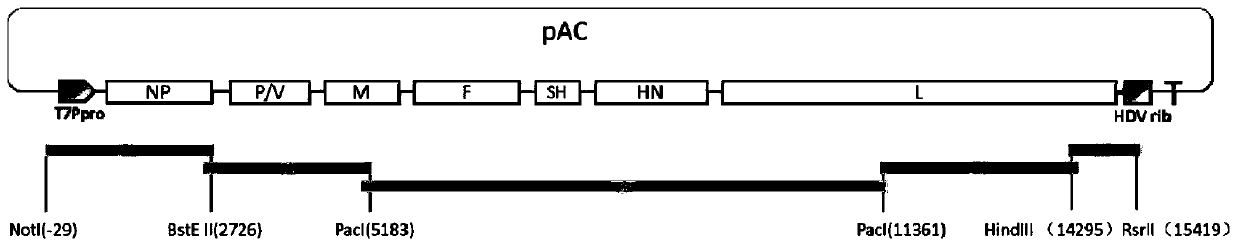
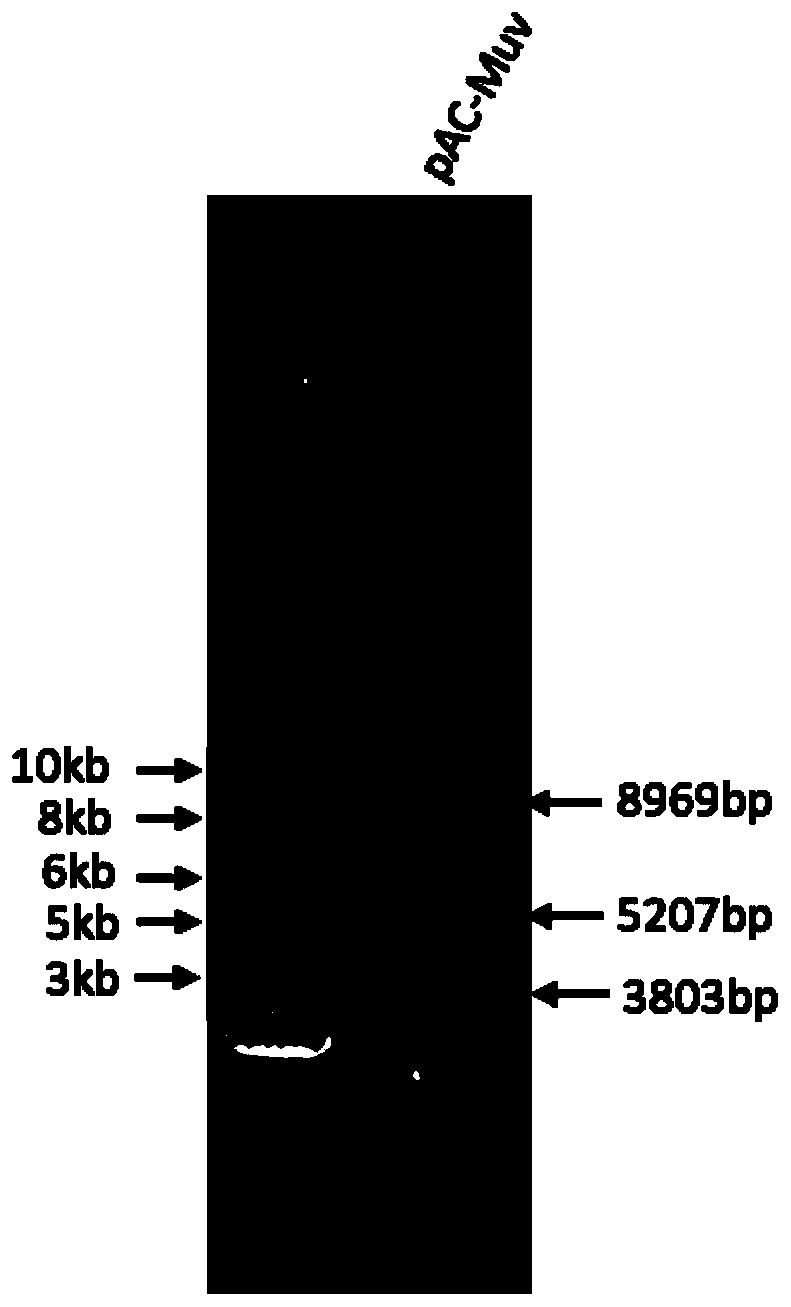
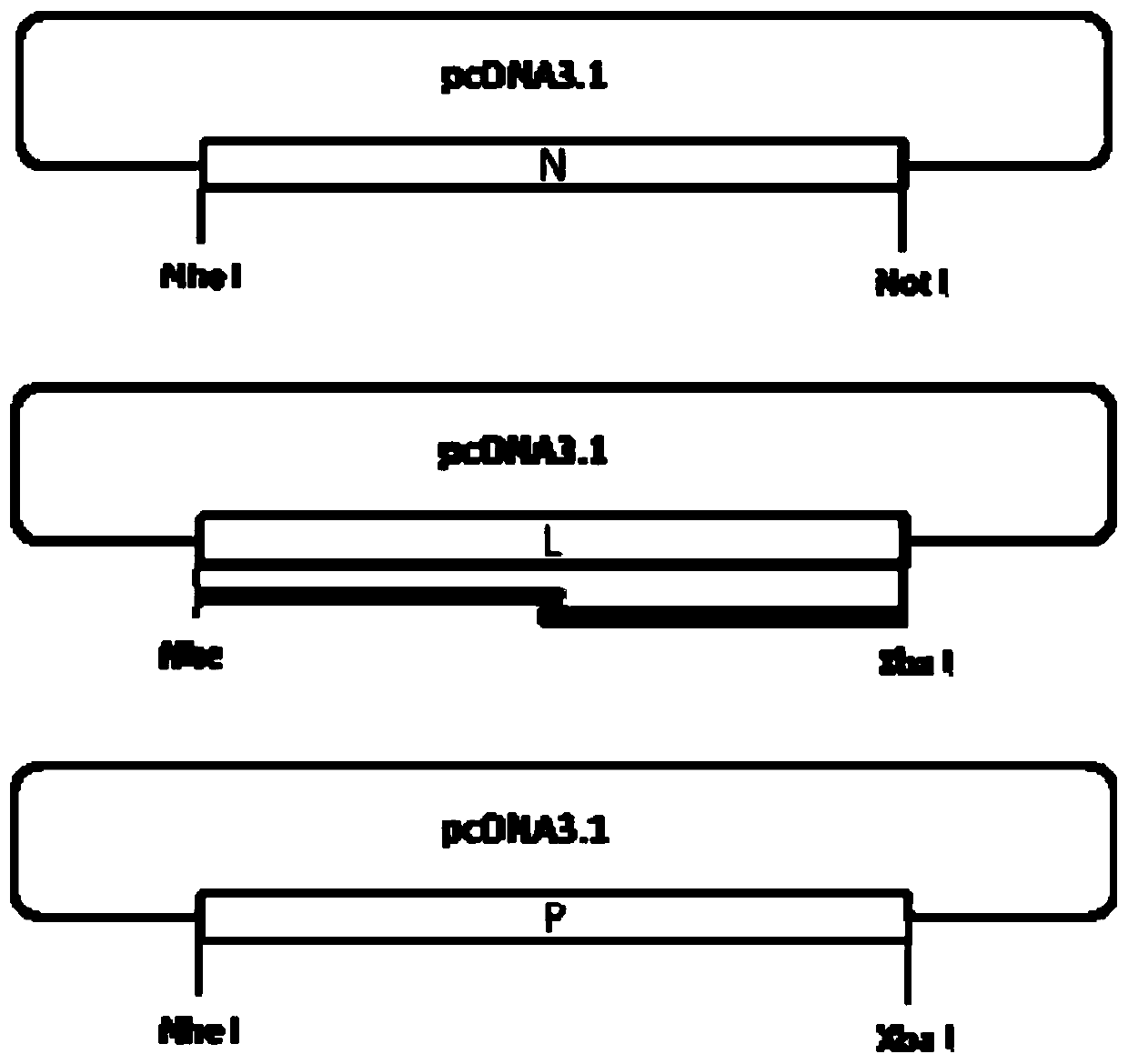
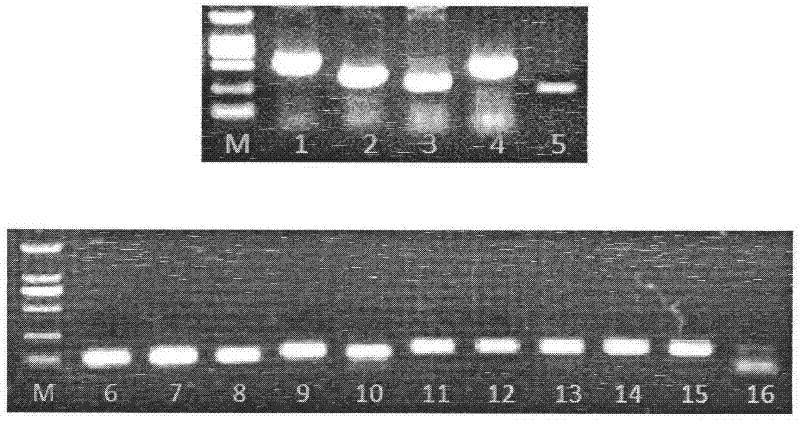
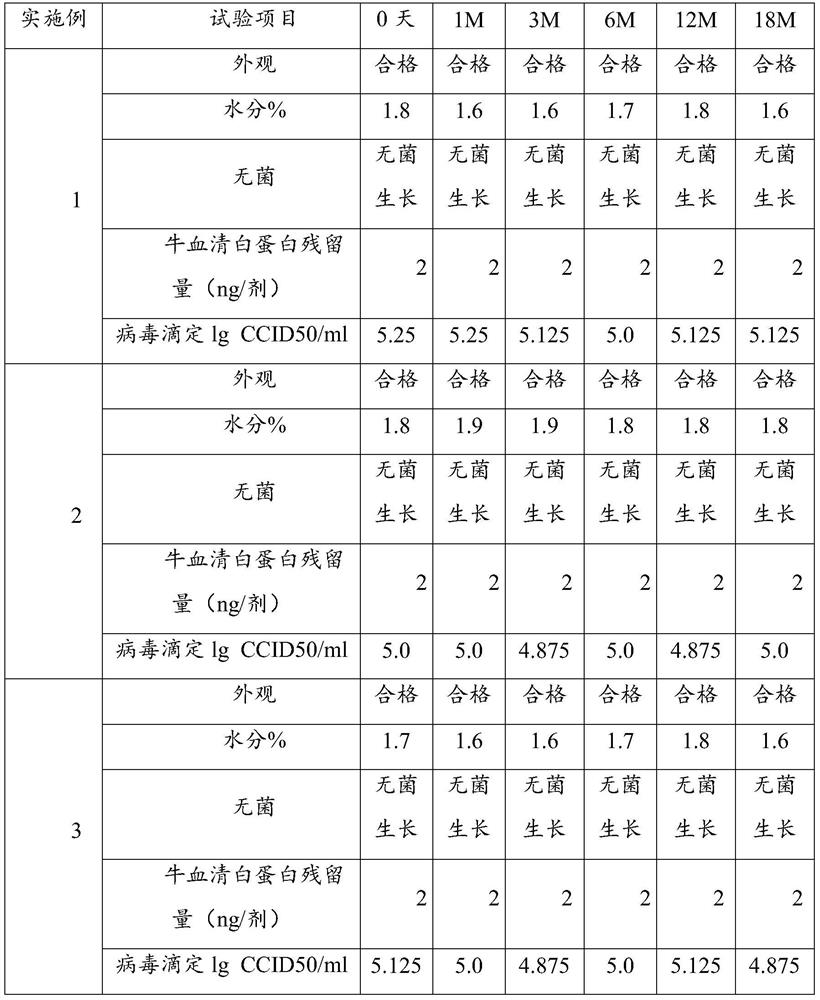
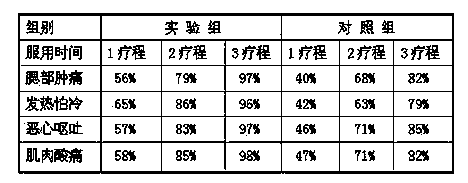
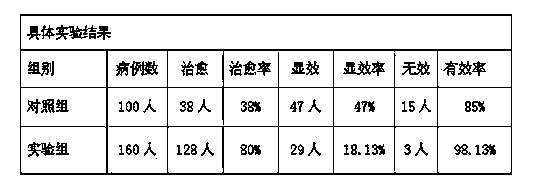
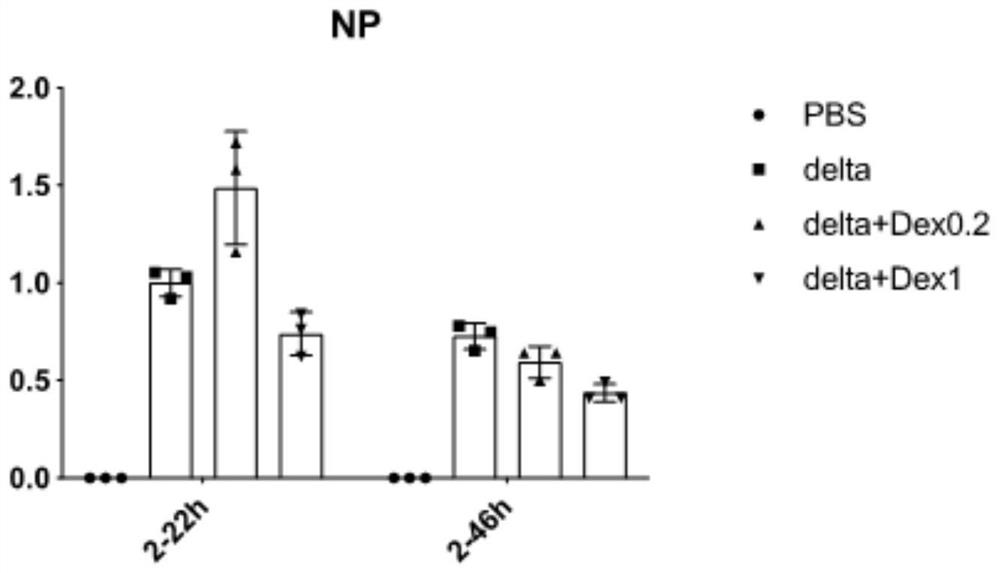
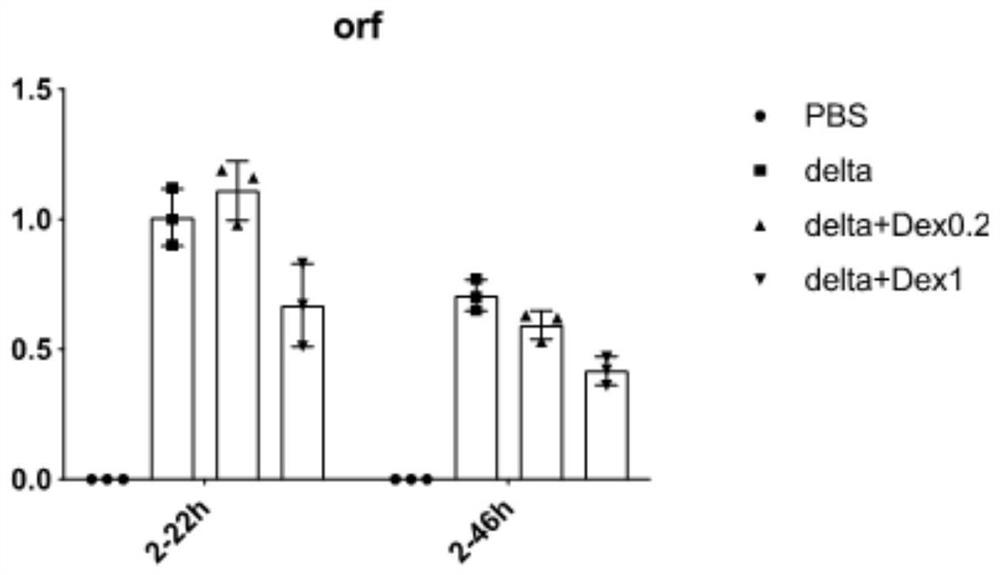
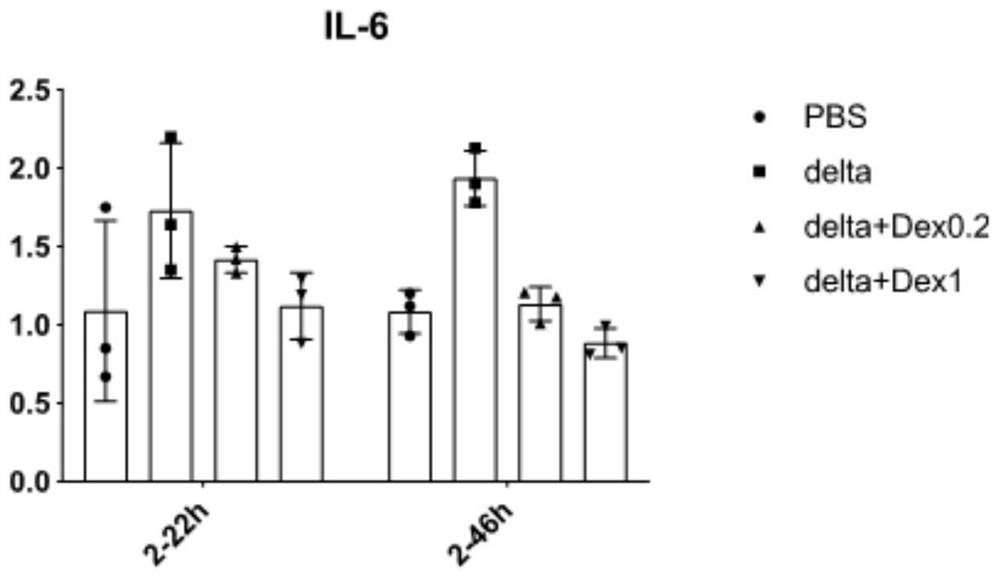
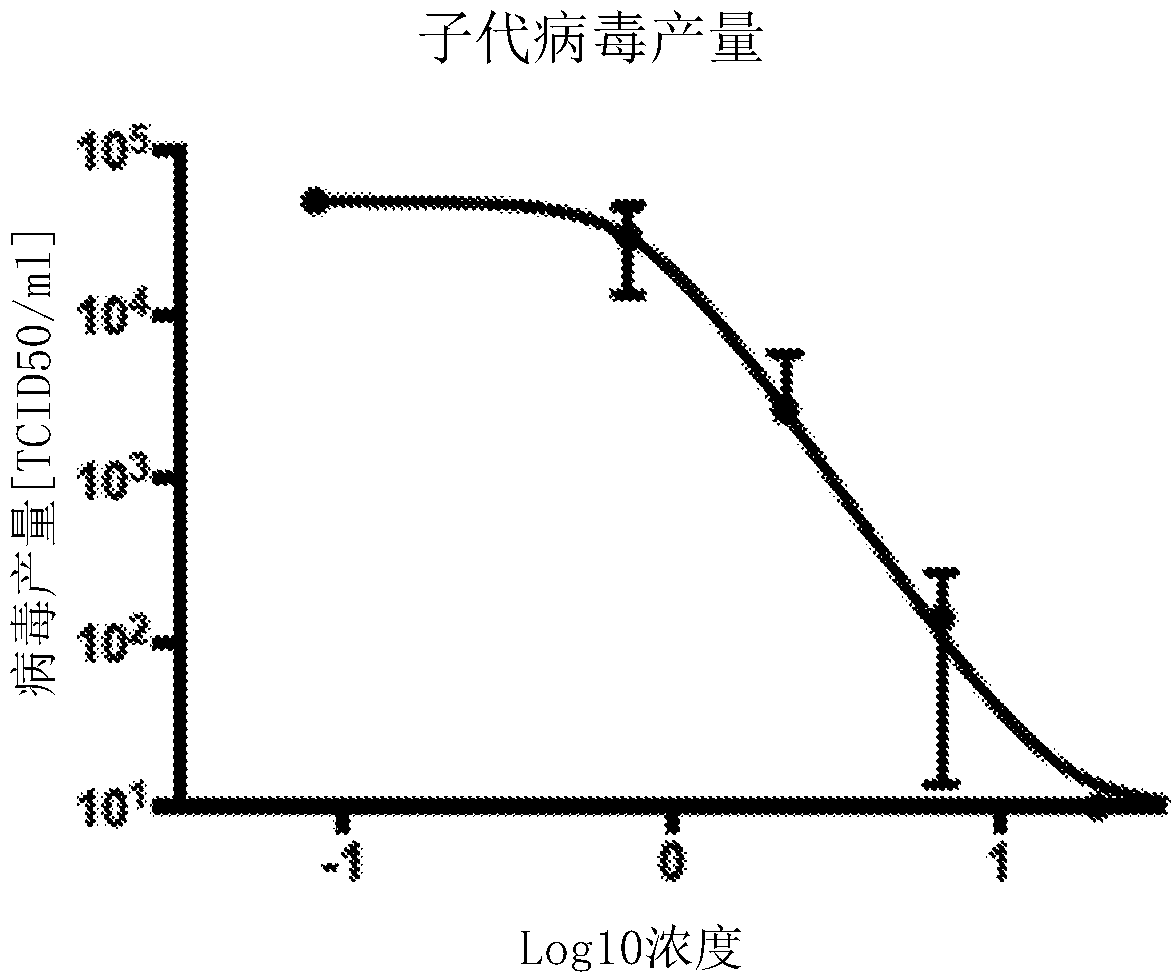
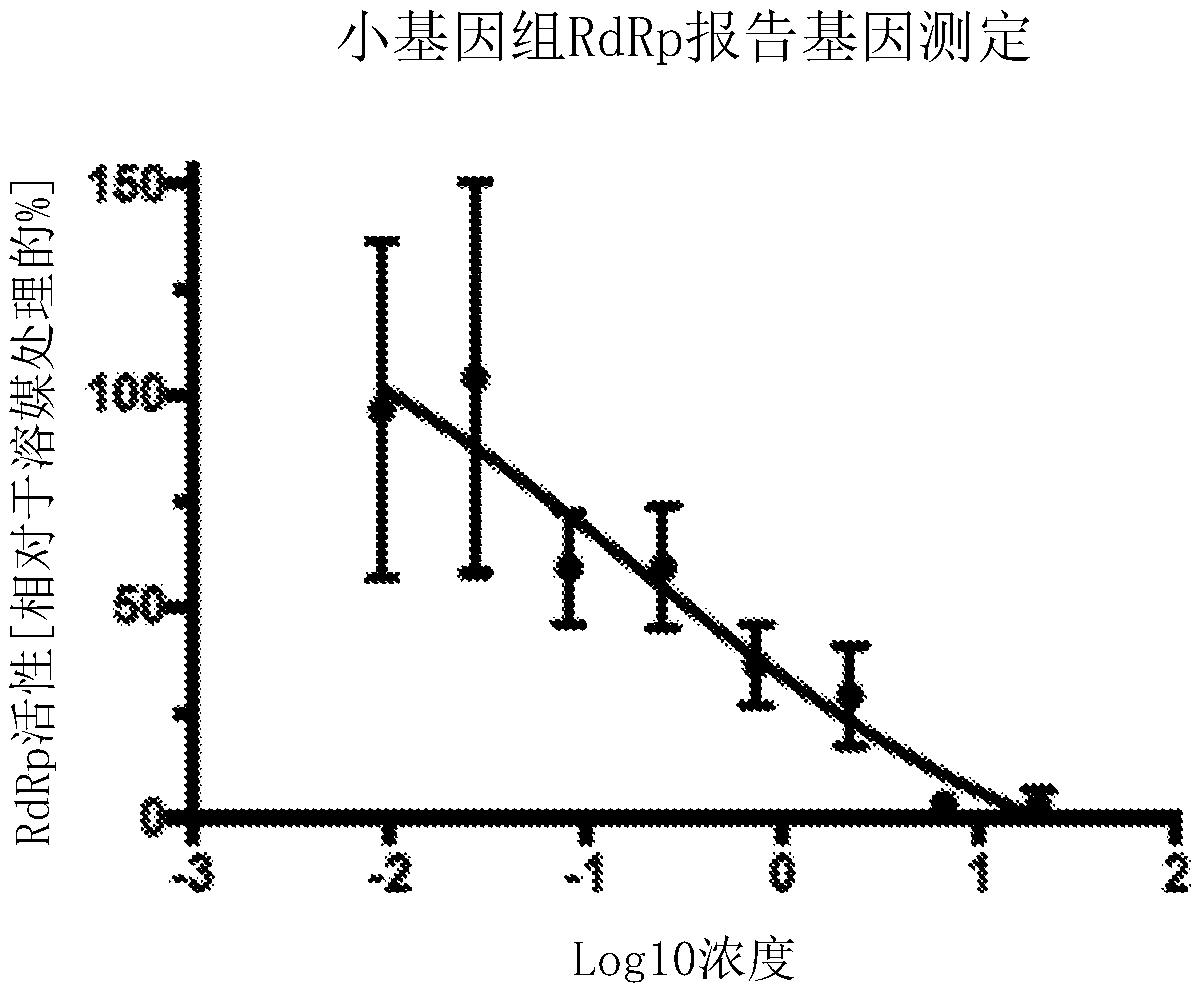
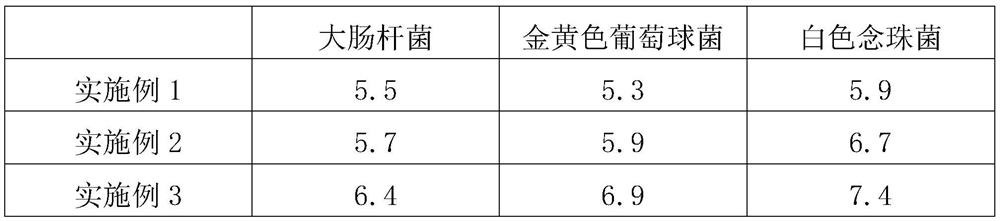
Patents
Literature
57 results about "Mumpsvax" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mumpsvax is a mumps vaccine made of mumps virus. Mumpsvax is a product of Merck & Co.,Inc. The vaccine is a component of Merck's three-virus MMR vaccine. Mumpsvax is administered by a subcutaneous injection of live virus reconstituted from freeze-dried vaccine. The dosage of the mumps vaccine component in MMR is the same as of Mumpsvax, "Each 0.5 mL dose contains not less than 20,000 TCID₅₀ of mumps virus." The Merck product information recommends MMR as the secondary vaccination treatment with Mumpsvax. Mumpsvax is produced from the Jeryl Lynn strain of mumps virus developed by Maurice Hilleman. When his daughter Jeryl Lynn Hilleman contracted Mumps Dr. Hilleman cultured the vaccine strain from her throat. The mumps virus strains were developed in embryonic hens' eggs and chick embryo cell cultures. The resulting strains of virus were less well-suited for human cells, and are thus said to be attenuated. They are sometimes referred to as neuroattenuated in the sense that these strains are less virulent to human neurons than the wild strains. The cells used in culture, virus stocks used, and animal fluids are all screened for extraneous material as part of the vaccine production.
Composition for killing virus and preventing influenza and cold prevention plaster prepared by composition
InactiveCN102349991AIncrease concentrationUniform slow releaseAntibacterial agentsBiocideDiseaseRespiratory tract disease
The invention relates to a composition for killing virus and preventing influenza and a cold prevention plaster prepared by the composition. An aromatic traditional Chinese medicine volatile extract product and fragrant plant essential oil are used as effective components of the plaster; high-molecular colloid, which can be compatible with the effective components and has a stereo network structure, and an excipient are used to wrap the effective components to prepare a microcapsule; the microcapsule is used to coat on a matrix to prepare a tablet; a double faced adhesive tape is used to plaster the tablet at the position close to mouth and nose (such as collar, shoulder, chest and the like) so as to allow the effective components to slowly release at uniform speed. The plaster can be used to kill pathogen and virus in the air and enhance the immunity of upper respiratory tract, has an obvious effect of preventing influenza, and has a certain prevention effect for common cold, hand-foot-mouth disease, varicella, measles, epidemic encephalitis B, epidemic mumps, chronic respiratory tract diseases and the like. The plaster is convenient to use, has fragrant smell and is safe without side-effect.
Owner:王红芳
Spleen extracts, its preparation method and use
ActiveCN1765371AThe preparation process is stable and matureImprove malignant transformationPowder deliveryPeptide/protein ingredientsDiseaseWhite blood cell
The invention discloses a spleen extract prepared from mammalsí» spleens through fat-removing, homogenating, comminuting, settling and hyperfiltrating, and comprises the following constituents: polypeptides, free amino acid, nucleic acid and total sugar. The spleen extract can be used for preparing medicament for the treatment of cell immunodeficiency diseases, respiratory tract and lung infection, chronic hepatitis B, mumps, aphtae, leucopenia, leukemia, regenerative anemia, and malignant tumour.
Owner:融致丰生制药有限公司
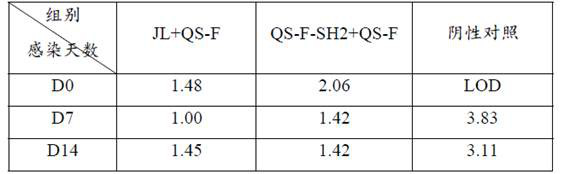
F genotype mumps virus attenuated strain as well as construction method and application thereof
The invention provides an F genotype mumps virus attenuated strain as well as a construction method and application thereof. Specifically, the invention provides an F genotype mumps virus attenuated strain and the attenuated strain is a mumps virus QS-F-SH2 with an accession number of CCTCC NO: V201950. The invention also provides a vaccine composition containing the F genotype mumps virus attenuated strain as an active ingredient and a preparation method thereof. The mumps virus attenuated vaccine strain disclosed by the invention can match the F type mumps virus predominantly popular in China, and the level of the mumps virus attenuated vaccine strain is equivalent to that of the current vaccine strain in the aspects of growth characteristics, immunogenicity, neurotoxicity and the like.In addition, the mumps virus genetic engineering attenuated strain screened by the invention can be stably produced in chick embryo cells, and the safety is high.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD +1
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Preparation method and application of gene chip for detecting important respiratory pathogenic viruses
InactiveCN102586475AStrong specificityImprove the consistency rateMicrobiological testing/measurementOligonucleotide chipMeasles virus IgG
The invention relates to a gene chip for detecting important respiratory pathogenic viruses. A preparation method of the gene chip comprises the following steps of: preparing a specific primer; preparing a virus specific probe; preparing an oligonucleotide chip; establishing an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) system; establishing a hybrid system; preparing a visual detection reagent; and establishing a developing method. The gene chip prepared by the invention can be used for simultaneously discriminating nine general respiratory pathogenic viruses comprising A and B type influenza viruses, parainfluenza viruses type 1 and 2, human metapneumovirus, respiratory syncytial virus, measles virus, rubella virus and mumps virus, provides a new solution for quickly detecting the general respiratory pathogenic viruses at high throughout and can provide guidance for monitoring, clinically diagnosing and treating the respiratory pathogenic viruses.
Owner:深圳市普瑞康生物技术有限公司 +1
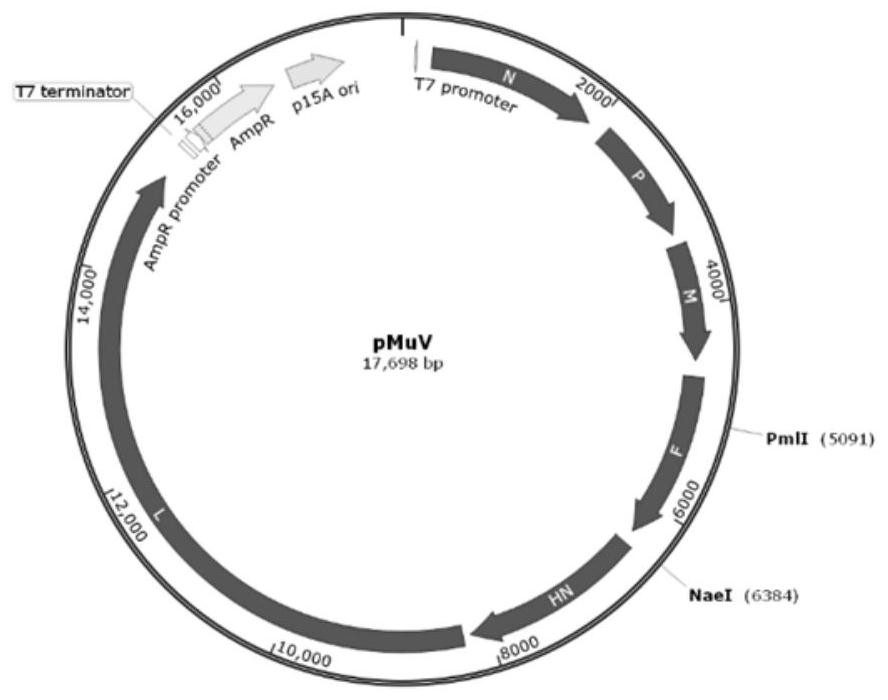
Rescue of mumps virus from cDNA
The present invention relates to a method of recombining and producing immunogenic composition by rescuing non-segmented antisense single-stranded RNA virus-mumps virus. Other embodiments relate to methods of making attenuated and / or infectious mumps viruses. Prepare recombinant viruses from cDNA clones to make specific changes, including deletions, insertions, substitutions and rearrangements of nucleotides / polynucleotides within the genome.
Owner:WYETH LLC
F-gene type attenuated live mumps vaccine and preparation method and application thereof
InactiveCN102018956AGood humoral immunityExcellent neutralization potencyInactivation/attenuationAntiviralsBiotechnologyGenotype
The invention discloses an F-gene type attenuated live mumps vaccine and a preparation method and application thereof, relating to an attenuated live mumps vaccine which is prepared in such a way that a separated F-gene type mumps attenuated live seed is inoculated to the human diploid cells (KMB17 strain) and is subjected to subsequent processes and the freezing-drying technology. The vaccine product has good immunogenicity and safety. The invention provides a cryoprotectant which does not contain glutin, thereby ensuring that the attenuated live mumps vaccine has good appearance, low moisture content and good stability.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
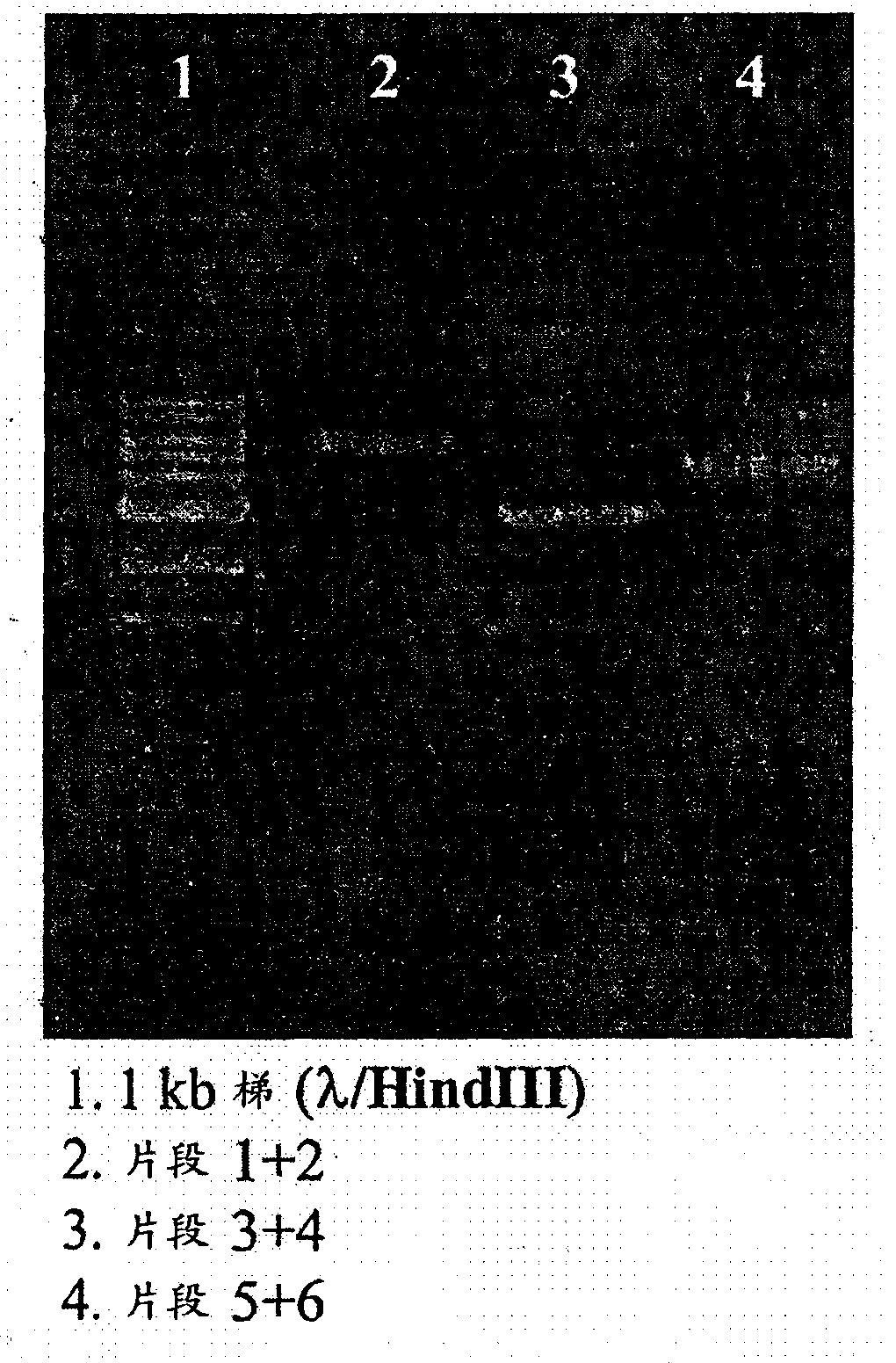
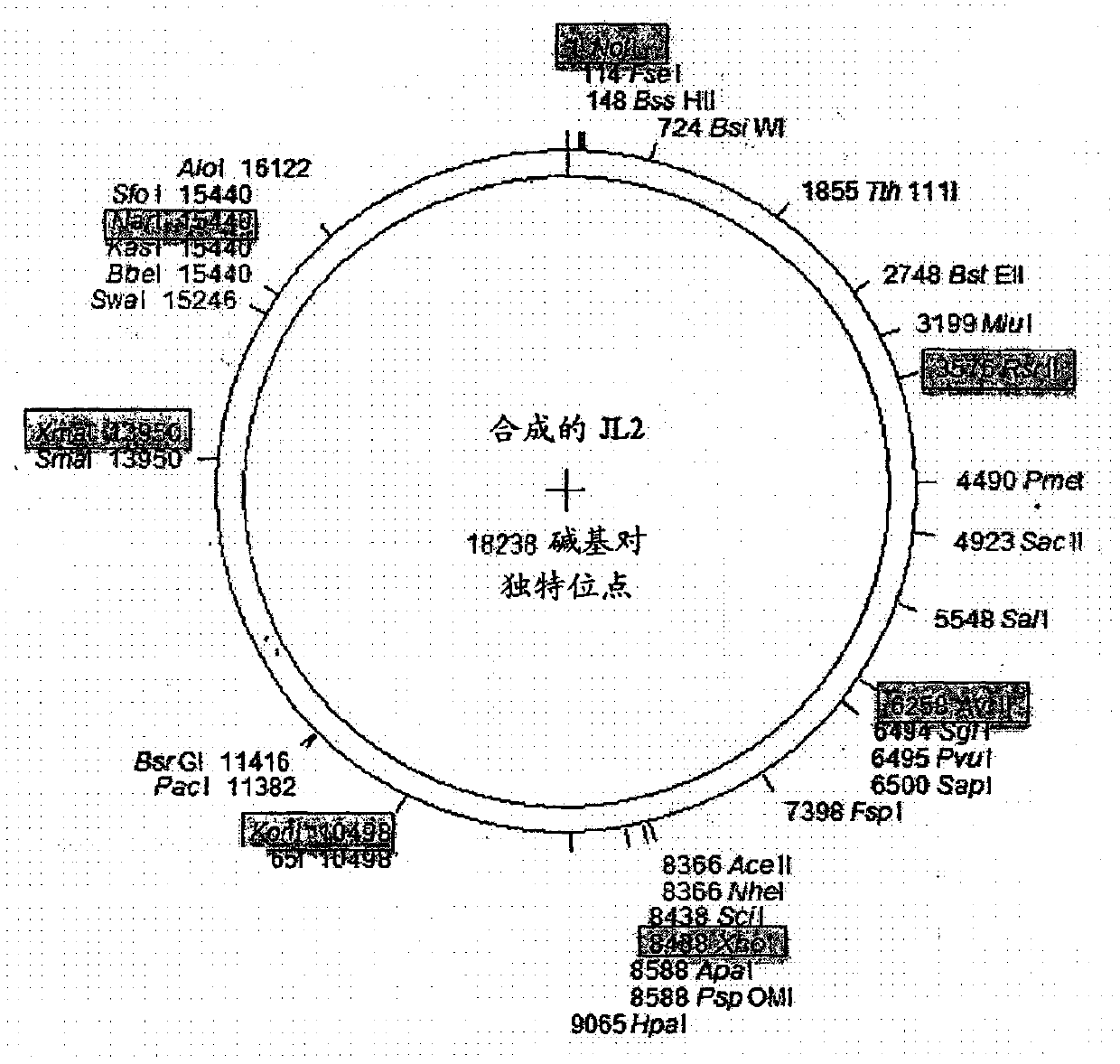
Recombinant mumps virus jeryl lynn 2 based vaccine
InactiveCN107667175AProperly obtainedSsRNA viruses negative-senseSenses disorderJeryl LynnImmunogenicity
The present invention provides a novel vaccine against mumps composed of highly immunogenic rMuVJL2 (recombinant mumps virus Jeryl Lynn2 based). Further, method to develop said immunogenic compositionis described in the present invention. The vaccine according to the present invention is safe, cost effective, highly efficacious and stable and consistent in terms of productivity.
Owner:CADILA HEALTHCARE LTD
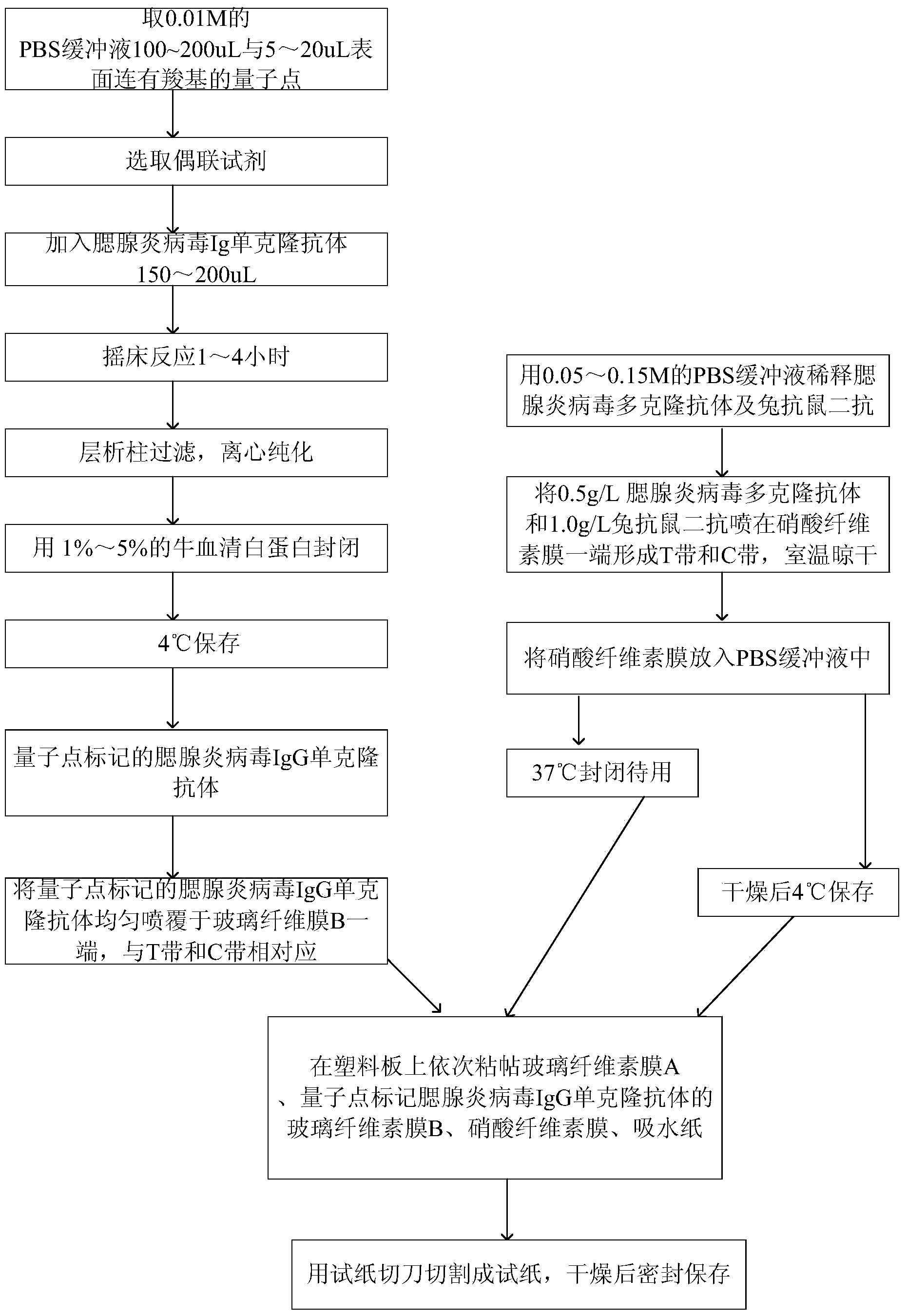
Method for detecting mumps virus, quantum-dot labeled immunochromatography test paper and preparation method thereof
ActiveCN103529216AHigh detection sensitivityHigh detection sensitivity than a rapid detection method commonly used at present - the detection sensitivity of colloidal goldBiological material analysisGlass fiberCellulose
The invention relates to a medical immunodetection method and particularly relates to a method for using quantum-dot labeled immunochromatography test paper to detect mumps virus by an immunological method. The quantum-dot labeled immunochromatography test paper is characterized in that a plastic board is stuck with a glass cellulose membrane A, a glass cellulose membrane B of a quantum-dot labeled mumps virus IgG monoclonal antibody, a nitrocellulose membrane and water absorbing paper from bottom to top in sequence, wherein one end of the nitrocellulose membrane is provided with mumps virus polyclonal antibody and rabbit antimouse second antibody so as to form a detecting band T and a quality control band C; the quantum-dot labeled mumps virus IgG monoclonal antibody is positioned at one end of the glass cellulose membrane and corresponds to the detecting band T and the quality control band C, and the quantum-dot labeled mumps virus IgG monoclonal antibody is positioned at one end of a sampling point. The method has the advantage that the detection sensitivity is about 1000 times higher than that of the commonly-used method.
Owner:北京华卫骥生物医药有限公司
Plaster for curing mumps
InactiveCN102008619AGood curative effectQuick resultsAmphibian material medical ingredientsHeavy metal active ingredientsCentipedeAconitum carmichaeli
The invention discloses a plaster for curing mumps, relating to a traditional Chinese medicine preparation. The plaster for curing mumps is prepared by processing the following components in parts by weight: 45-55 parts of squama manitis, 250-350 parts of Chinese angelica, 150-300 parts of fossil fragments, 150-250 parts of elephant hide, 100-250 parts of rosin, 15-25 parts of musk, 1500-3000 parts of minium, 50-150 parts of spina gleditsiae, 70-150 parts of sangusis draconis, 50-150 parts of aconitum wardii, 50-150 parts of aconitum kusnenzoffii reichb, 250-350 parts of radix achyranthis bidentatae, 3 long-noded pit vipers, 20 centipedes, 30-60 parts of venenum bufonis, 70-150 parts of coptis, 50-150 parts of nux vomica, 150-300 parts of radix angelicae and 5-6.5 parts of sesame oil. When in use, the plaster is molten, spread on a white cotton cloth and pasted on a wounded part. The plaster can be used for curing mumps at one time without pains and side effect, can become effective immediately, and has the curative effect of 100%.
Owner:买广益
Large-scale preparation method of F genotype mumps attenuated live vaccine
PendingCN111840534AImprove effectivenessEnsure safetySsRNA viruses negative-senseViral antigen ingredientsCell factoryGenotype
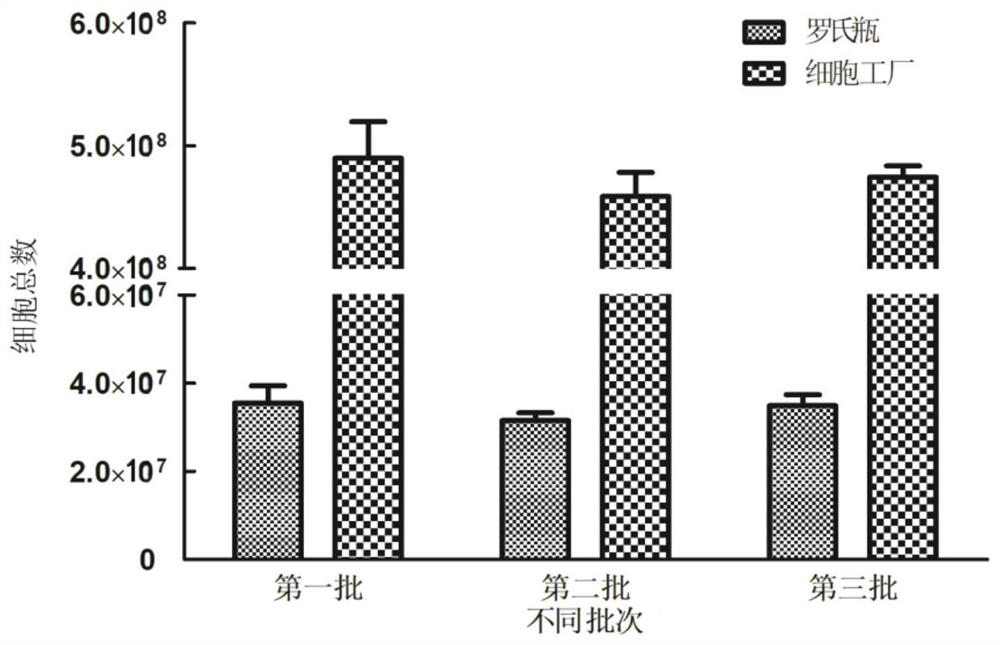
The invention discloses a large-scale preparation method of an F genotype mumps attenuated live vaccine. The preparation method comprises the following steps: passage and counting of KMB-17 cells, preparation of a virus harvesting liquid, preparation of a vaccine stock solution, preparation of a vaccine semi-finished product and preparation of a finished product. The F genotype mumps virus attenuated live vaccine is prepared on a large scale by taking human diploid cells as a matrix in a cell factory for the first time, so that the production cost can be reduced and the production time can besaved while the safety and the quality stability of the vaccine are ensured. Compared with traditional Roche flask culture, the method has the advantages that the amount of required virus seeds is small, 60000 vaccine finished products can be obtained only by 60-84 mL of virus seeds in each batch, the yield is high, the cost is low, the quality is stable, and the like, and the method has good application prospects in preventing and controlling infection and transmission of epidemic parotitis in China.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Recombinant virus and application thereof in mumps virus neutralizing antibody detection
ActiveCN113999822AShielding limitationsThe result is highly consistentSsRNA viruses negative-sensePeptidesNeutralising antibodyTiter
The invention relates to a recombinant virus and application thereof in mumps virus neutralizing antibody detection, and belongs to the technical field of biology. The invention provides a recombinant virus and a method for detecting a neutralizing antibody of a mumps virus by using the recombinant virus as a neutralizing virus. The recombinant virus takes the mumps virus as a live vector to express a marker gene; the traditional mumps virus neutralizing antibody detection method needs 7 days for final judgment of the result, while the neutralizing antibody detection method disclosed by the invention only needs 3 days, and the result conformity is extremely high; moreover, according to the neutralizing antibody detection method, the neutralizing titer of the antibody in the to-be-detected sample is calculated through fluorescence, the neutralizing antibody detection method can be used in a high-flux fluorescence detection instrument, data can be automatically and electronically stored, and meanwhile, the limitation of human factors in result judgment is shielded.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD
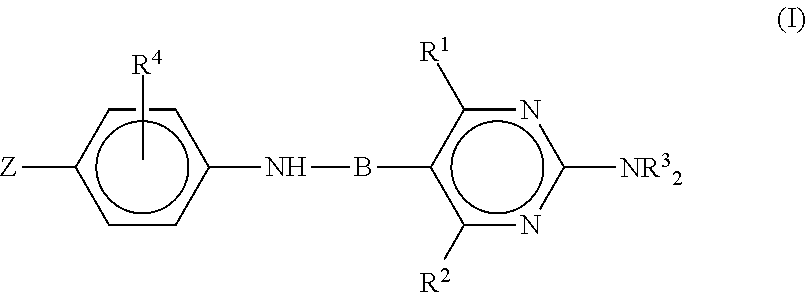
Anti-influenza compounds
The present invention provides pyrimidinyl compounds of formula (I) and pharmaceutically acceptable salts thereof. These compounds may be used for the inhibition of influenza. In particular, the compounds of the invention may be used for the treatment or prophylaxis of influenza A, most particularly H1N1 or H5N1 influenza. The compounds of the invention can also be used for the treatment or prophylaxis of a disease caused by Vibrio cholerae, Clostridium perfringens, Streptococcus pneumoniae, Arthrobacter sialophilus, an orthomyxovirus, a paramyxovirus, a parainfluenza virus, mumps virus, Newcastle disease virus, fowl plague virus or Sendai virus.
Owner:VERSITECH LTD +1
Mumps virus HN antigen and application of antigen in detection of mumps-resisting virus antibody
The invention discloses a mumps virus HN antigen capable of detecting a mumps-resisting virus antibody, especially a mumps-resisting virus neutralized antibody and further discloses application of the antigen in preparation of detection agent for detecting the mumps-resisting virus neutralized antibody and a corresponding detection kit.
Owner:北京市华信行生物科技有限公司
Vaccine against mumps containing a JERYL-LYNN virus strain
InactiveCN1763181AProtective responseCompensate for interferenceViruses/bacteriophagesAntibody medical ingredientsSerum igeJeryl Lynn
The present invention proposes a novel mumps vaccine comprising a homogeneously purified isolate derived from the Jeryl-Lynn strain of mumps virus. The vaccine in the preferred embodiment of the invention produces higher seroconversion rates and antibody titers than known commercial vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Mumps virus attacking animal model and establishment method thereof
ActiveCN114794018AIncrease capacityEffective infectionCompounds screening/testingAnimal husbandryIntramuscular injectionIntravenous IG
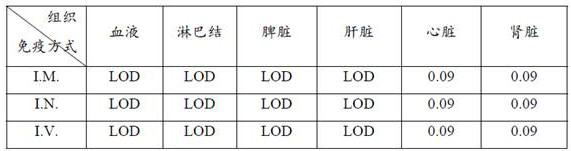
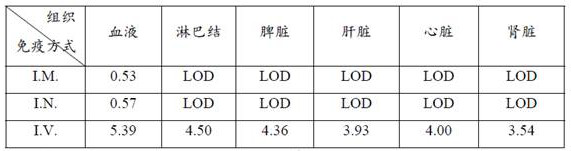
The invention relates to a mumps virus attacking animal model and an establishment method thereof, and belongs to the technical field of biology. The invention provides a mumps virus counteracting animal model. The mumps virus counteracting animal model is an interferon receptor deletion animal which is intravenously injected with mumps virus. Research finds that compared with common mice, the mumps virus load in blood of the interferon receptor-deleted mice is remarkably improved after the mumps virus is attacked by the interferon receptor-deleted mice, and compared with intramuscular injection and intranasal injection, the mumps virus load in blood of the interferon receptor-deleted mice is remarkably improved after the mumps virus is injected into the interferon receptor-deleted mice through caudal veins, so that the mumps virus load in blood of the interferon receptor-deleted mice is remarkably improved. Intravenous injection of the mumps virus to the interferon receptor-deficient animal can break through species limitation, effective infection of the mumps virus in the animal body is realized, and symptoms similar to human infection (for example, remarkable replication in various visceral organs) are formed, so that the mumps virus challenge animal model can be used as the mumps virus challenge animal model.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD +1
Medicinal composition, its preparation method and use
InactiveCN1253175CEnhance immune functionImprove protectionPharmaceutical delivery mechanismAntiviralsMedicinal herbsDisease
Owner:吉林威威药业股份有限公司
Recombinant F genotype mumps virus live vector measles vaccine
ActiveCN113293144AEasy to produceEasy quality controlSsRNA viruses negative-senseViral antigen ingredientsMeasles virus MVGenotype
The invention provides a recombinant F genotype mumps virus live vector measles vaccine. Specifically, the invention provides a recombinant F genotype mumps virus strain, the virus strain is a recombinant F genotype mumps virus strain with a preservation number of CCTCC NO: V202102, and a measles virus H gene is integrated in a genome. The invention also provides a vaccine composition containing the recombinant F genotype mumps virus strain as an active ingredient and a preparation method of the vaccine composition. The vaccine provided by the invention can be better matched with predominant epidemic F-type mumps viruses in China regions, can prevent mumps and measles at the same time, and is equivalent to the current vaccine strains in the aspects of growth characteristics, immunogenicity and the like. In addition, only one virus strain is needed during preparation of the vaccine, production is simple, and quality control is easy.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD +1
Concentration and preservation method of mumps attenuated live vaccine viruses
ActiveCN111909905ANo difference in titerNo lossSsRNA viruses negative-senseViral antigen ingredientsUltrafiltrationVaccine virus
The invention discloses a concentration and preservation method of mumps attenuated live vaccine viruses. The method comprises the following steps of preparing a cell suspension, preparing monolayer cells, preparing a virus solution, carrying out ultrafiltration concentration, preparing a stock solution, and carrying out cryopreservation to obtain a mumps attenuated live vaccine virus concentratedsolution with extremely low virus titer loss rate, and then a semi-finished product preparation and freeze-drying method is combined, and therefore, it can be guaranteed that the virus titer of the mumps attenuated live vaccine virus vaccine is almost not lost in the long-time storage process.
Owner:天津中逸安健生物科技有限公司
Recombinant parotitis virus particle, composition and application thereof
PendingCN114316071AHigh poisoning rateImprove stabilityInactivation/attenuationAntiviralsBiomedical engineeringMumps virus
The invention relates to a recombinant mumps virus particle, a composition and application thereof. The recombinant mumps virus particles have the effects of high efficiency, good preventability and good safety. Therefore, the recombinant mumps virus particles and the composition comprising the same of the present invention can effectively prevent and treat new coronavirus.
Owner:ZHEJIANG UNIV
Agentia for treating mumps and preparation
InactiveCN104306666ASignificant effectReliable and reliableDispersion deliveryAntiviralsPinelliaCurative effect
The invention discloses agentia for treating mumps. The agentia is prepared through, by weight, 0.6-1.2 parts of rhizoma anemarrhenae, 0.5-1.5 parts of gentians, 0.4-0.8 part of coptis chinensis, 0.2-0.6 part of uroctea, 0.8-1.6 parts of rhizoma dioscoreae nipponicae, 0.5-1.2 parts of manglietia fordiana fruits, 0.8-1.6 parts of catharanthus roseus, 0.5-1.5 parts of Chinese mosla herbs, 0.8-1.2 parts of pinellia ternate, 0.4-0.8 part of cynanchum paniculatum, 0.8-1.2 parts of momordica grosvenori and 0.4-1.0 part of radix angelicae. Traditional Chinese medicine raw materials are compatible in a discriminated mode and supplement each other in function, the effects of relieving exterior syndrome and diffusing wind pathogen, managing qi and activating blood, removing stasis and carrying out detumescence and clearing heat-fire are achieved, the curative effect of treating the mumps is obvious, the function is reliable, and no side effect exists.
Owner:郝建友
Application of glucocorticoid in preparation of medicine for treating and/or preventing respiratory virus infection
PendingCN113855685AAvoid infectionInhibition of replicationOrganic active ingredientsPharmaceutical delivery mechanismEcho virusesEnterovirus
The invention relates to application of glucocorticoid in preparation of a medicine for treating and / or preventing respiratory virus infection. The respiratory viruses are selected from SARS-CoV virus, SARS-CoV-2 virus, Middle East respiratory syndrome coronavirus, influenza virus, measles virus, parotitis virus, respiratory syncytial virus, parainfluenza virus, human metapneumovirus, Hendea virus, Nipie virus, rubella virus, rhinovirus, adenovirus, reovirus, coxsackie virus and ECHO virus.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Bicyclic fused pyrazole derivatives for the treatment of rsv
Disclosed herein are compounds and compositions for treating or inhibiting RSV and related members of the pneumovirus and paramyxovirus families such as human metapneumovirus, mumps virus, human parainfluenzaviruses, and Nipah and hendra virus, and methods of treatment or prevention thereof.
Owner:GEORGIA STATE UNIV RES FOUND INC
Medicine for treating tuberculosis of cervical lymph nodes, cyclomastopathy and mumps
ActiveCN102526440ARelieve painPromote absorptionAmphibian material medical ingredientsAntibacterial agentsTreating tuberculosisMammary gland
The invention discloses a medicine for treating tuberculosis of cervical lymph nodes, cyclomastopathy and mumps. The medicine is prepared by using the following raw materials by weight part: 4-5 parts of Chinese alangium leaves, 5-8 parts of omoto nipponlily roots, 8-10 parts of rumex madaio, 4-5 parts of Paris rhizomes, 8-10 parts of pokeberry roots, 4-5 parts of radix semiaquilegiae, 8-10 partsof dysosma versipellis and 3-4 parts of toads. A preparation method for the medicine provided by the invention comprises the following steps: weighting all the raw materials by weight part, washing the raw materials and then drying at the temperature below 35 DEG C; milling the raw materials into fine powder and screening by using a 100 mesh sieve; adding 60%-70% alcohol into the powder, wherein the weight of the alcohol is 3-4 times of the total weight of the powder; mixing and soaking for 5-7 days; removing the alcohol; and adding a suitable amount of honey and preparing into paste for externally applying. The medicine provided by the invention has the advantages that only few medicines are externally applied; the pains of a patient caused by taking medicines, injection and surgery are avoided; the application scope is wide; and the medicine is easily absorbed, quickly becomes effective, is high in cure rate, is low in cost and is free from side effect.
Owner:唐启裕
Disinfectant
InactiveCN112167273AChange permeabilityGood sterilization and antibacterial effectBiocideDisinfectantsBiotechnologyAntimicrobial action
The invention provides a disinfectant. The disinfectant comprises the following components: citric acid, lactic acid, malic acid, baicalin alcohol extract, chinaroot greenbrier alcohol extract, bruceajavanica alcohol extract, echinacea purpurea alcohol extract, chloroquine phosphate and water. Through the synergistic effect of citric acid, lactic acid and malic acid, the sterilization and antibacterial effects on bacteria are well achieved, and after baicalin alcohol extract, brucea javanica alcohol extract and echinacea purpurea alcohol extract are compounded, the obvious killing effect on respiratory syncytial viruses and influenza viruses can be well achieved; through the compounding effect of chloroquine phosphate, the inhibition effect on herpes viruses and parotitis viruses is improved, the chinaroot greenbrier alcohol extract plays a good role in inhibiting bacteria, new bacteria and viruses are prevented from growing in the sterilized environment again, and the sterilized environment is kept from being polluted.
Owner:重庆赛力格柯网络科技有限公司
Bicyclic fused pyrazole derivatives for the treatment of rsv
Disclosed herein are compounds and compositions for the treatment or inhibition of RSV and related members of the Pneumoviridae and Paramyxoviridae families, such as human metapneumovirus, mumps virus, human parainfluenza virus, and Nipah and Hendra viruses , and methods of treating or preventing it.
Owner:GEORGIA STATE UNIV RES FOUND INC
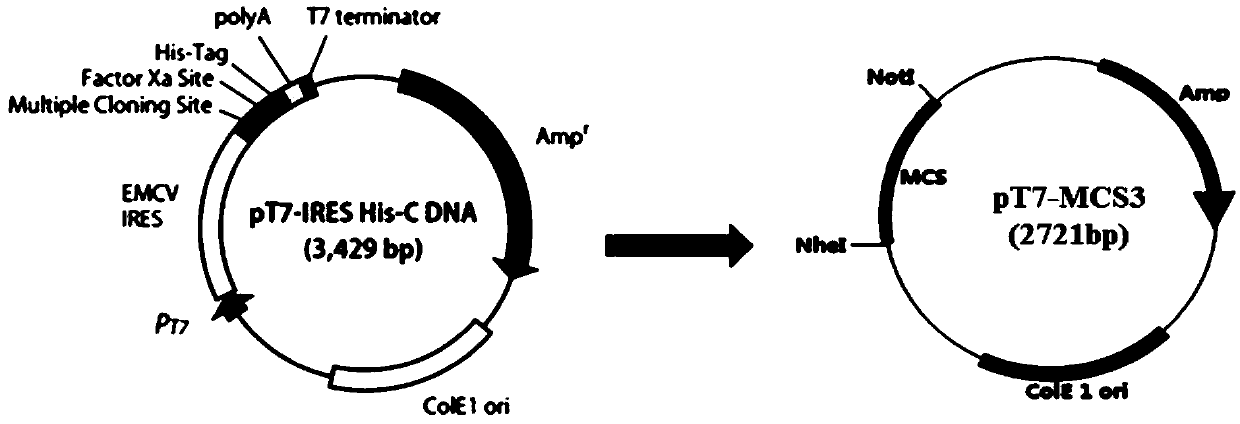
System and method for rescuing mumps virus
PendingCN111218473AEffective rescueHigh titerSsRNA viruses negative-senseFermentationA-DNAImmunogenicity
The invention provides a system and method for preparing a mumps virus. A mumps virus full-length plasmid having a T7 promoter and auxiliary plasmids are constructed, and the mumps virus is prepared by a reverse genetics method, wherein the four auxiliary plasmids are as follows: a NP plasmid, a P plasmid, an L plasmid and a T7RNAP plasmid, and the T7RNAP plasmid has the sequence of a CMV promoterand the sequence of a DNA for encoding T7 polymerase. The system and method can effectively prepare the mumps virus with higher immunogenicity, and have good application prospects.
Owner:CHENGDU INST OF BIOLOGICAL PROD
Method for subculturing and purifying mumps virus by dilution method
PendingCN114457046AHigh titerImprove stabilitySsRNA viruses negative-senseRecovery/purificationTiterViral culture
The invention discloses a method for subculturing and purifying mumps virus by a dilution method, and belongs to the technical field of attenuated live vaccines. Comprising the following steps: gradually diluting mumps virus mother liquor to 10 <-1 >-10 <-9 > dilution, respectively inoculating obtained virus solutions with 10 <-5 >-10 <-9 > dilution to a cell matrix, culturing at 32-37.0 DEG C, harvesting virus culture supernatant with cell fusion lesion, and detecting virus titer; diluting the harvested viruses step by step again until the dilution degree is 10 <-1 > to 10 <-9 >; culturing at 32-37.0 DEG C, harvesting a virus culture supernatant with obvious cell fusion lesion, and detecting virus titer; and if the virus titer is lower than 6.5 lgCCID50 / ml, repeating the operation steps until high-quality virus seeds are obtained. The virus titer of the virus seeds obtained by the method is obviously improved, the stability is good, the gene is not changed, and the effect of improving the quality of attenuated live vaccines is obvious.
Owner:SINOVAC DALIAN VACCINE TECH
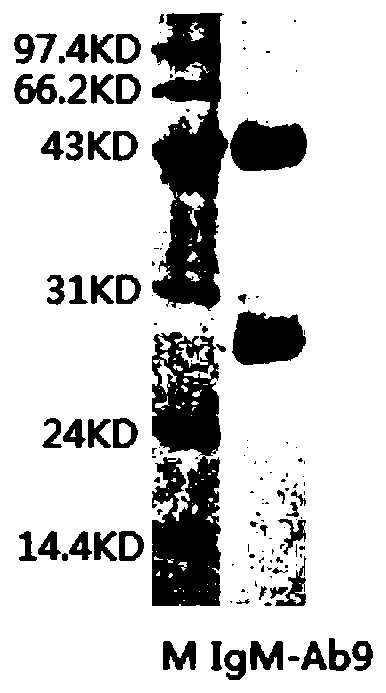
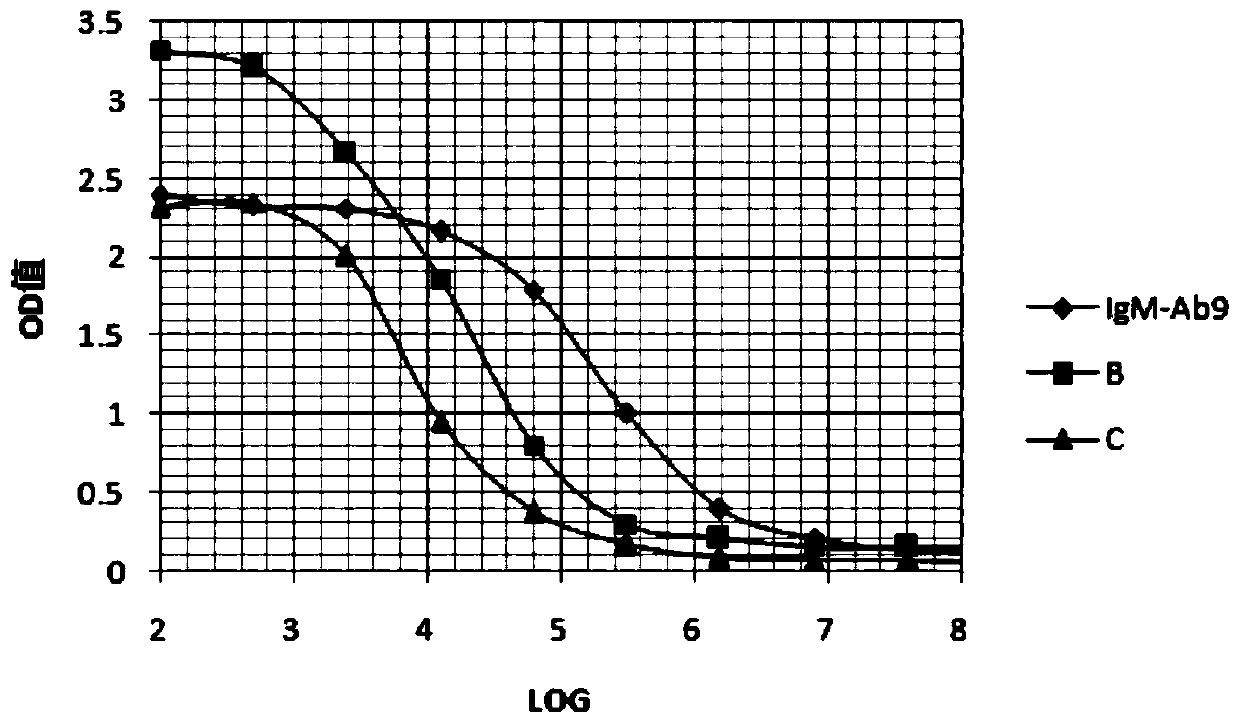
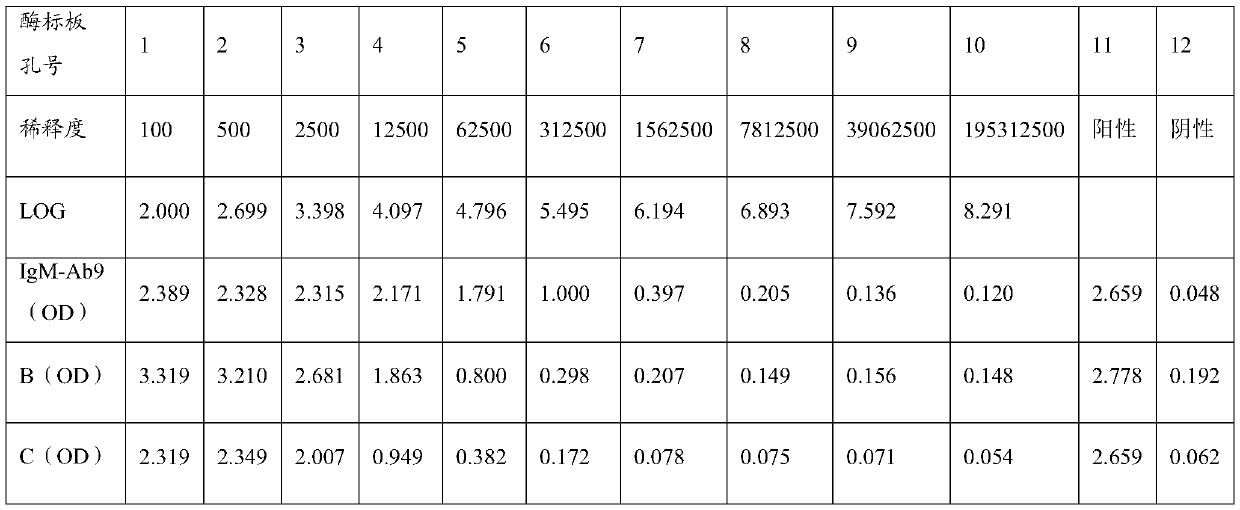
Anti-human igm monoclonal antibody, its hybridoma cell line and application
ActiveCN108531459BImprove immunityGood specific binding abilityMicroorganism based processesTissue cultureAntiendomysial antibodiesCell strain
The present invention relates to a hybridoma cell strain and a monoclonal antibody secreted thereof, the monoclonal antibody can specifically bind to human IgM and can be used for in vitro detection of the human IgM, and is particularly suitable for early diagnosis of mumps virus infection. The invention also relates to a kit comprising the hybridoma cell strain or the monoclonal antibody.
Owner:SICHUAN ANKERUI NEW MATERIAL TECH CO LTD
Medicine for treating tuberculosis of cervical lymph nodes, cyclomastopathy and mumps
ActiveCN102526440BRelieve painPromote absorptionAntibacterial agentsAmphibian material medical ingredientsAlcohol ethylPharmaceutical drug
The invention discloses a medicine for treating tuberculosis of cervical lymph nodes, cyclomastopathy and mumps. The medicine is prepared by using the following raw materials by weight part: 4-5 parts of Chinese alangium leaves, 5-8 parts of omoto nipponlily roots, 8-10 parts of rumex madaio, 4-5 parts of Paris rhizomes, 8-10 parts of pokeberry roots, 4-5 parts of radix semiaquilegiae, 8-10 parts of dysosma versipellis and 3-4 parts of toads. A preparation method for the medicine provided by the invention comprises the following steps: weighting all the raw materials by weight part, washing the raw materials and then drying at the temperature below 35 DEG C; milling the raw materials into fine powder and screening by using a 100 mesh sieve; adding 60%-70% alcohol into the powder, wherein the weight of the alcohol is 3-4 times of the total weight of the powder; mixing and soaking for 5-7 days; removing the alcohol; and adding a suitable amount of honey and preparing into paste for externally applying. The medicine provided by the invention has the advantages that only few medicines are externally applied; the pains of a patient caused by taking medicines, injection and surgery are avoided; the application scope is wide; and the medicine is easily absorbed, quickly becomes effective, is high in cure rate, is low in cost and is free from side effect.
Owner:唐启裕
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com