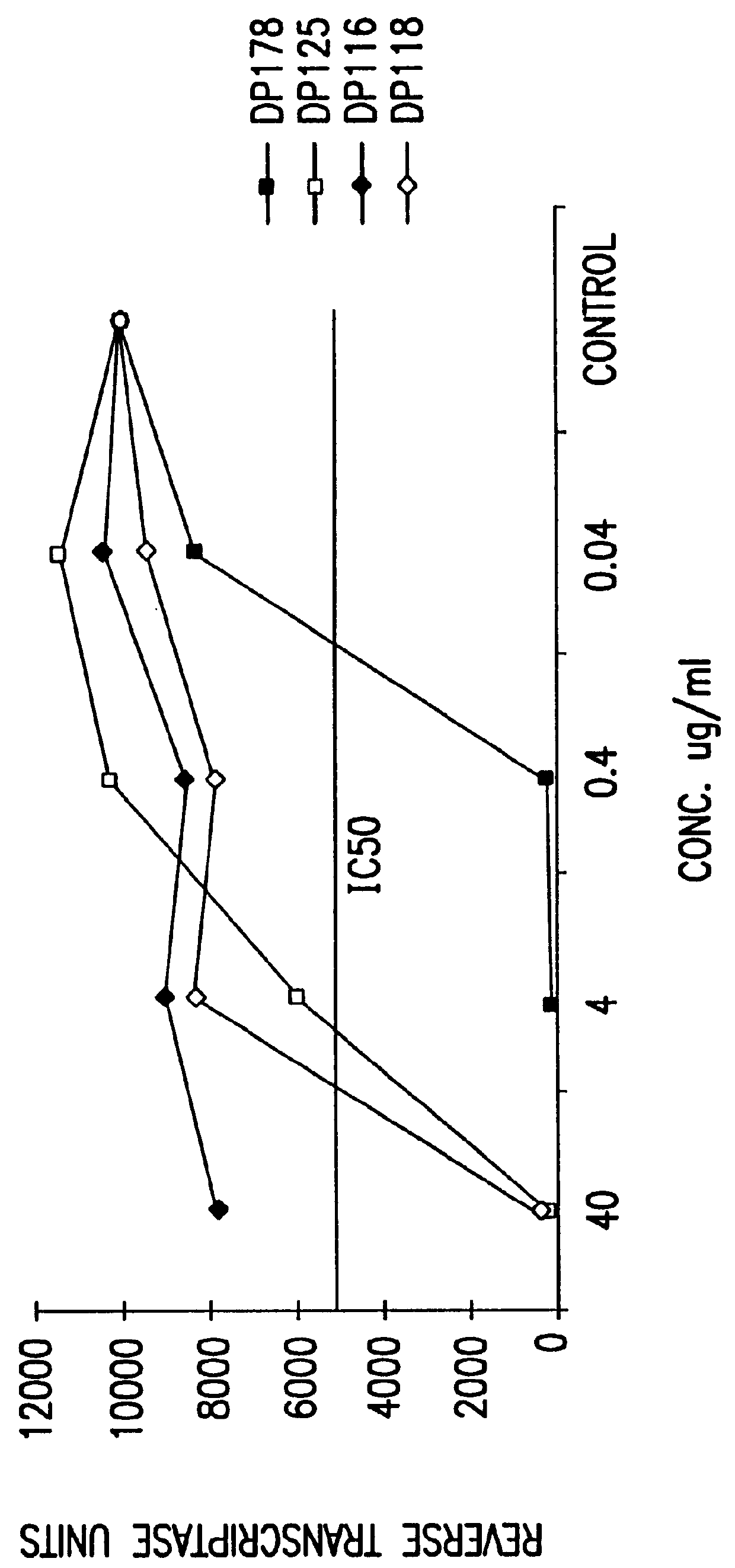
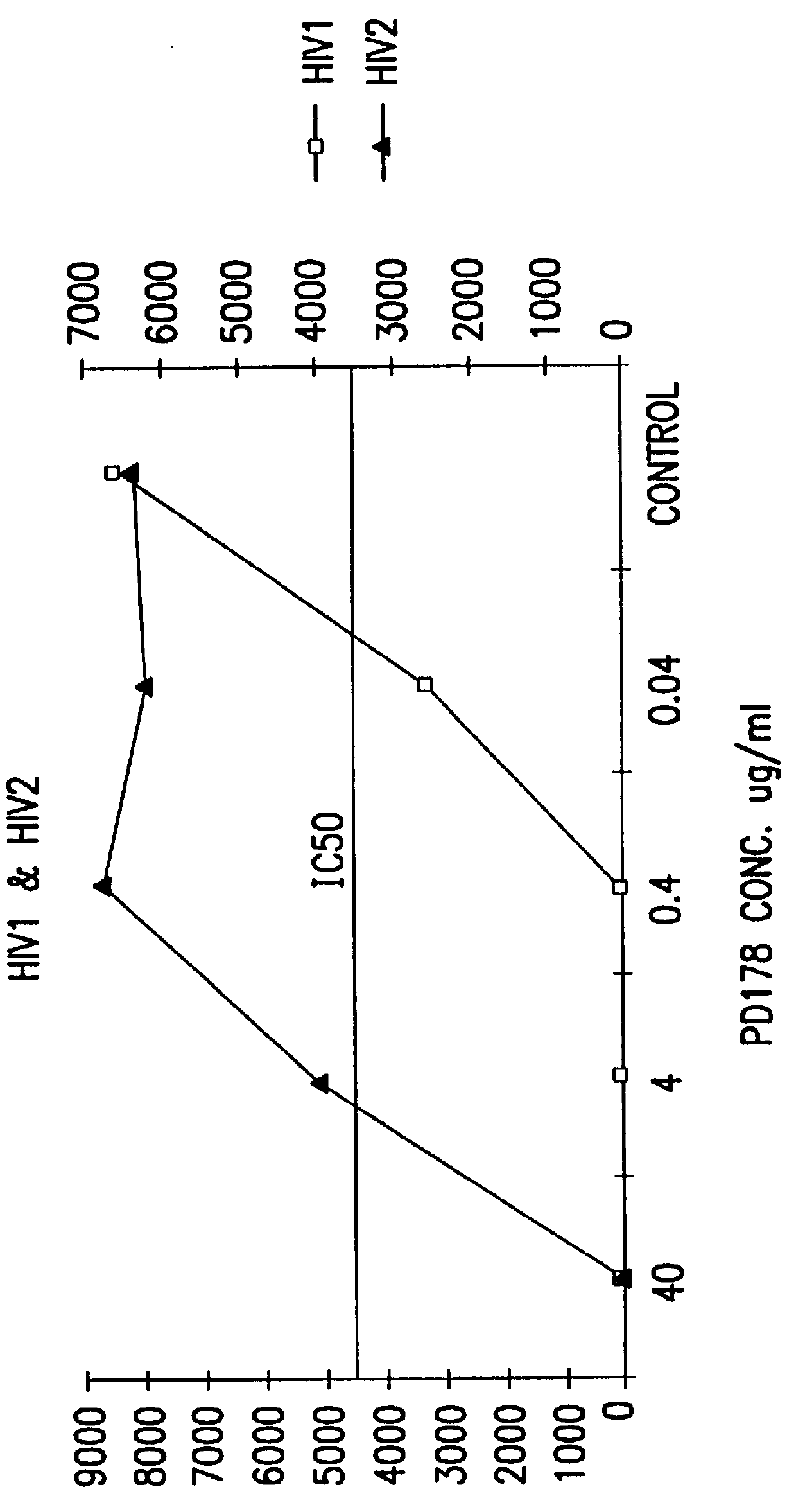
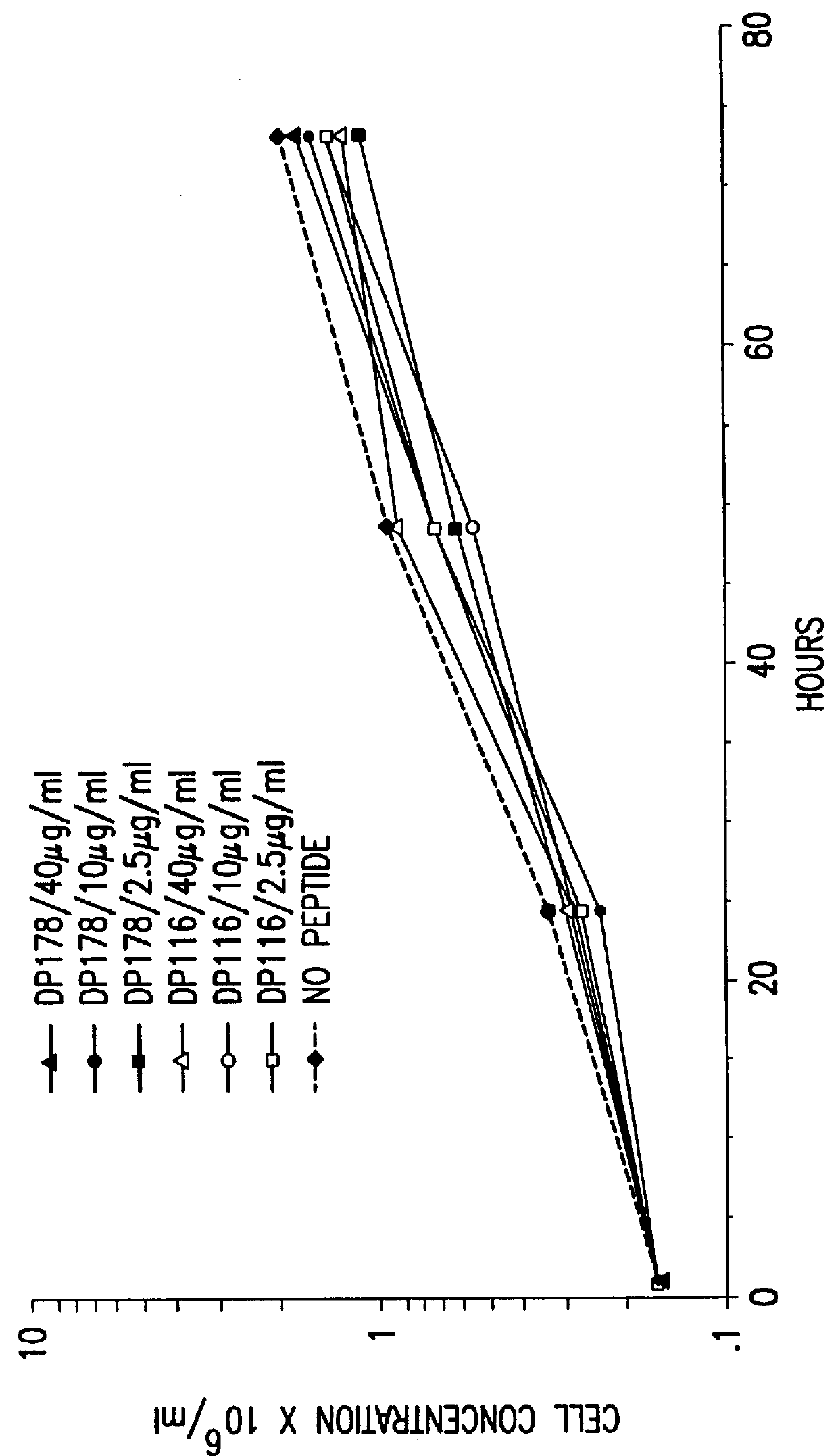
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Long Acting Biologically Active Conjugates
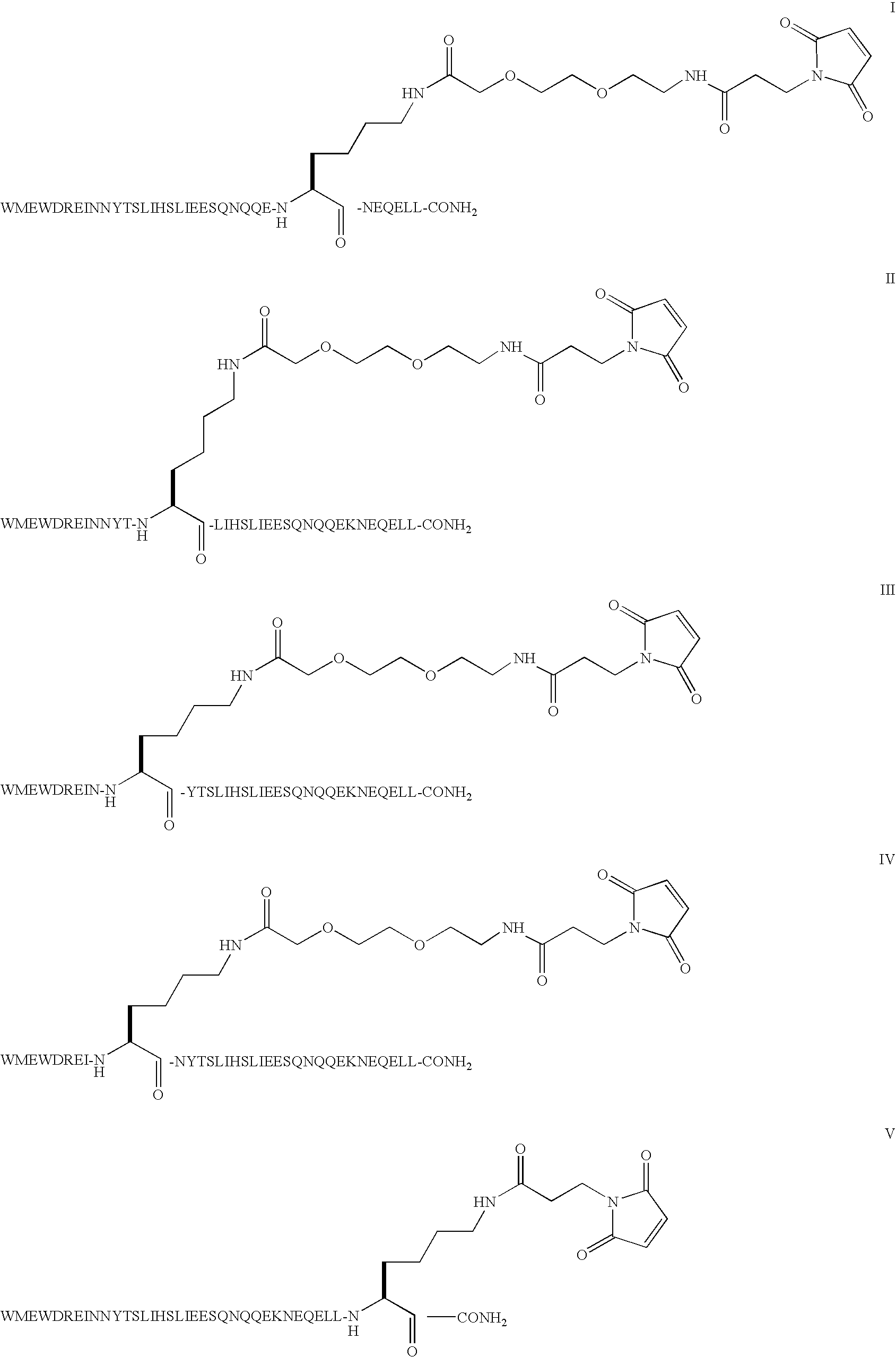
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
Anti-viral treatment and assay to screenfor Anti-viral agent
InactiveUS20130085133A1Improve palatabilityImprove stabilityBiocideSugar derivativesCytopathic effectAntiviral drug
The present disclosure relates to novel compounds of formulas (1) through (19) and to a method for treating humans infected with a virus including various respiratory viruses such as members of the Paramyxoviridae family (respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human parainfluenza virus (HPIV), measles virus, and mumps virus) with a compound of formulas (1) through (19). The present disclosure also relates to a cytopathic effect (CPE)-based assay that will assess virus-induced CPE for screening of compounds for treating viral diseases or inhibiting a virus.
Owner:SOUTHERN RES INST & IP
Immunogenic peptide composition comprising measles virus Fprotein Thelper cell epitope (MUFThl-16) and N-terminus of β-amyloid peptide
InactiveUS6906169B2Increase the gapHigh cross reactivityNervous disorderPeptide/protein ingredientsFibrilDisease patient
The present invention relates to a composition comprsing a peptide immunogen useful for the prevention and treatment of Alzheimer's Disease. More particularly, the peptide immunogen comprises a main functional / regulatory site, an N-terminal fragment of Amyloid β (Aβ) peptide linked to a helper T cell epitope (Th) having multiple class II MHC binding motifs. The peptide immunogen elicit a site-directed immune response against the main functional / regulatory site of the Aβ peptide and generate antibodies, which are highly cross-reactive to the soluble Aβ1-42 peptide and the amyloid plaques formed in the brain of Alzheimer's Disease patients. The antibodies elicited being cross reactive to the soluble Aβ1-42 peptide, promote fibril disaggregation and inhibit fibrillar aggregation leading to immunoneutralization of the “soluble Aβ-derived toxins”; and being cross-reactive to the amyloid plaques, accelerate the clearance of these plaques from the brain. Thus, the composition of the invention comprising the peptide immunogen is useful for the prevention and treatment of Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
Method for limiting the growth of cancer cells using an attenuated measles virus
A method for treating cancer cells is provided comprising directly or systemically administering a therapeutically effective dose of an attenuated measles virus. In one embodiment, the therapeutically effective dose is from about 103 pfus to about 1012 pfus and is delivered by direct injection into a group of cancer cells or via intravenous injection.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Long lasting fusion peptide inhibitors for hiv infection
InactiveUS20050065075A1Prolong half-life in vivoBiocidePeptide/protein ingredientsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Ordered molecular presentation of antigens, method of preparation and use
The invention provides compositions and processes for the production of ordered and repetitive antigen or antigenic determinant arrays. The compositions of the invention are useful for the production of vaccines for the prevention of infectious diseases, the treatment of allergies and the treatment of cancers. Various embodiments of the invention provide for a virus, virus-like particle, viral capsid particle, phage or recombinant form thereof coated with any desired antigen in a highly ordered and repetitive fashion as the result of specific interactions. In one specific embodiment, a versatile new technology based on a cassette-type system (AlphaVaccine Technology) allows production of antigen coated viral particles. Other specific embodiments allow the production of antigen coated hepatitis B virus-like particles or antigen coated Measles virus-like particles.
Owner:CYTOS BIOTECHNOLOGY AG
Cysteic acid derivatives of Anti-viral peptides
InactiveUS20090088377A1Easy to processEasy to purifyPeptide/protein ingredientsVirus peptidesFeline immunodeficiency virusImmunodeficiency virus
This invention relates to C34 peptide derivatives having improved aqueous solubility that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory synctial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Combined measles-human papilloma vacine
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
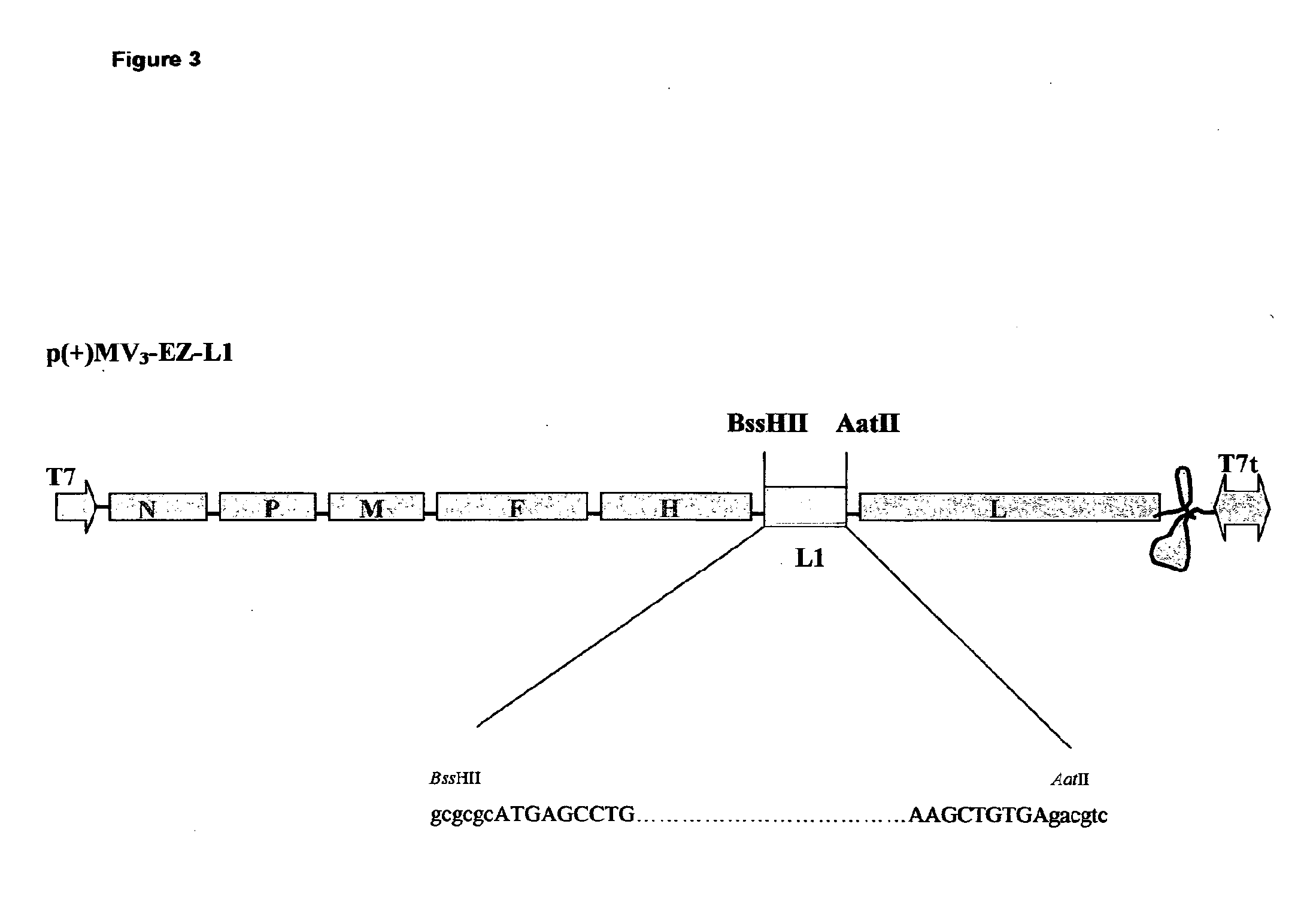
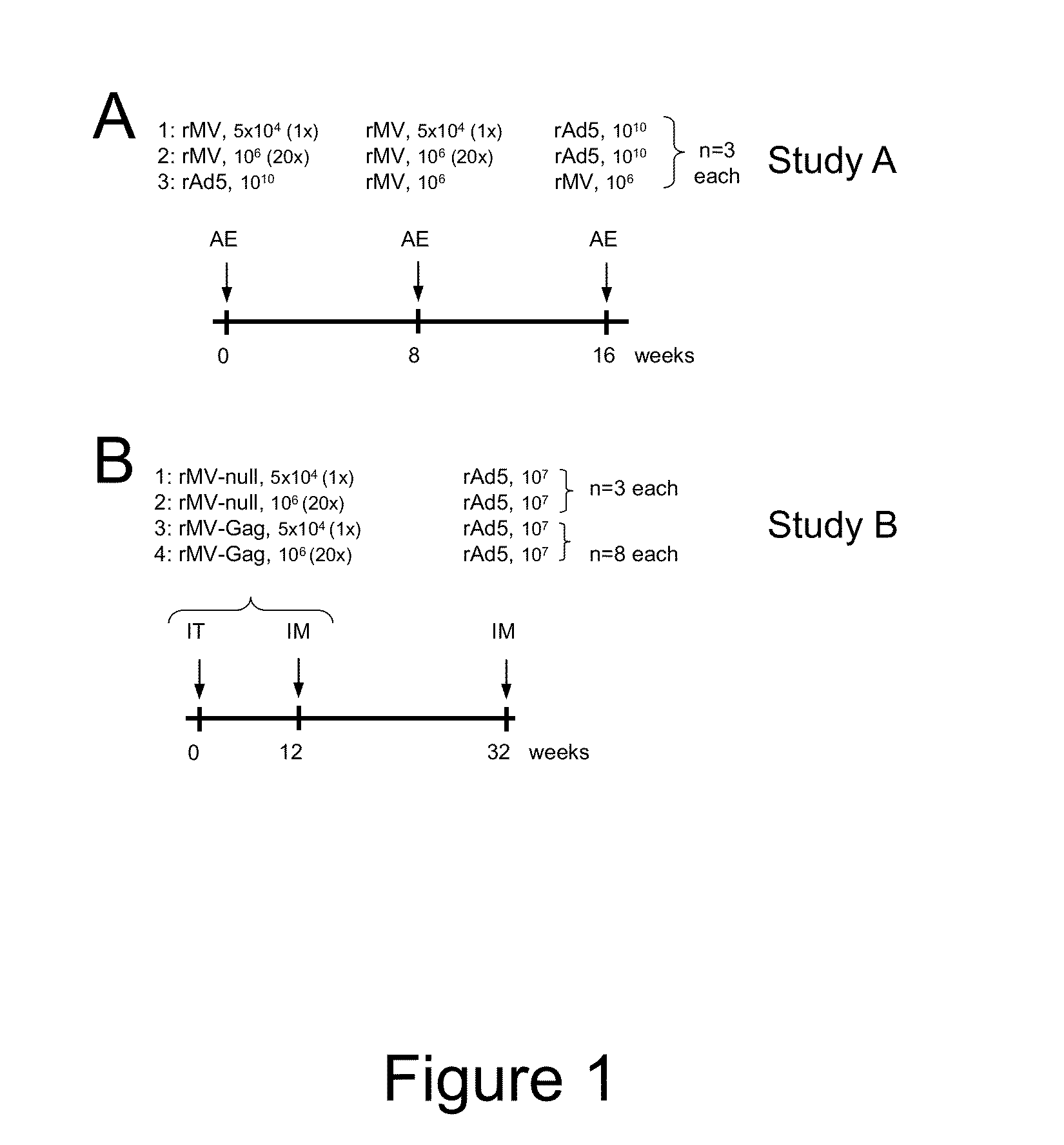
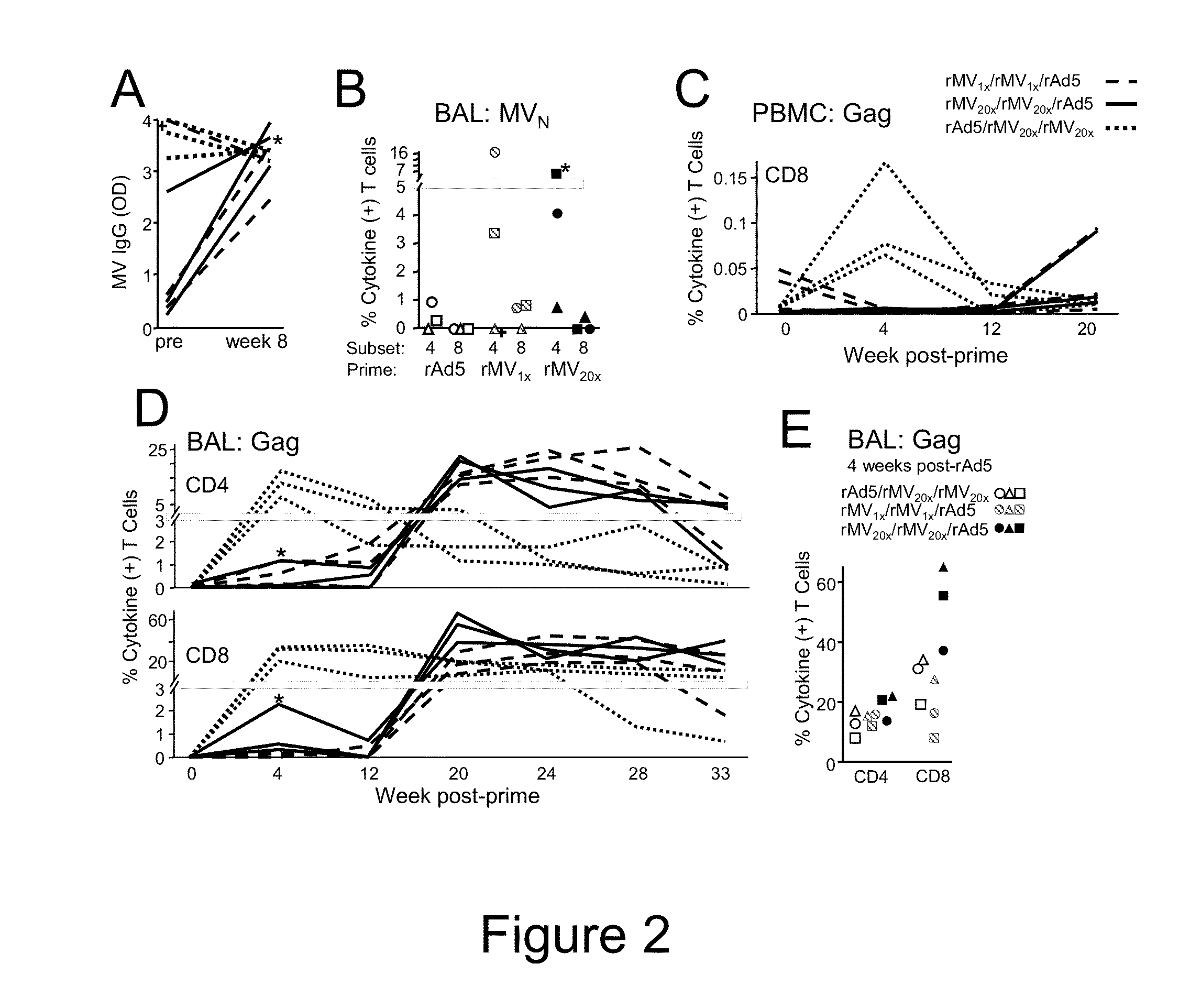
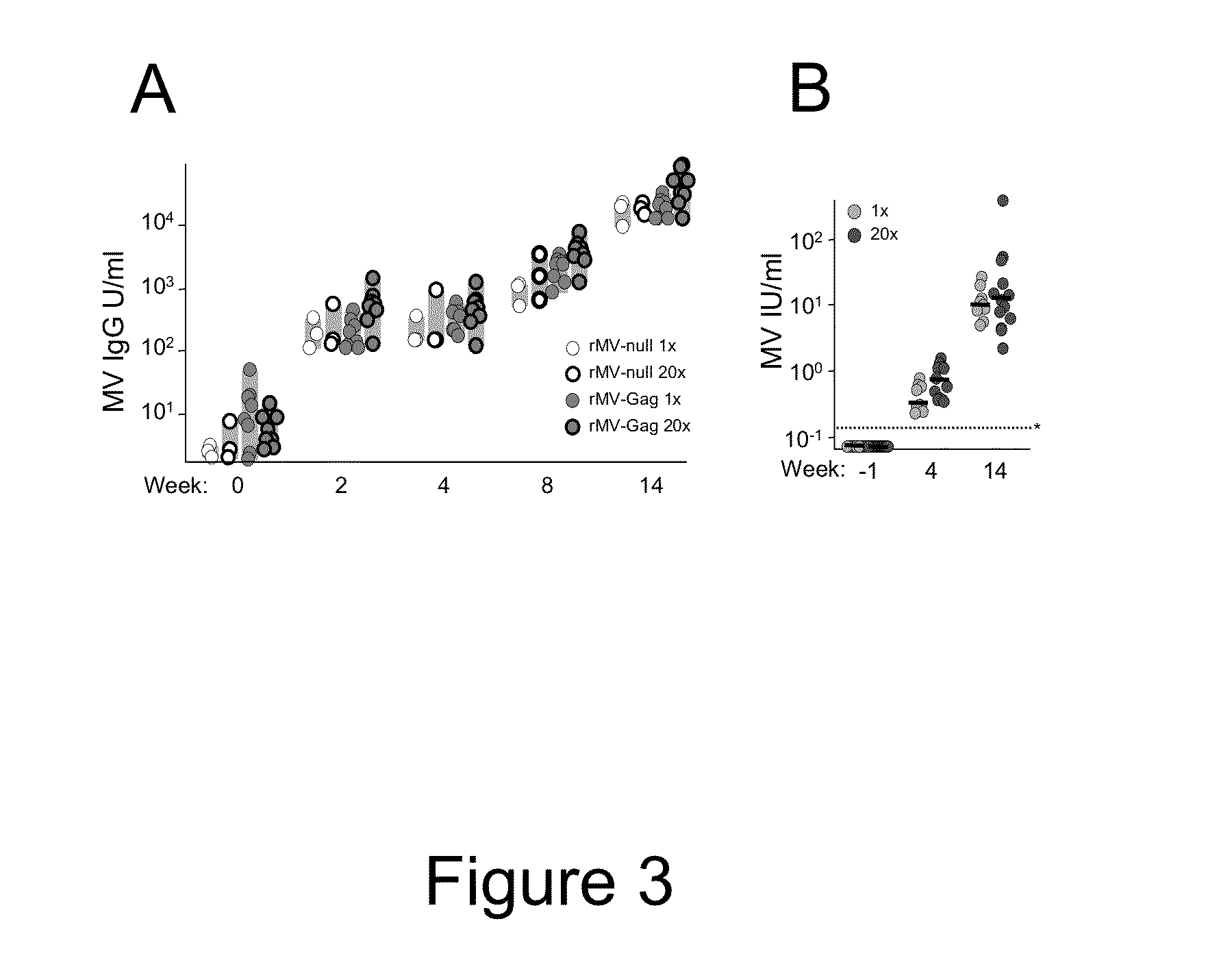
Heterologous prime-boost immunization using measles virus-based vaccines
InactiveUS20130122038A1Enhanced Gag-specificImprove responseSsRNA viruses negative-senseViral antigen ingredientsAntigenPrime boost
The invention provides reagents and methods for heterologous prime-boost immunization regimens. In particular, the invention provides reagents and methods for use in a paramyxovirus-based prime and adenovirus-based boost immunization system, wherein the immunization induces an immune response to a foreign antigen.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
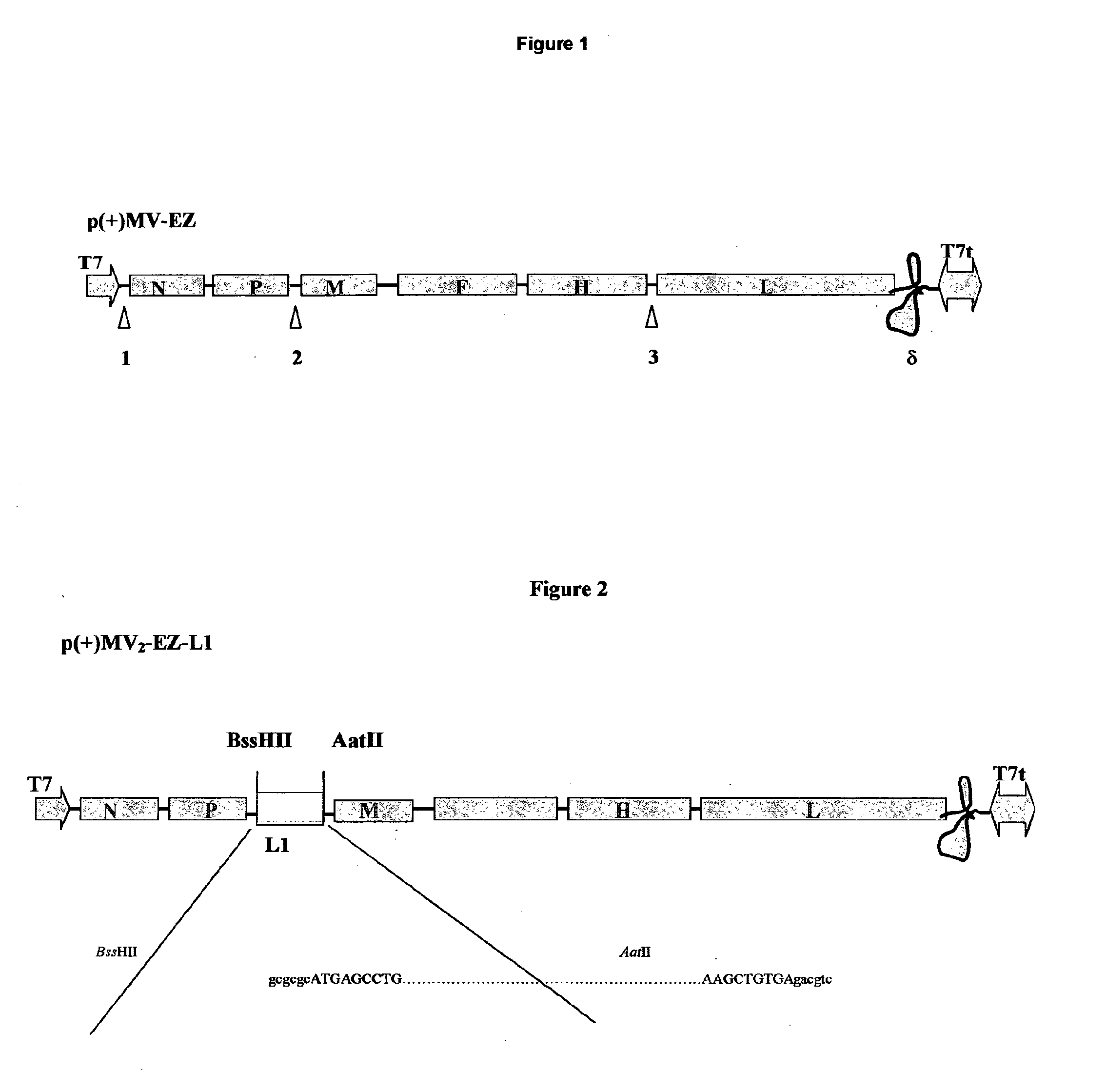
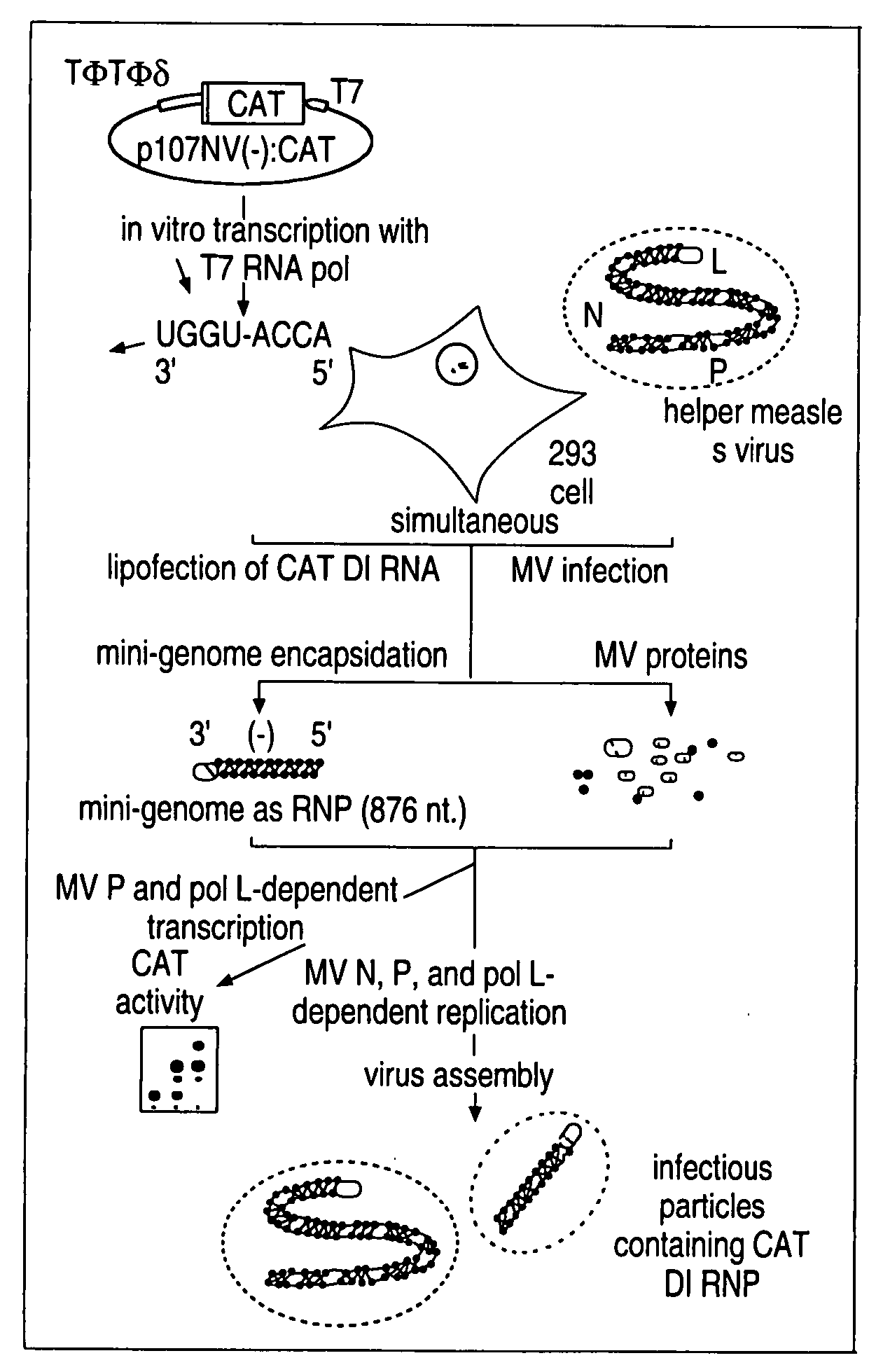
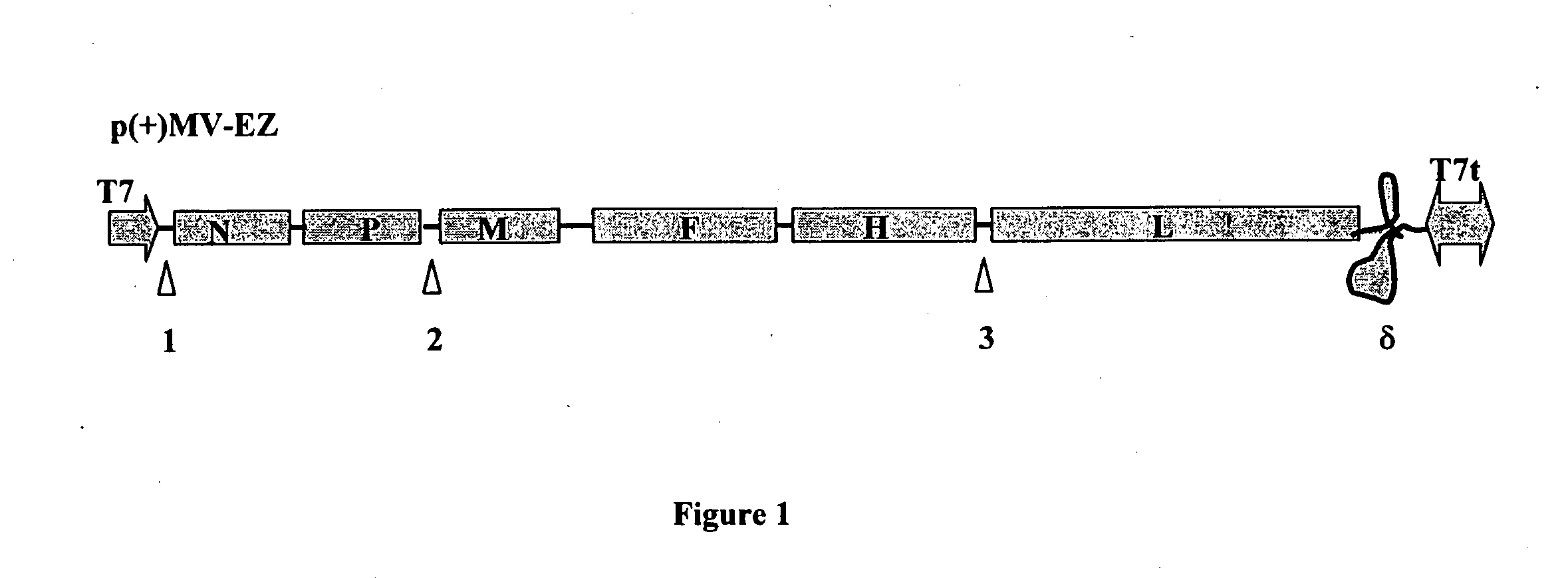
cDNA corresponding to the antigenome of nonsegmented negative strand RNA viruses and process for the production of such viruses
The present invention relates, in general, to a methodology or the generation of nonsegmented negative-strand RNA viruses (Pringle, 1991) from cloned deoxyribonucleic acid (cDNA). Such rescued viruses are suitable for use as vaccines, or alternatively, as plasmids in somatic gene therapy applications. The invention also relates to cDNA molecules suitable as tools in this methodology and to helper cell lines allowing the direct rescue of such viruses. Measles virus (MV) is used as a model for other representatives of the Mononegavirales, in particular the family Paramyxoviridae.
Owner:CRUCELL SWITZERLAND AG
Complementing cell lines
A packaging cell line that complements recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells that are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 that expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell lines can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. Also, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, and measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Combined measles-malaria vaccine
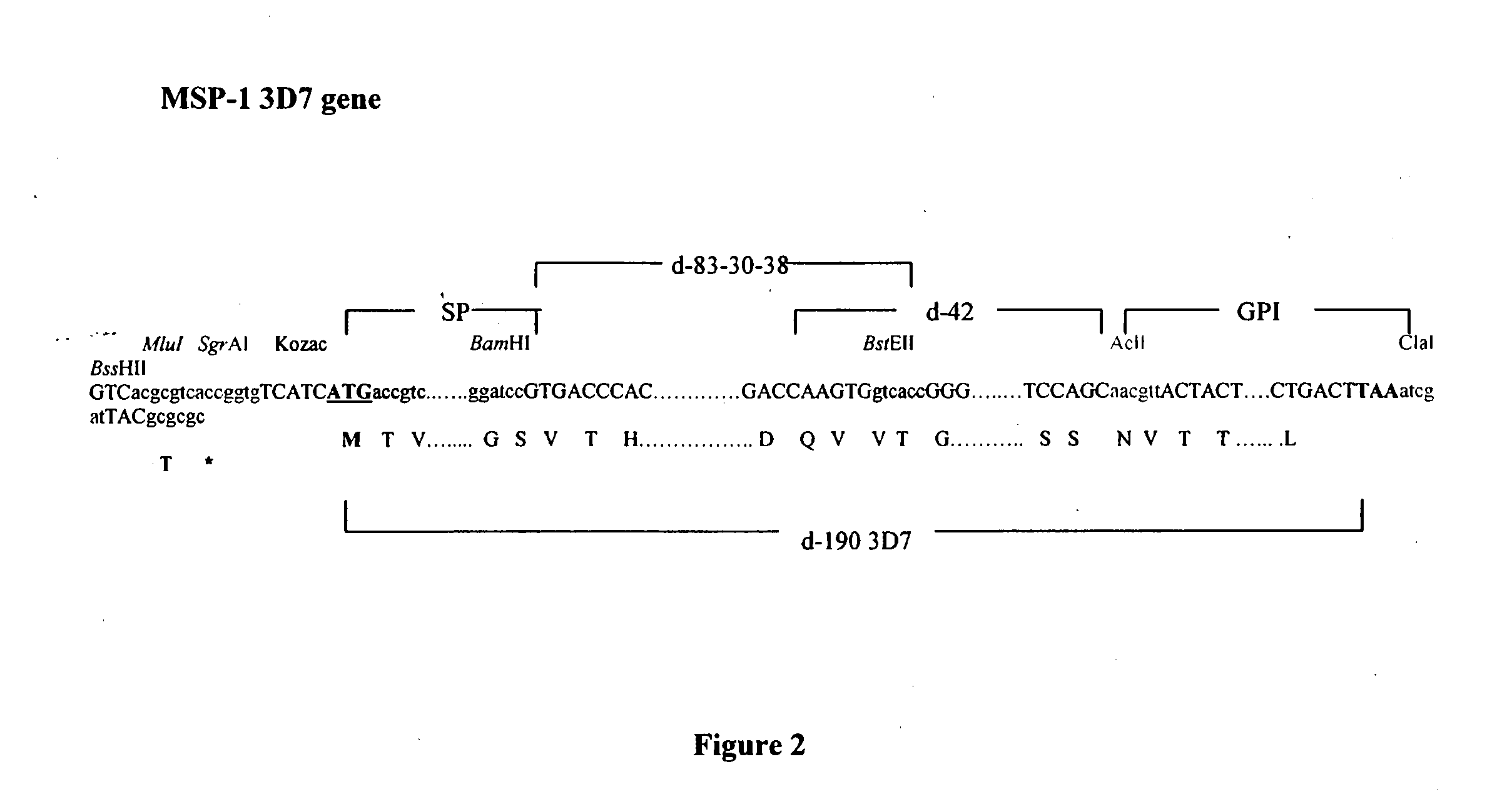
A combined measles-malaria vaccine containing different attenuated recombinant measles-malaria vectors comprising a heterologous nucleic acid encoding several Plasmodium falciparum antigens is described. Preferably, it relates to viral vectors that comprise nucleic acids encoding the circumsporozoite (CS) protein of P. falciparum, the merozoite surface protein 1 (MSP-1) of P. falciparum, and its derivatives (p-42; p-83-30-38) in its glycosylated and secreted forms, and apical membrane antigen1 (AMA1) of P. falciparum, in its anchored or secreted form. The viral vector stems from an attenuated measles virus, based on a strain that is used as a vaccine and is efficient in delivering the gene of interest and that binds to and infects the relevant immune cells efficiently.
Owner:CADILA HEALTHCARE LTD
Test paper strip for rapidly detecting morbilli and rubella virus IgG antibody colloidal gold
ActiveCN101363856AHigh sensitivityImprove featuresMaterial analysisRubulavirus InfectionsSpecific igg
The invention provides a test strip for simultaneous detection of measles and rubella virus specific IgG antibodies, which comprises a reaction film and a conjugate release pad. The reaction film has a detection band simultaneously coated with measles virus H antigen and rubella virus E1 specific antigen, and a quality control band coated with double-antibody IgG. The conjugate release pad is coated with colloidal gold labeled anti-human IgG. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base and site detection and epidemiological investigation, has auxiliary and differential diagnosis effects on measles and rubella virus infection, and can be used for the immune effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
Composition for recovering measles virus, and kit, purpose and method thereof
ActiveCN103160473ARescue is easy and convenientAcquire infectivityAntiviralsViruses/bacteriophagesGenomeNucleocapsid Proteins
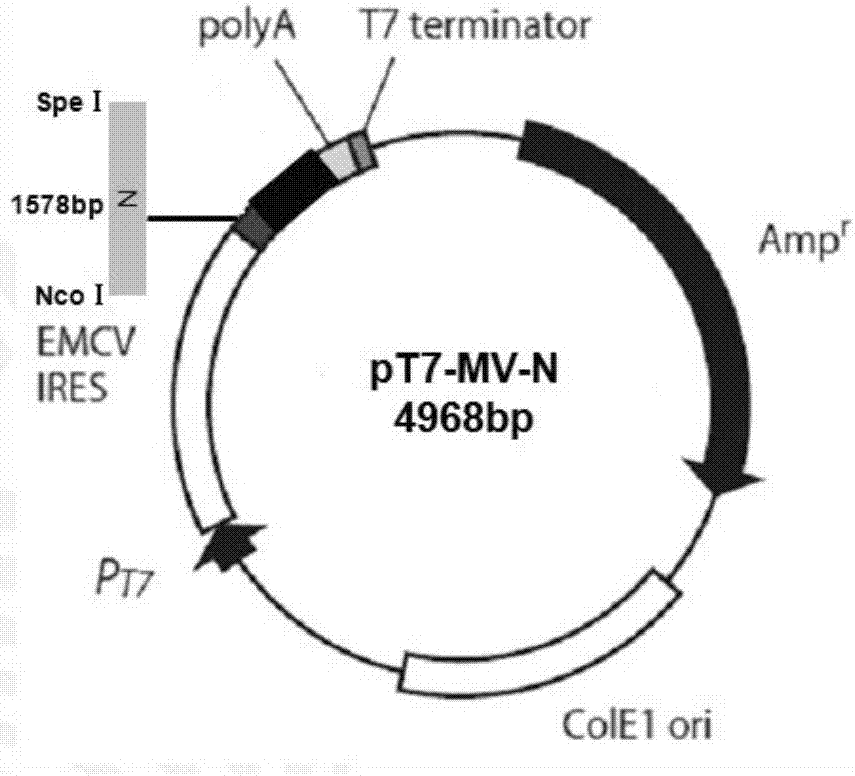
The present invention provides a composition for recovering measles virus, and a kit, a purpose and a method thereof. The composition for recovering measles virus of the present invention comprises: 1) a recombinant transcription vector, which contains the measles virus whole genome cDNA, wherein the 5' and 3' ends of the measles virus whole genome respectively includes a ribozyme sequence with self-cleavage function; 2) a first recombinant expression vector, which includes an encoding gene of nucleocapsid protein of measles virus; 3) a second recombinant expression vector, which includes the encoding gene of measles virus phosphoprotein; and 4) a third recombinant expression vector, which includes an encoding gene of RNA polymerase of measles virus. The composition of the present invention can simply and easily recover measles virus, and the recovered measles virus can be used for preparing a measles vaccine. The composition is suitable for industrial applications.
Owner:NAT VACCINE & SERUM INST
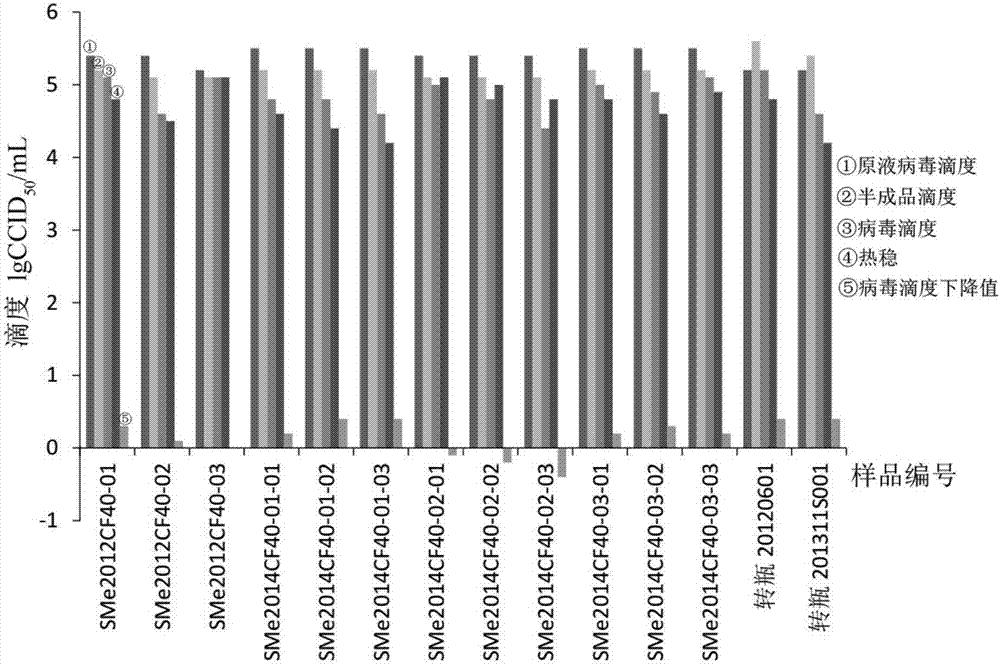
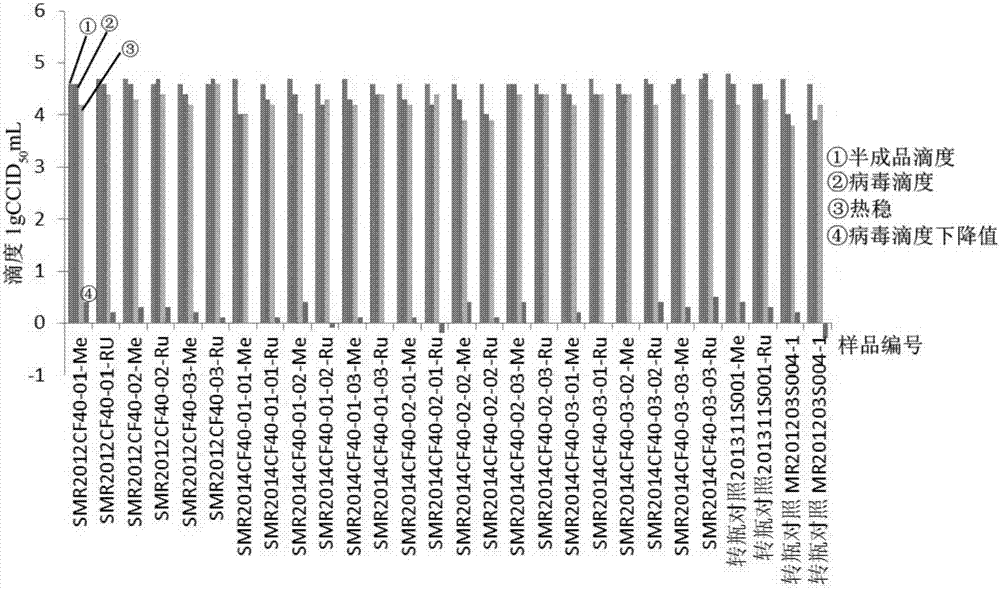
Cell-factory-based measles virus stock solution and measles-series attenuated live vaccine preparation
InactiveCN107418936AAchieve trainingSmall batch-to-batch varianceSsRNA viruses negative-senseSsRNA viruses positive-senseCell factoryCulture fluid
The invention provides a cell-factory-based measles virus stock solution and a measles-series attenuated live vaccine preparation. A preparation method of the measles virus stock solution includes selecting SPF chick-embryo cells, and adding cell culture fluid to prepare cell suspension liquid, and adding the cell suspension liquid into a cell factory; inoculating the cell factory with working seeds of measles viruses and the cell suspension liquid according to the ratio of the working seeds to the cell suspension liquid being (0.005-0.05):1, and standing and cultivating the cell factory at the temperature of 33+ / -1 DEG C for 3-4 days, pouring out archeocyte culture fluid, and replacing with fresh cell growth liquid for continuous cultivation; during cytopathy, collecting single measles virus liquid step by step. In an equal production scale, the batch number of cell dissociation is reduced, and the high-titer measles virus liquid is obtained, so that quality uniformity of measles-series vaccine products is improved effectively, and product yield is increased.
Owner:BEIJING BIOLOGICAL PROD INST CO LTD
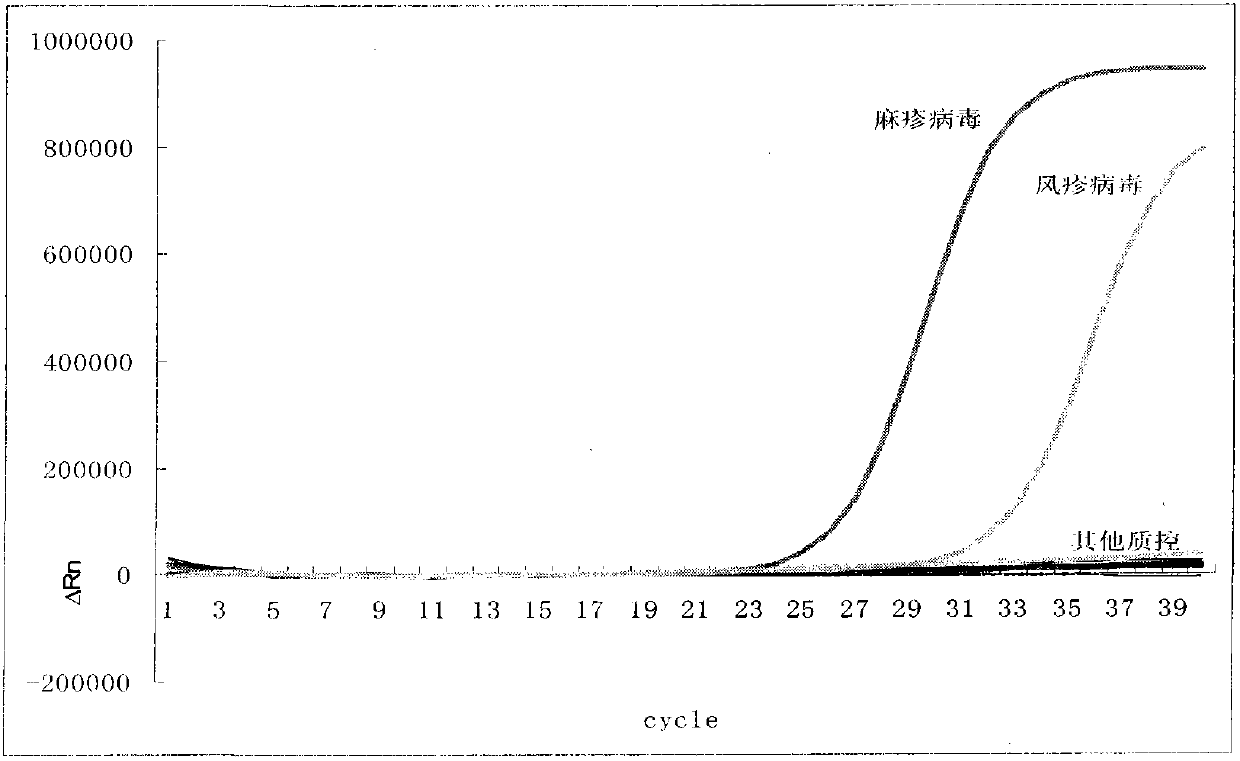
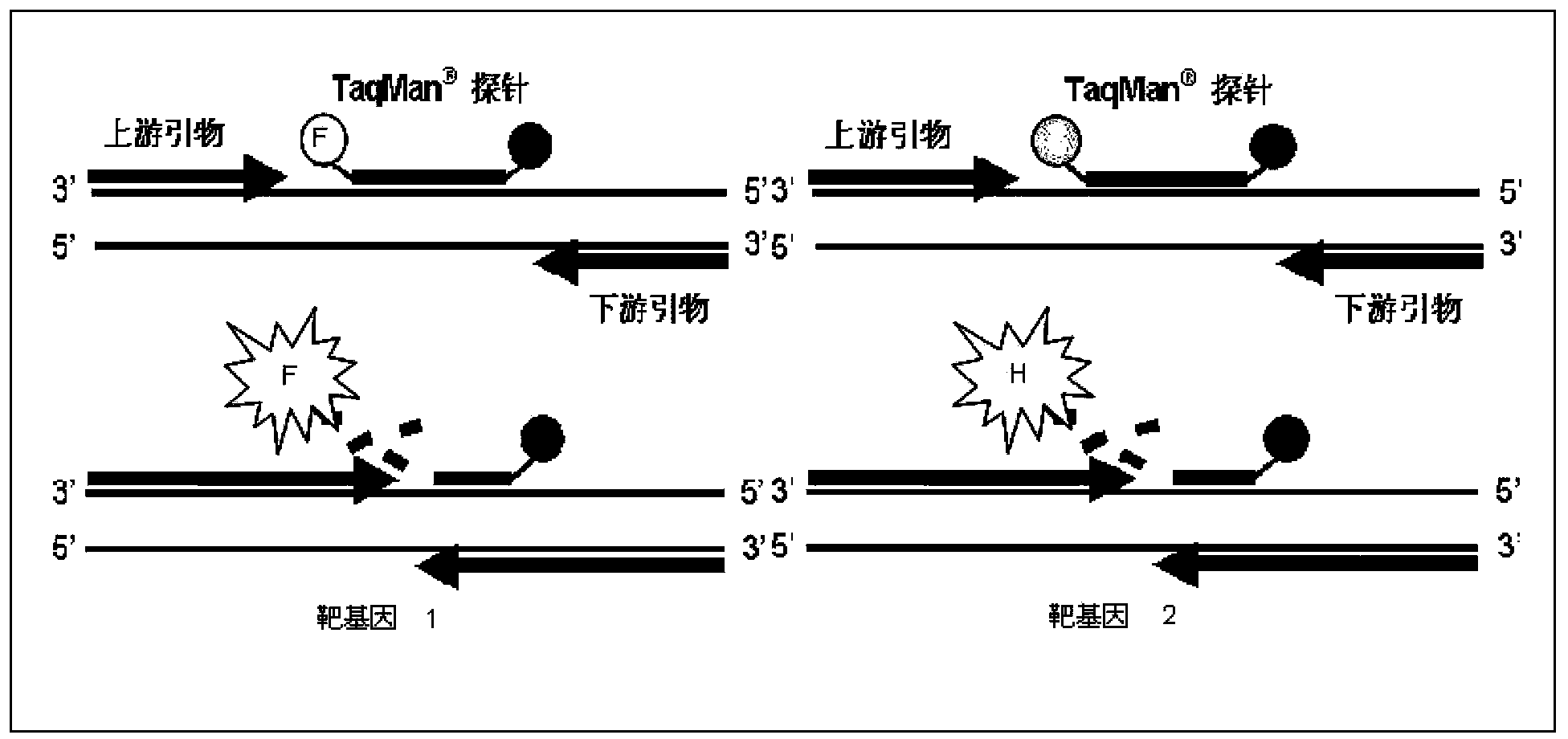
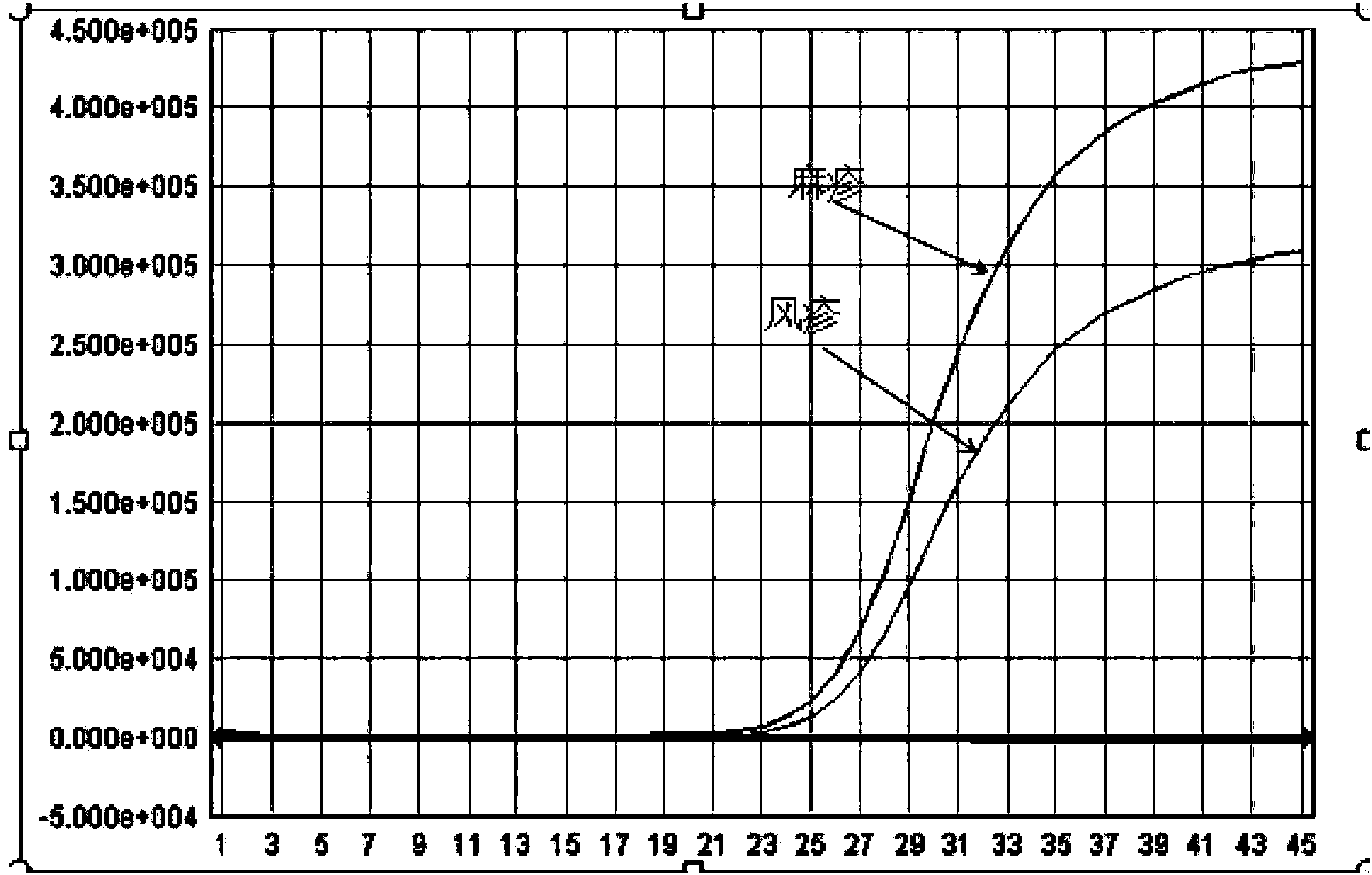
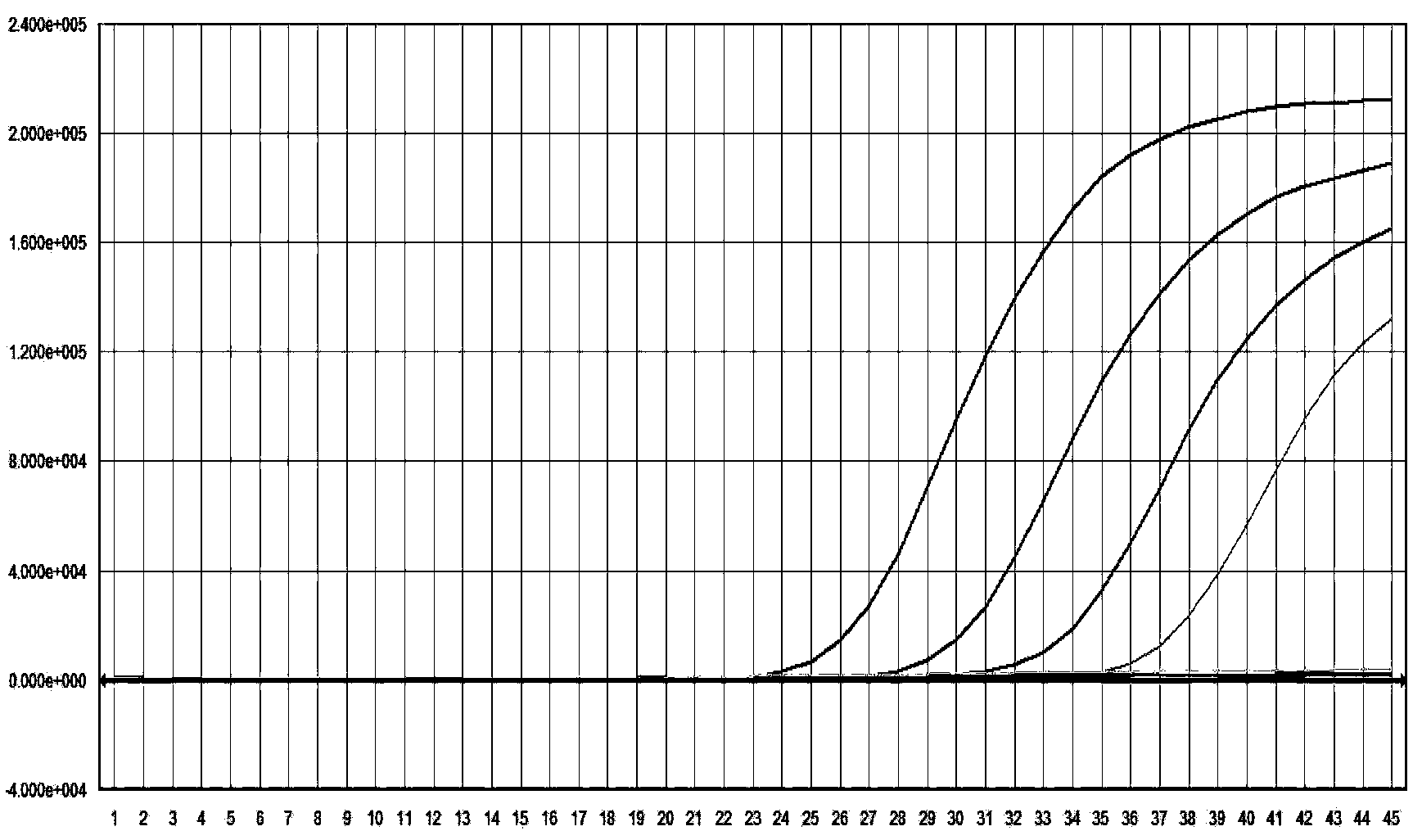
Primer probe combination for specific detection of measles virus and rubella virus and kit
ActiveCN102634610ALow detection costStrong specificityMicrobiological testing/measurementMicroorganism based processesDisease monitoringSpecific detection
The invention relates to an oligonucleotide sequence combination for specific detection of nucleic acid of measles virus and rubella virus in a sample by utilizing a fluorescent PCR (polymerase chain reaction) technology, as well as a kit including the combination. The kit can sensitively detect and identify the nucleic acid of the measles virus and the rubella virus in the sample, the detection lower limit is 20 copies per reaction system, and the kit further has an important application value in the fields of disease monitoring, clinical diagnosis and the like.
Owner:JIANGSU UNINOVO BIOLOGICAL TECH
Enzyme immunological method for quickly detecting rubella virus antibody
InactiveCN1434297AFor point-of-care testingImproved color development timeBiological testingViral antibodyRubella virus antibody
The invention discloses an enzyme immunological method of rapidly detecting the antibody of the measles virus, mainly using PEG to speed up the antigen-antibody reaction, thus shortening the reactiontime of routine ELISA. Firstly, wrap the soluble antigen in the enzyme-mark board; secondly made up the PBS lotion, PH9.6, and the sample diluent containing PEG; thirdly use the sample diluent to dilute the measured blood serum and the positive and negative comparison blood serum, fourthy add the diluted blood serum into the hole of the enzyme-mark board for warm cultivation; fifthly throw off the antigen solution in the hole, and use PBS lotion to wash; sixthly use the sample diluent to dilute the known enzyme-mark antibody; seventhly add the diluted enzyme-mark antibody in the hole for warmcultivation.
Owner:WUHAN UNIV
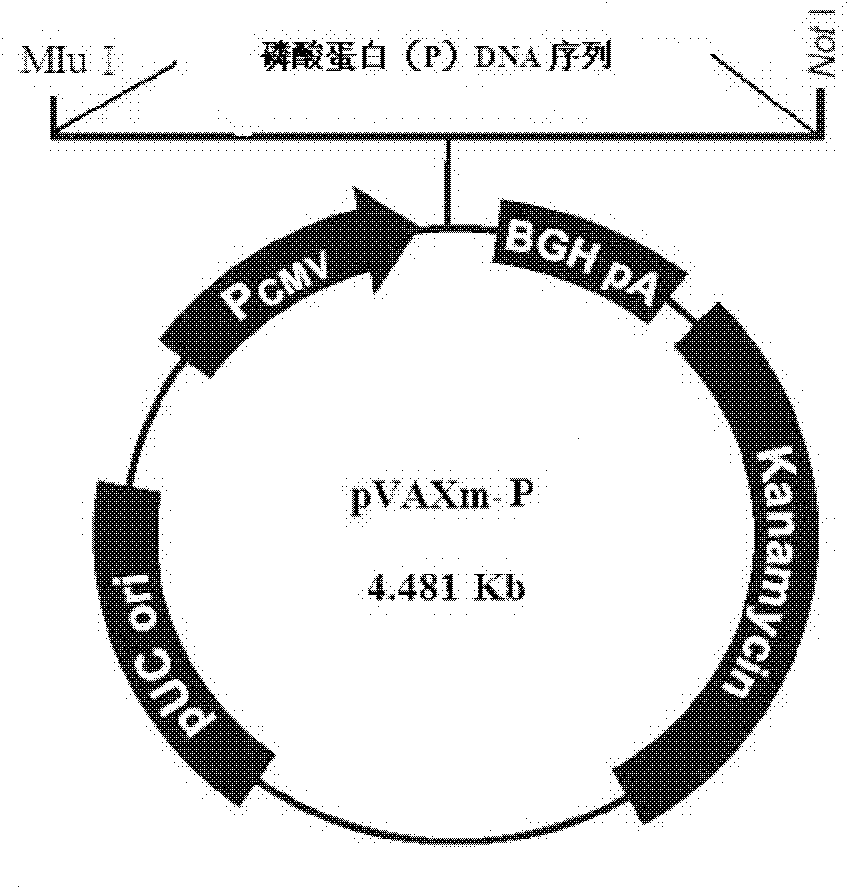
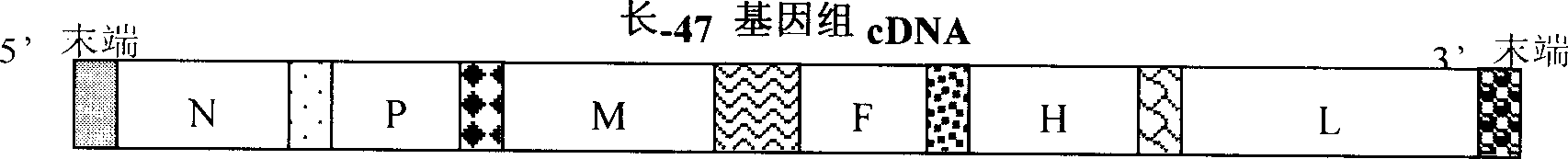
Non-coding region and P.M gene sequence and vector construction of long-47 measles virus
The present invention provides coding sequences and structures of all the non-coding regions and P and M coding regions of Longchun-47 measles virus, protein amino acid sequence and structure of P and M coding regions, the use of the non-coding region sequences in constituting the vaccine virus carrier and the constituting process of the carrier. These data and method may be used in recombining multiple effect vaccine or gene treatment via utilizing the vaccine strain virus to constitute expression vector and may be also used in molecular biological diagnosis and reagent development for measles virus infection.
Owner:中国预防医学科学院病毒学研究所
Antiviral polypeptides and use
InactiveCN101284869AReduce manufacturing costGood water solubilityPeptide/protein ingredientsAntiviralsChemical synthesisDisease
The invention discloses scorpion toxin antiviral polypeptide and a use of the same. Scorpion toxin antivirotic polypeptide (AVP-W2) is obtained through a molecular biological and chemical synthesis method, and then the antiviral activity of the AVP-W2 on measles virus (MeV) and human immunodeficiency virus (HIV) is measured through adopting an antibody neutralization method; moreover, the measurement shows that the AVP-W2 can effectively inhibit virus infection at a low concentration. The AVP-W2 has a tremendous prospect in making applied medicine which can cure or prevent a disease caused by virus. Moreover, the antiviral peptide of the invention has high activity on MeV and HIV and a simple method, and can be developed as antiviral medicine.
Owner:WUHAN UNIV
System and method for rescuing measles viruses and recombinant measles viruses
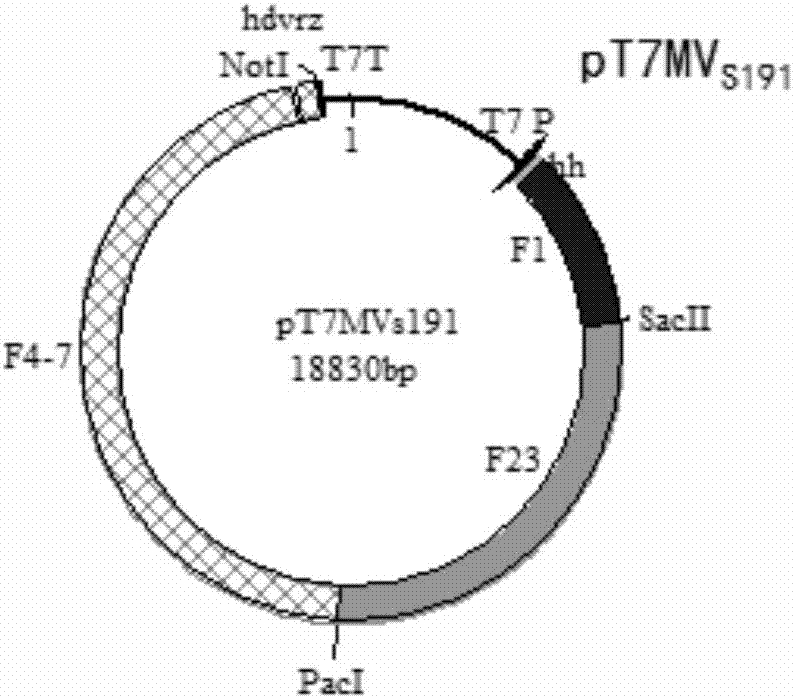
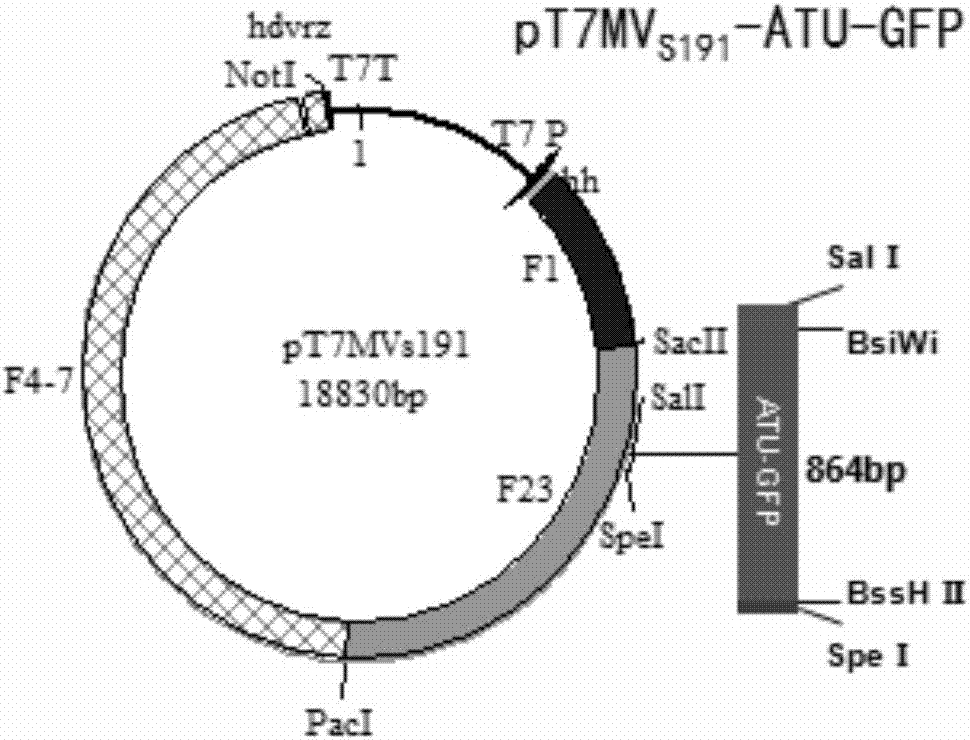
PendingCN106957859AImprove rescue efficiencyImprove stabilitySsRNA viruses negative-senseInactivation/attenuationGreen fluorescent proteinPolymerase L
The invention discloses a system and a method for rescuing measles viruses and recombinant measles viruses for expressing GFP (Green Fluorescent Protein) and application. The system provided by the invention comprises the following components: 1) a full-length transcription plasmid pT7MVS191 or pT7MVS191-ATU-GFP containing a measles virus anti-genome; 2) a helper plasmid 1 containing an encoding gene of nucleocapsid protein of the measles viruses; 3) a helper plasmid 2 containing an encoding gene of phosphoprotein of the measles viruses; and 4) a helper plasmid 3 containing an encoding gene of RNA (Ribonucleic Acid) polymerase of the measles viruses. The invention further provides a method for rescuing the measles viruses and the recombinant measles viruses. By adopting the system and the method, the measles viruses and the recombinant measles viruses can be effectively rescued; and the system and the method can be used for developing and applying therapeutic or preventive recombinant measles virus products.
Owner:CHENGDU INST OF BIOLOGICAL PROD
Nucleic acid detection kit for quickly detecting measles virus/rubella virus
ActiveCN103409554ASimple and fast operationAvoid pollutionMicrobiological testing/measurementMicroorganism based processesPositive controlDisease surveillance
The invention discloses a nucleic acid detection kit for quickly detecting a measles virus / a rubella virus. The kit comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, an enzyme mixed liquid, a measles virus / rubella virus reaction liquid, positive control and negative control. The kit disclosed by the invention overcomes the deficiencies that the prior art, almost measles virus / rubella virus detection kits are poor in specificity, relatively low in sensitivity and the like, has the advantages of high throughput, strong repeatability, quick and objective detection result and the like, is simple and convenient to operate and has huge application prospect in the fields such as clinical diagnosis and disease surveillance.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
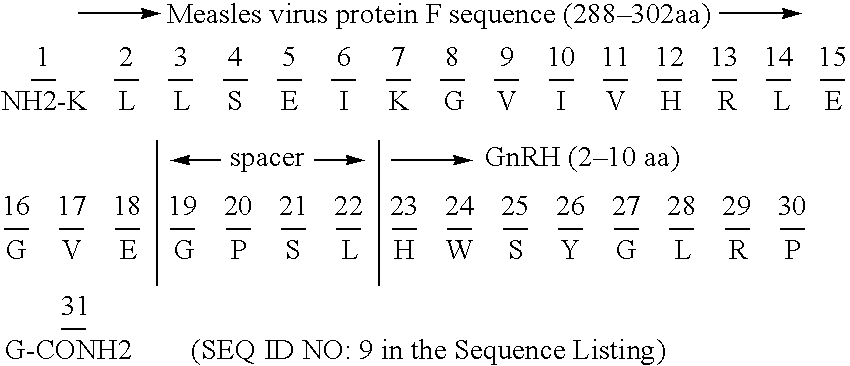
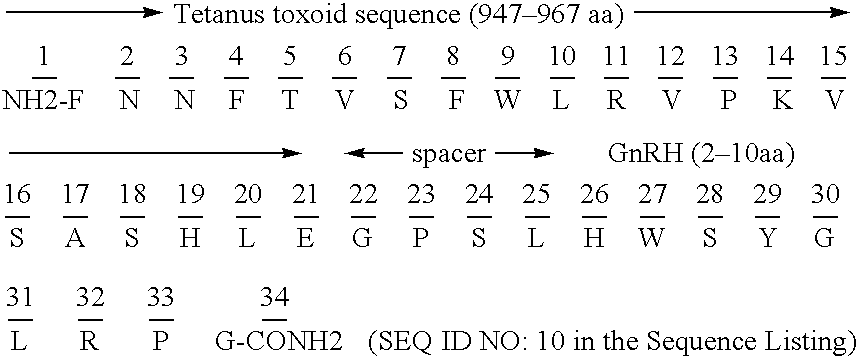
Chimeric peptide immunogens
InactiveUS20050106137A1Suppresses antibody responseSuppressing antibody responseAntibody mimetics/scaffoldsVaccinesTetanus toxoidsMalaria
Chimeric peptide epitopes can serve as effective immunogens against hormones and other small peptides or proteins. Immunogenic peptides are selected from promiscuous helper T-lymphocyte epitopes and synthesized together with self antigenic peptide sequences optionally fused through a spacer moiety. Examples of the chimeric peptide immunogens of the invention include peptide sequences which may be from any antigen, such as a gonadotropin releasing hormone (GnRH), linked with an immunogenic peptide sequence such as a promiscuous helper T-lymphocyte epitope of measles virus protein F, tetanus toxoid, or malaria protein CSP. Compositions of the chimeric immunogen are found effective in eliciting high and specific anti-GnRH antibody titers. This invention also relates to compositions and methods for synergistically enhancing or suppressing an immune response to a target antigen.
Owner:APHTON
Human embryonic lung fibroblastic cell SV-7 and application thereof
ActiveCN103387958ANo pollution in the processEasy to trainViruses/bacteriophagesEmbryonic cellsEnterovirusRotavirus RNA
The invention provides a human embryonic lung fibroblastic cell SV-7 with collection number CGMCC No.6956 and an application thereof. The SV-7 cell has obvious characteristics, vigorous growth and long life cycle; the average population doubling level is the 60th generation; the SV-7 cell is pure without pollution, and the culture method is simple; and moreover, the SV-7 cell has adaptability to multiple viruses, can be used for culturing varicella-zoster virus, enterovirus type 71, poliovirus, coxsackie virus A16, rubella virus, hepatitis A virus, rabies virus, rotavirus and measles virus, and can be further used for developing and preparing vaccines against the viruses. The cell culture cost is low, and the SV-7 has good economic values and broad application prospects.
Owner:SINOVAC RES & DEV
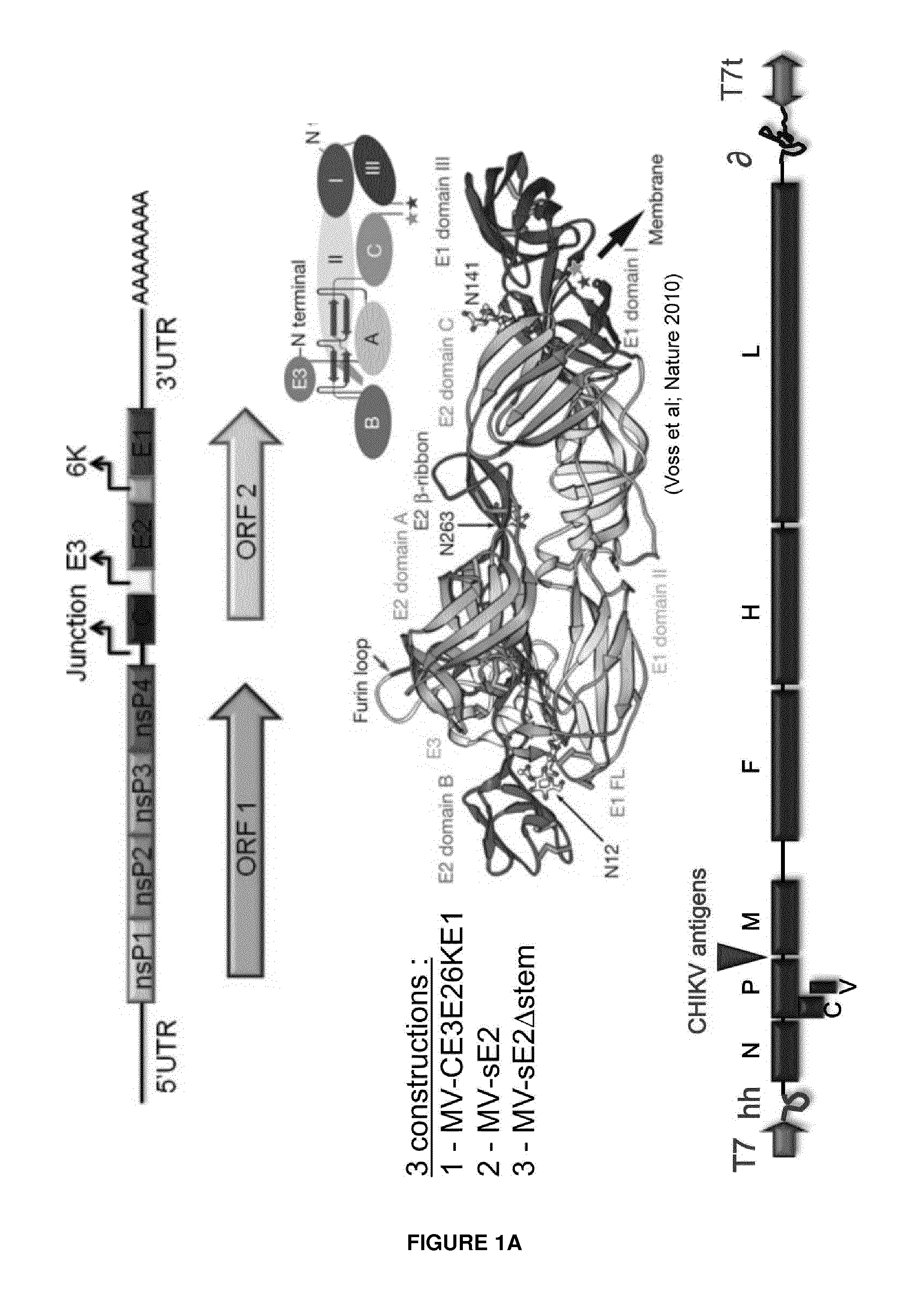
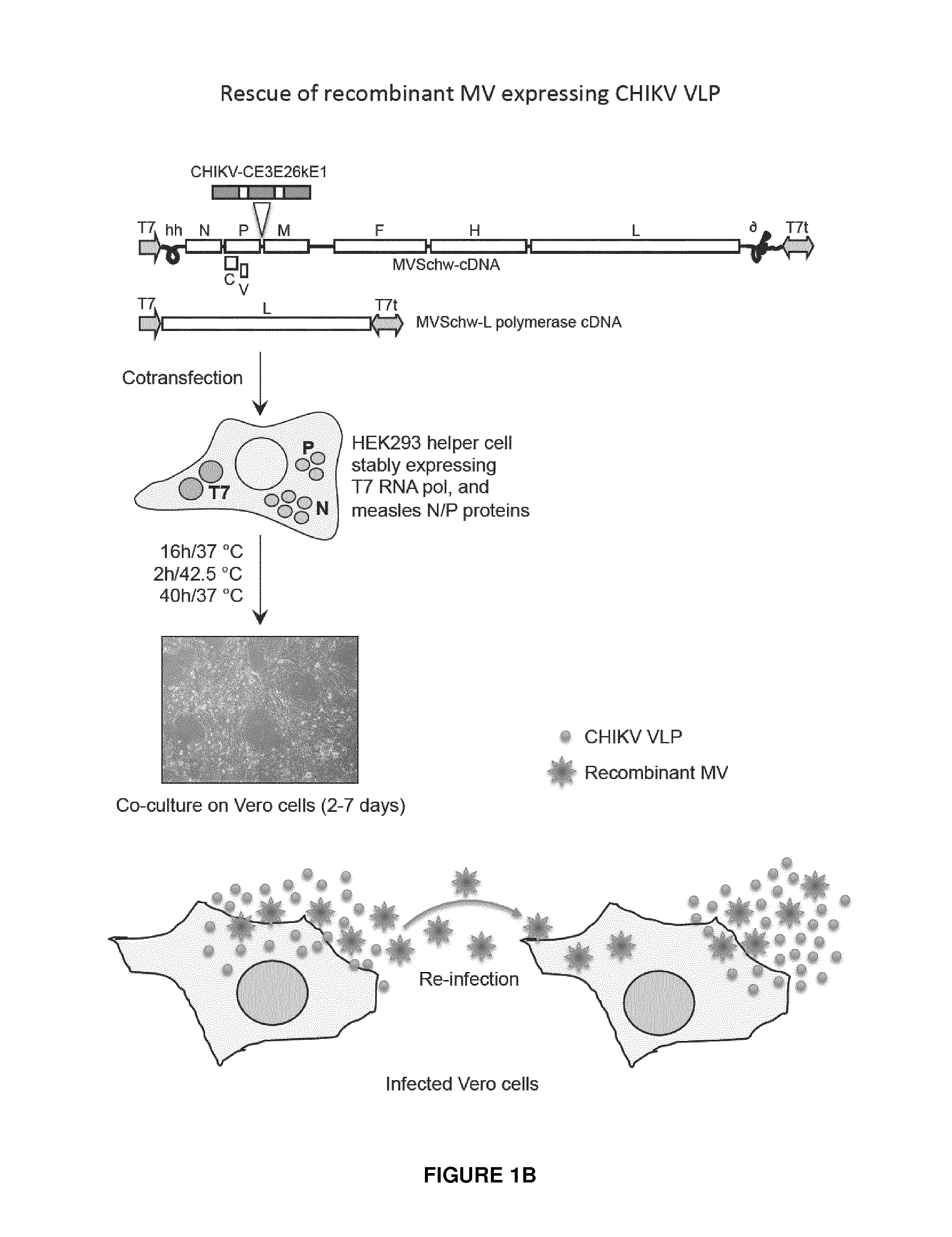
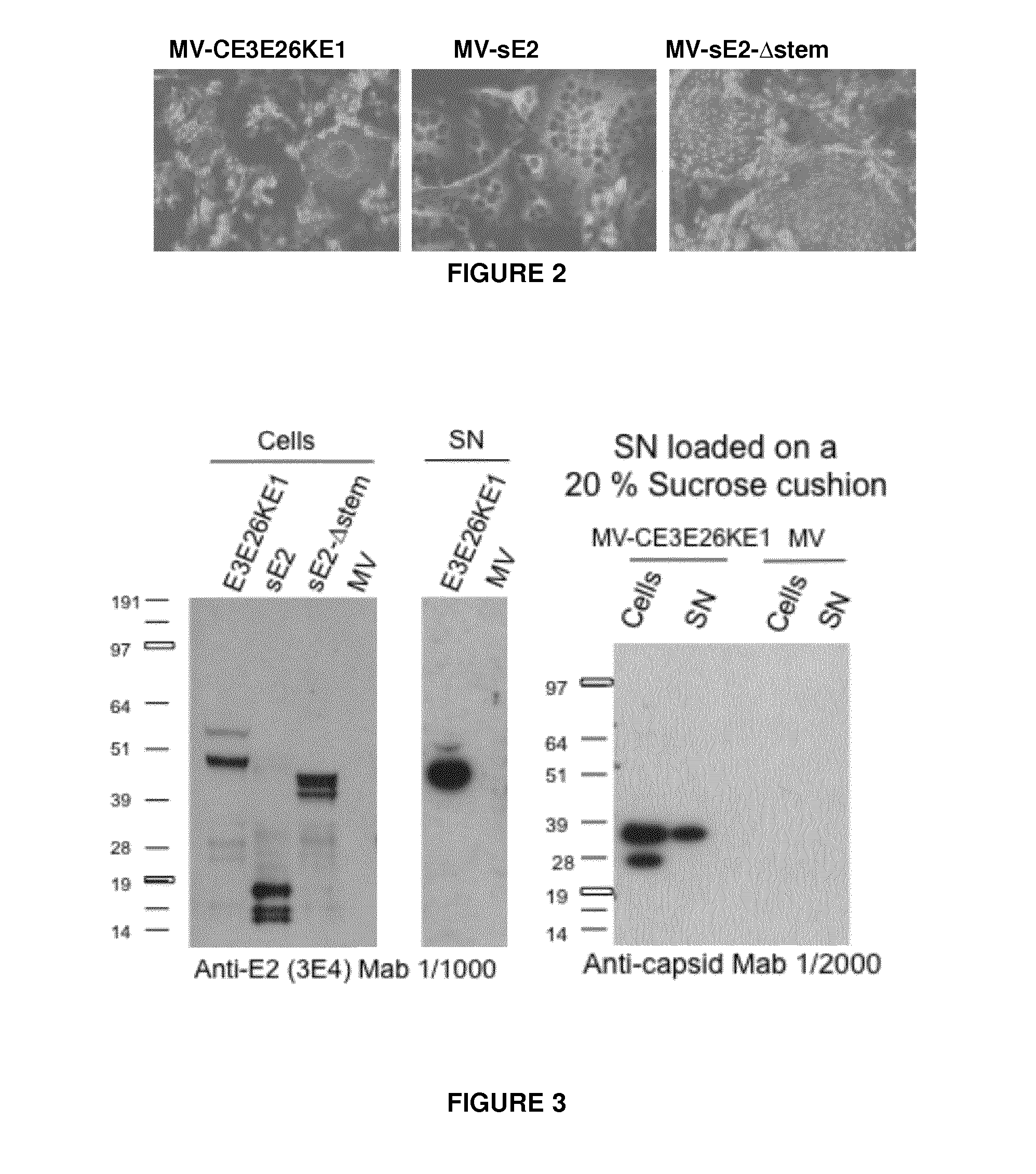
Recombinant measles virus expressing chikungunya virus polypeptides and their applications
ActiveUS20150238592A1Efficient expressionEasy to insertSsRNA viruses negative-senseBiocideVirus-like particleChikungunya fever
The invention relates to recombinant Measles virus expressing Chikungunya virus polypeptides, and concerns in particular virus like particles (VLP) that contain envelope and capsid proteins of a Chikungunya virus at their surface. These particles are recombinant infectious particles able to replicate in a host after an administration. The invention provides means, in particular nucleic acids, vectors, cells and rescue systems to produce these recombinant infectious particles. The invention also relates to the use of these recombinant infectious particles, in particular under the form of a composition, more particularly in a vaccine formulation, for the treatment or prevention of an infection by Chikungunya virus.
Owner:MERCK SHARP & DOHME LLC +2
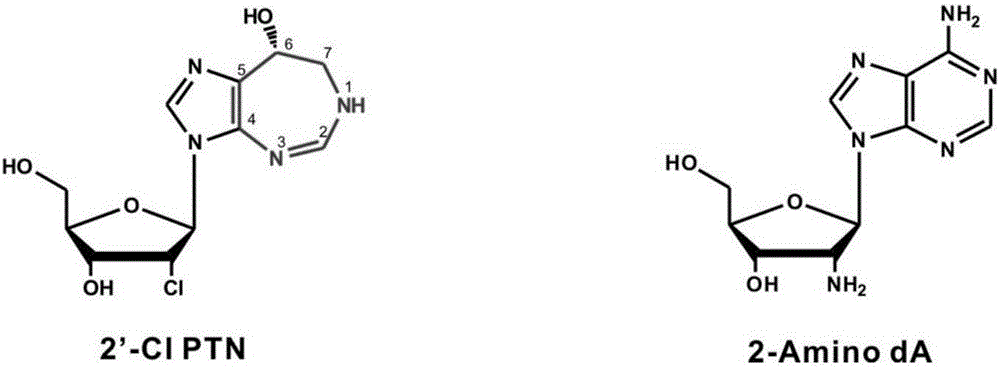
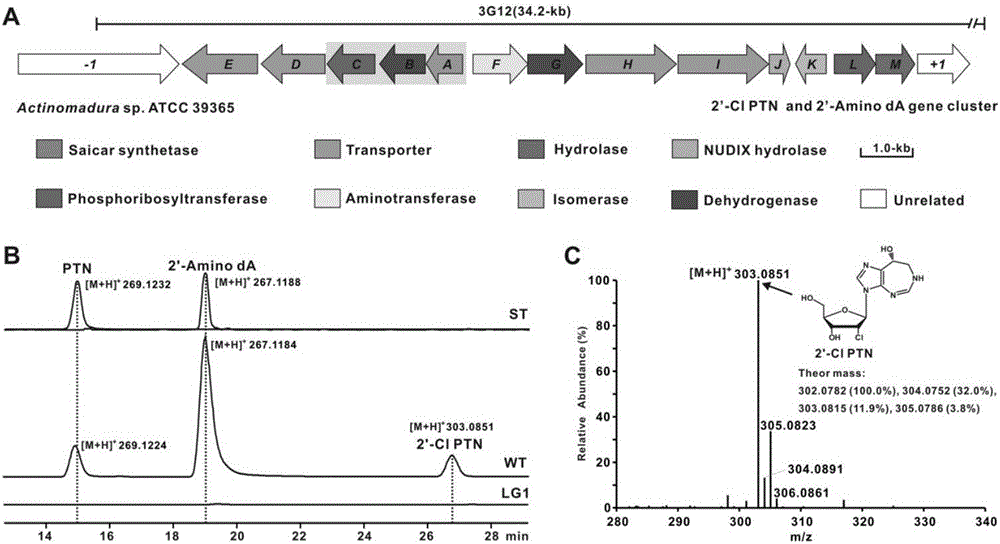
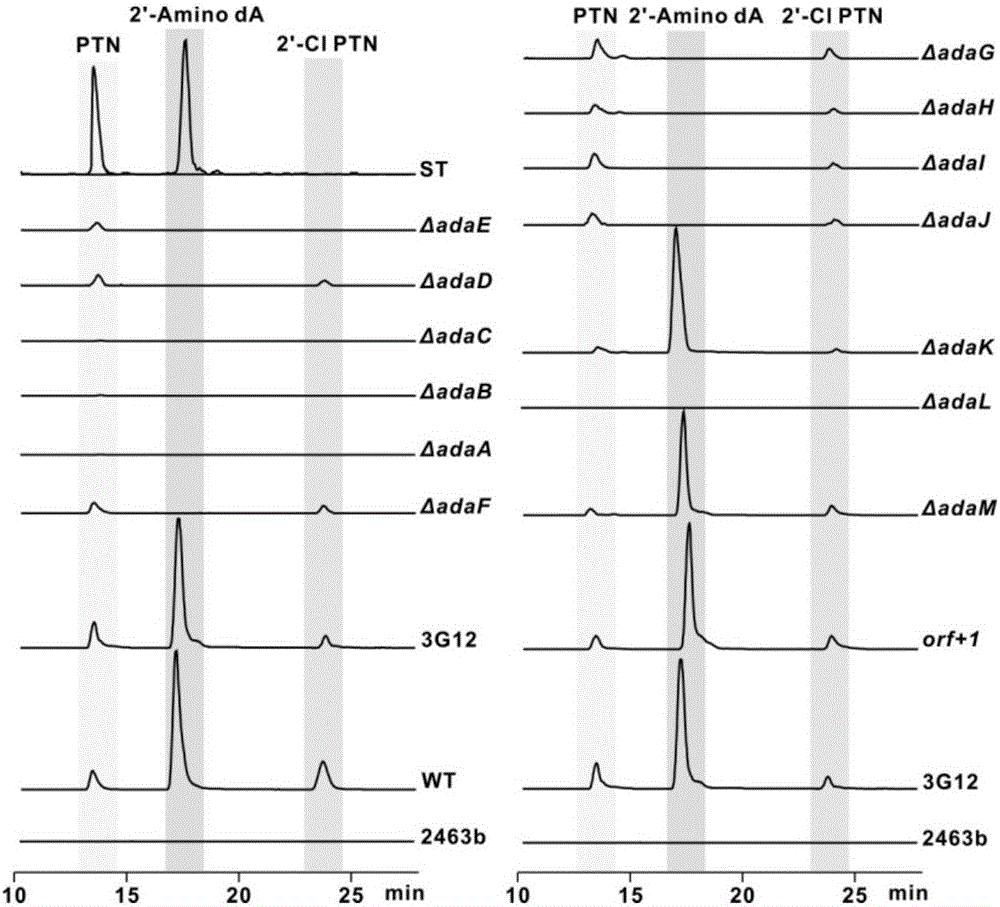
Biosynthesis gene cluster of 2'-chloropentostatin and 2'-amino-2'-deoxyadenosine and application thereof
ActiveCN106676115AUnderstanding Biosynthetic MechanismsImprove dynamic characteristicsHydrolasesMicroorganism based processesActinomadura maduraeBiosynthetic genes
The invention relates to cloning, sequencing, analysis and functional research of a biosynthesis gene cluster of 2'-chloropentostatin and 2'-amino-2'-deoxyadenosine and application thereof. The 2'-chloropentostatin is a natural product produced by actinomadura madurae ATCC 39365 and is used for treating leukemia; the 2'-amino-2'-deoxyadenosine is a natural product of RNA (ribonucleic acid) viruses of anti-mycoplasma virus, measles virus and the like. The whole gene cluster contains 13 genes, wherein five genes are related with synthesis of the 2'-chloropentostatin; four genes are related with synthesis of the 2-amino-2'-deoxyadenosine; four genes are related with transfer and regulation of the 2'-chloropentostatin and the 2-amino-2'-deoxyadenosine. The biosynthesis of the 2'-chloropentostatin and the 2-amino-2'-deoxyadenosine is blocked or improved by the genetic manipulation on the biosynthesis gene. The gene and the protein thereof can be applied to the gene engineering, protein expression, enzyme catalytic reaction and the like of the compound, and be used for looking and finding compounds, genes and proteins for medicines, industry or agriculture.
Owner:WUHAN UNIV
Immunization compositions and methods
ActiveUS8697353B2SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
A dengue virus chimeric polyepitope composed of fragments of non-structural proteins and its use in an immunogenic composition against dengue virus infection
ActiveUS20170158740A1SsRNA viruses negative-senseSsRNA viruses positive-senseStructural proteinPolynucleotide
The present invention is directed to a dengue virus chimeric polyepitope composed of fragments of non-structural proteins and its use in an immunogenic composition against dengue virus infection. The present invention provides means, in particular polynucleotides, vectors, cells and methods to produce vectors expressing said chimeric polyepitopes, in particular vectors consisting of recombinant measles virus (MV) particles. The present invention also relates to the use of the recombinant MV particles, in particular under the form of a composition or of a vaccine, for the prevention and / or treatment of a dengue virus infection.
Owner:INST PASTEUR +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com