Salmonella choleraesuis attenuated vaccine expressing Haemophilus parasuis surface antigen
A technology of Haemophilus suis and Salmonella, applied in the direction of bacterial antigen components, bacteria, antibacterial drugs, etc., can solve the problems of bacteriolysis and death, and achieve the effect of convenient operation, broad application prospects, and good humoral immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
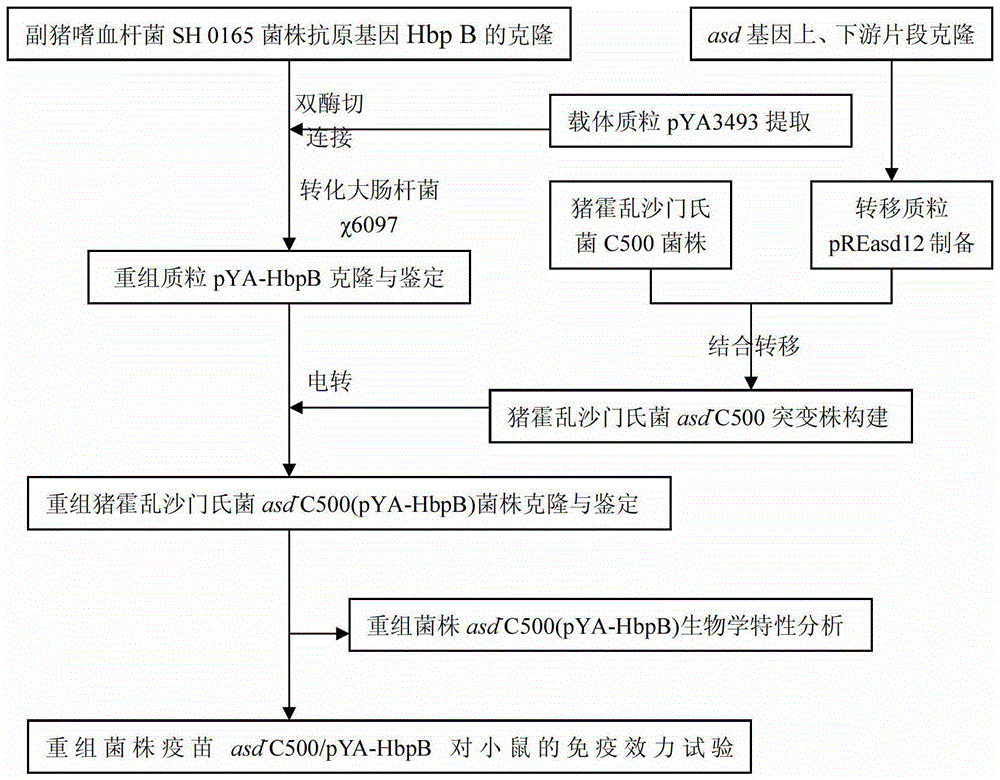
[0035] Example 1 Salmonella choleraesuis asd gene deletion strain asd - Construction of the C500
[0036] 1. Main experimental materials
[0037] Salmonella choleraesuis attenuated vaccine strain C500 was purchased from China Veterinary Drug Control Institute. pBluescriptSK(+) carrier plasmid (see attached Figure 13 ) was purchased from Stratagene, USA. Suicide plasmid pRE112 (see attached Figure 12 ), Escherichia coli χ7213 were kindly donated by Professor Dr.Roy Curtiss III, University of Washington, USA.
[0038] PCR-related reagents such as Taq enzyme, endonucleases such as BamH I and Hind III and related Buffer, T4 ligase and Buffer, DH5α competent cells, etc. are all products of Treasure Bioengineering (Dalian) Co., Ltd. Bacterial Genomic DNA Extraction Kit was purchased from Tianjin Tiangen Biochemical Technology (Beijing) Co., Ltd. UNIQ-10 Column DNA Gel Recovery Kit was purchased from Shanghai Sangon Bioengineering Technology Co., Ltd. DNA Marker (2K or 2K Pl...
Embodiment 2
[0051] Embodiment 2: Cloning of the outer membrane protein gene HbpB of Haemophilus parasuis
[0052] 1. Main experimental materials
[0053] NAD (nicotinamide adenine dinucleotide) and DAB chromogenic diagnostic kits are all products of Sigma Company, and newborn bovine serum is a product of Hangzhou Sijiqing Biological Products Co., Ltd. TSB, TSA for Difco TM product. Refer to Example 1 for the rest of the main experimental materials.
[0054] 2. Target gene analysis and primer design
[0055] The sequence registration number of the target antigen HbpB involved in the present invention and its application in Haemophilus parasuis SH0165 strain (Cai Xuwang, Research on the isolation and identification of Haemophilus parasuis and its diagnostic method and inactivated vaccine, June 2006, Huazhong Agricultural University Doctoral dissertation, National Library of China, National Digital Library of China (http: / / res4.nlc.gov.cn / home / search.trs?method=showDetail&channelid=3&id=...
Embodiment 3
[0065] Embodiment 3: Construction of recombinant Salmonella choleraesuis asd-C500 / pYA-HbpB strain
[0066] 1. Main experimental materials
[0067] Escherichia coli χ6097 (ara Δ (lac-pro)rpslΔasdA4Δ[zhf-2.∵Tn10]thiΦ80d / lacZΔM15) was kindly provided by Dr. Roy CurtissIII, University of Washington, USA. CaCl 2 , Glycerin and other reagents are products of Shanghai Sinopharm Chemical Reagent Co., Ltd. The other main experimental reagents are shown in Example 1.
[0068] 2. Preparation of competent state of χ6097 (CaCl 2 Law)
[0069] Using CaCl 2 Escherichia coli χ6097(ara Δ (lac-pro)rpslΔasdA4Δ[zhf-2.∵Tn10] thiΦ80d / lacZΔM15) competent cells, the specific steps are as follows: take out Escherichia coli χ6097 from -80℃ refrigerator Handed over to China.Wuhan.Wuhan University China Type Culture Collection Center for preservation, its preservation number is CCTCCNO: M2012344) freeze-dried powder, after thawing, put a line on the LB plate, and incubate at a constant temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com