F-gene type attenuated live mumps vaccine and preparation method and application thereof
A technology for attenuated live vaccines and mumps, which can be used in inactivation/attenuation, antiviral agents, pharmaceutical formulations, etc., and can solve problems such as allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] ——Cultivation and harvesting of virus stocks
[0028] The working seeds of the 24th generation were inoculated with mumps vaccine virus and diluted in serum-free cell maintenance solution at 0.1-0.25 moi, and directly inoculated into monolayer KMB17 cells without adsorption. Culture at 37°C for 7 to 9 days, and collect the harvested liquid after the cells are completely damaged. The harvested virus stock solution was centrifuged at 5000 rpm for 30 min to remove cell debris.
Embodiment 2
[0030] ——Preparation of semi-finished product of live attenuated mumps vaccine
[0031] Use MEM nutrient solution to dilute the virus stock solution to 5.0 lgCCID50 / ml, add an equal amount of lyoprotectant, and it is a semi-finished product. The formula of lyoprotectant is (2×): 8% sucrose, 8% trehalose, 2% glycine, 2% dextran, 1% human albumin, 0.2% arginine, 1% sodium glutamate
Embodiment 3
[0033] ——Detection of live attenuated mumps vaccine virus stock solution and semi-finished products
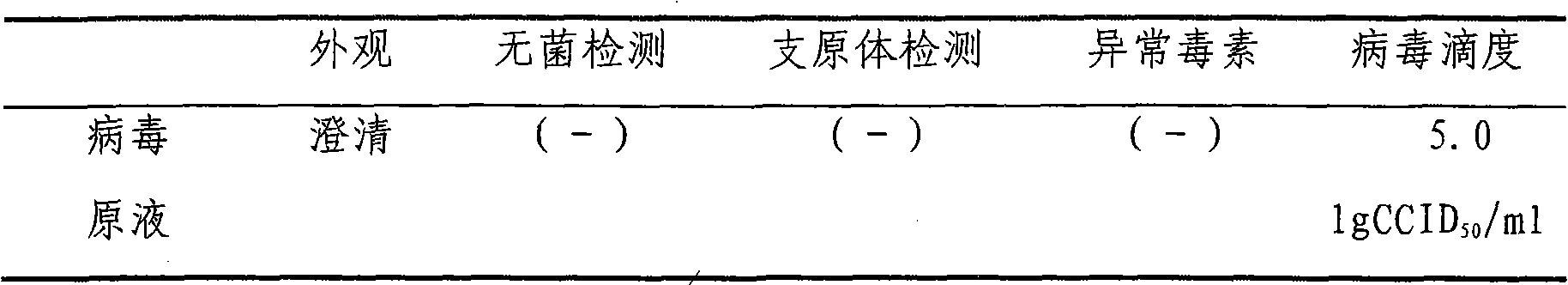
[0034] (1) The virus stock solution is a clear liquid without foreign matter and precipitation. The sterility test and mycoplasma test should be negative, without abnormal toxins, and the virus titer must be greater than 5.0 lgCCID50 / ml (see Table-1).
[0035] Table-1: Test results of virus stock solution
[0036]
[0037] (2) The sterility test of semi-finished products should be negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com