Recombinant virus and application thereof in mumps virus neutralizing antibody detection
A mumps virus, recombinant virus technology, applied in the direction of virus/phage, application, virus, etc., can solve the problems of low detection efficiency, manual judgment, high professional requirements of experimenters, etc., and achieve the effect of accurate detection results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: A kind of recombinant virus
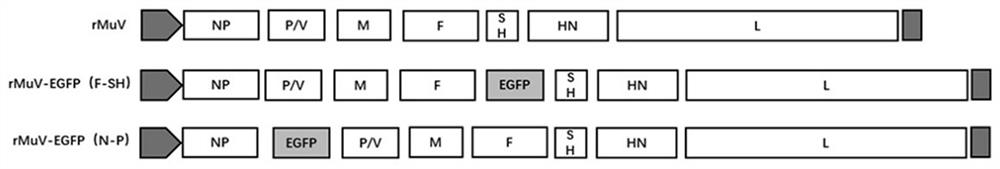
[0042] This embodiment provides a recombinant virus, which uses mumps virus strain QS-F (preservation number is CCTCC No: V201948, recorded in the patent application text with publication number CN111019910A) as a living vector to express the green The gene of fluorescent protein (the amino acid sequence is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.2), wherein the gene encoding green fluorescent protein is inserted into the NP segment and P / Between the V segments (recombinant virus genome architecture pattern see figure 1 ).
[0043] The preparation method of described recombinant virus comprises the steps:
[0044] 1. Construction of pAC-rMuV-eGFP (N-P) full-length clone
[0045] Synthesize DNA fragments F1, F2; use restriction enzyme P ml I andN ae I (purchased from Takara Company) digested the recombinant plasmid pAC-MuV (recorded in the patent application text with publication number ...
Embodiment 2
[0051] Embodiment 2: A kind of recombinant virus
[0052] This embodiment provides a recombinant virus, which uses mumps virus strain QS-F (preservation number is CCTCC No: V201948, recorded in the patent application text with publication number CN111019910) as a living vector to express the green The gene of fluorescent protein (the amino acid sequence is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.2), wherein the gene encoding green fluorescent protein is inserted into the F segment and SH segment of the mumps virus genome Between (recombinant viral genome architecture pattern see figure 1 ).
[0053] The preparation method of described recombinant virus comprises the steps:
[0054] 1. Construction of pAC-rMuV-eGFP (F-SH) full-length clone
[0055] Synthesize DNA fragments F3, F4, and F5; use F3 and F4 as templates, and F3-GFP-F, F4-GFP-R as primers, perform fusion PCR to obtain fragments F3+F4; use restriction endonuclease X ma I and S a...
experiment example 1
[0061] Experimental Example 1: Performance Growth Verification of Recombinant Viruses
[0062] Vero cells (purchased from ATCC) were 8*10 5 The inoculum of each individual was plated in advance in a six-well plate containing 2000 μL / well DMEM medium (purchased from Gibco), and cultured at 37°C until the cells covered a monolayer; Influenza virus strain QS-F) was used as a control, and the recombinant virus pAC-rMuV-eGFP (N-P) and the recombinant virus pAC-rMuV-eGFP (F-SH) were inoculated to Vero In the cells, after adsorption and infection at 37°C for 1 hour, the DMEM medium was sucked off with a pipette gun, and the cells containing 2% (v / v) fetal bovine serum were added to the six-well plate at an addition amount of 2000 μL / well. The maintenance solution (the cell maintenance solution is DMEM medium purchased from Gibco Company) was cultured at 34°C, and the culture supernatant was collected on the 1d, 2d, 3d, 4d, and 5d of the virus inoculation, and the virus titer was det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com