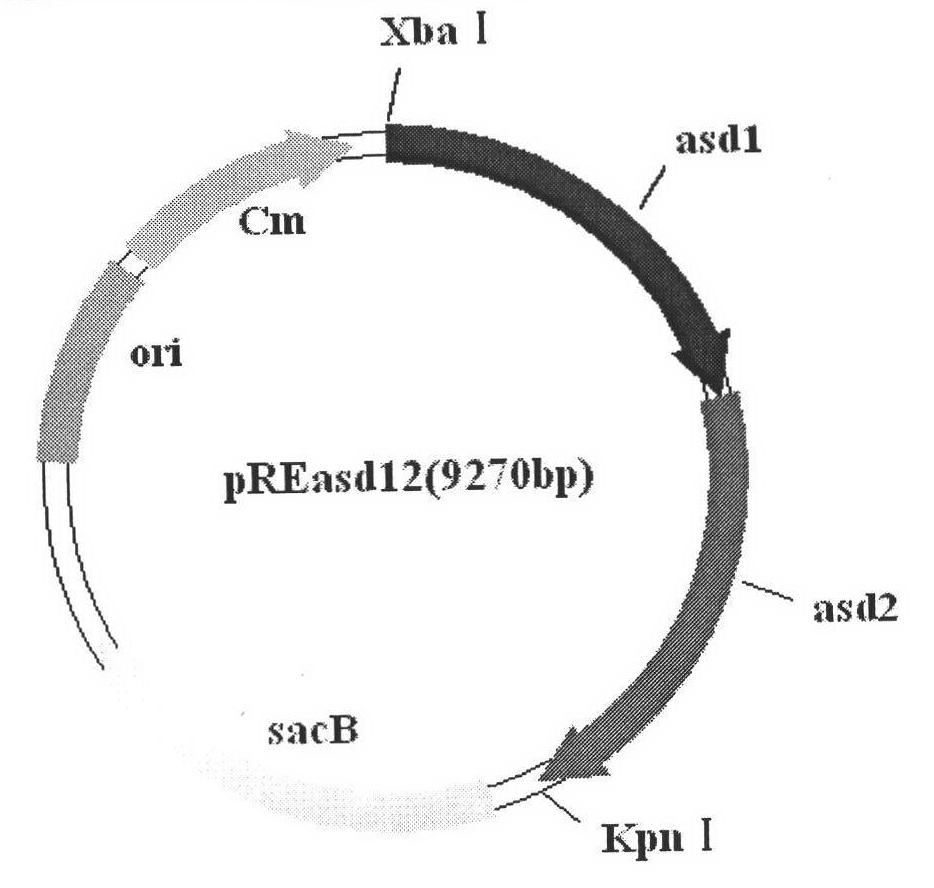
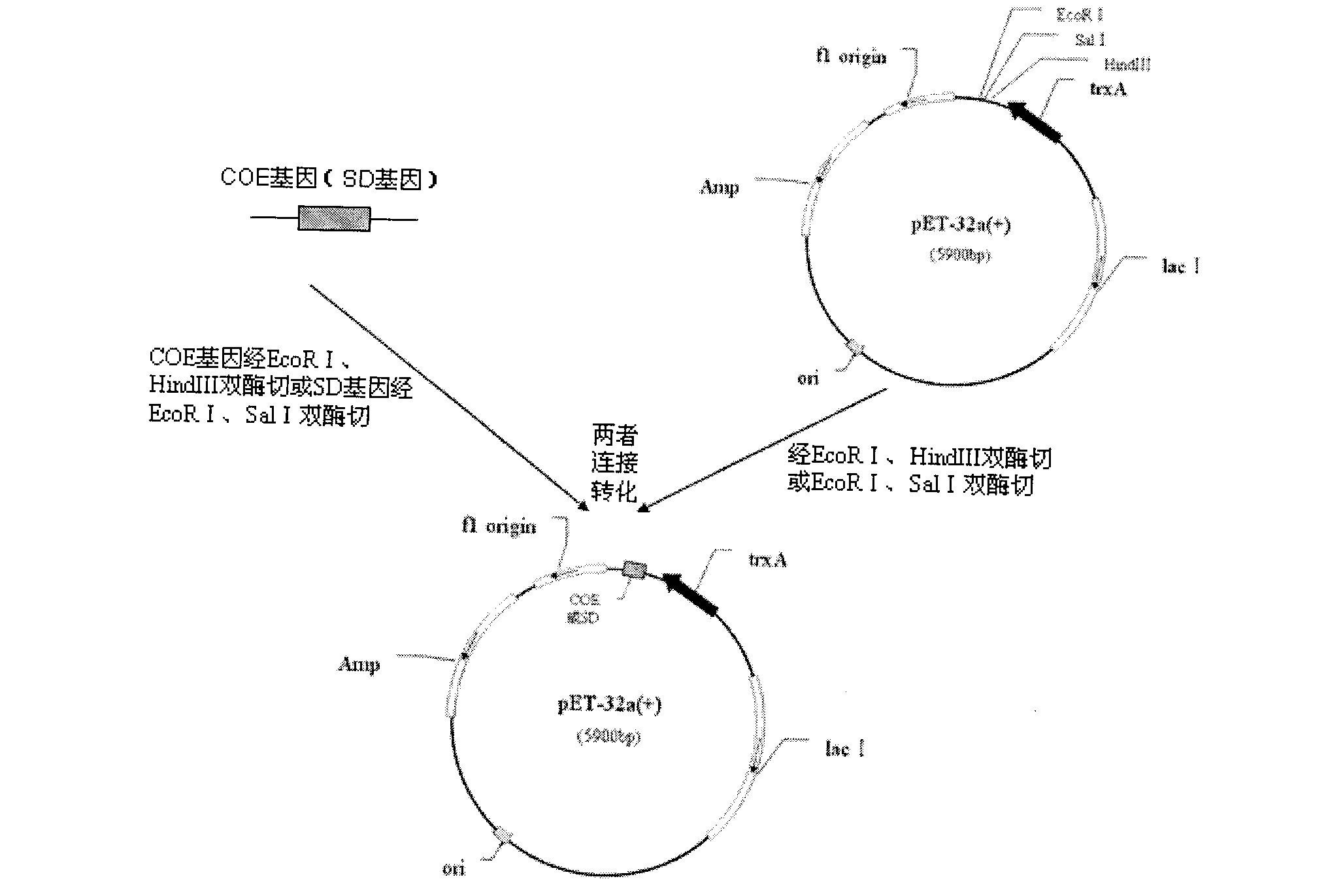
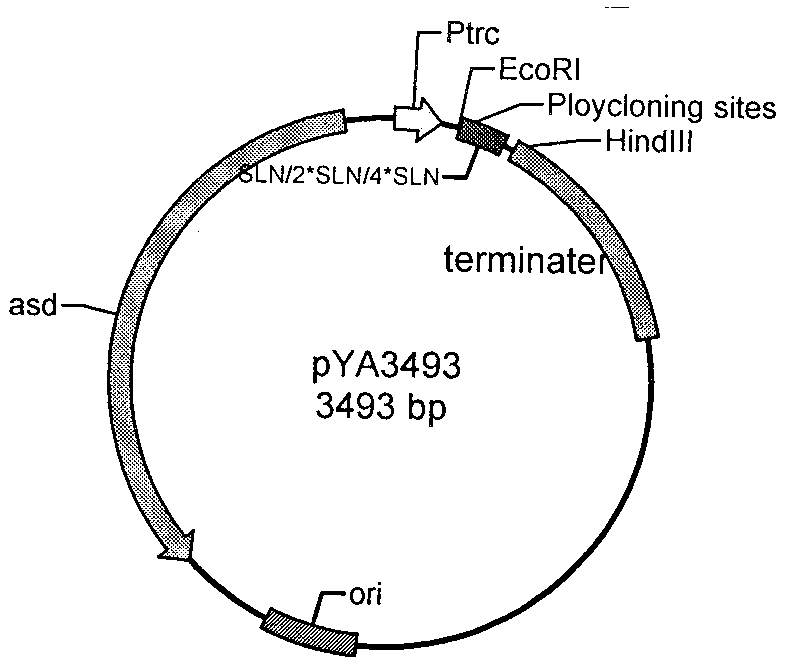
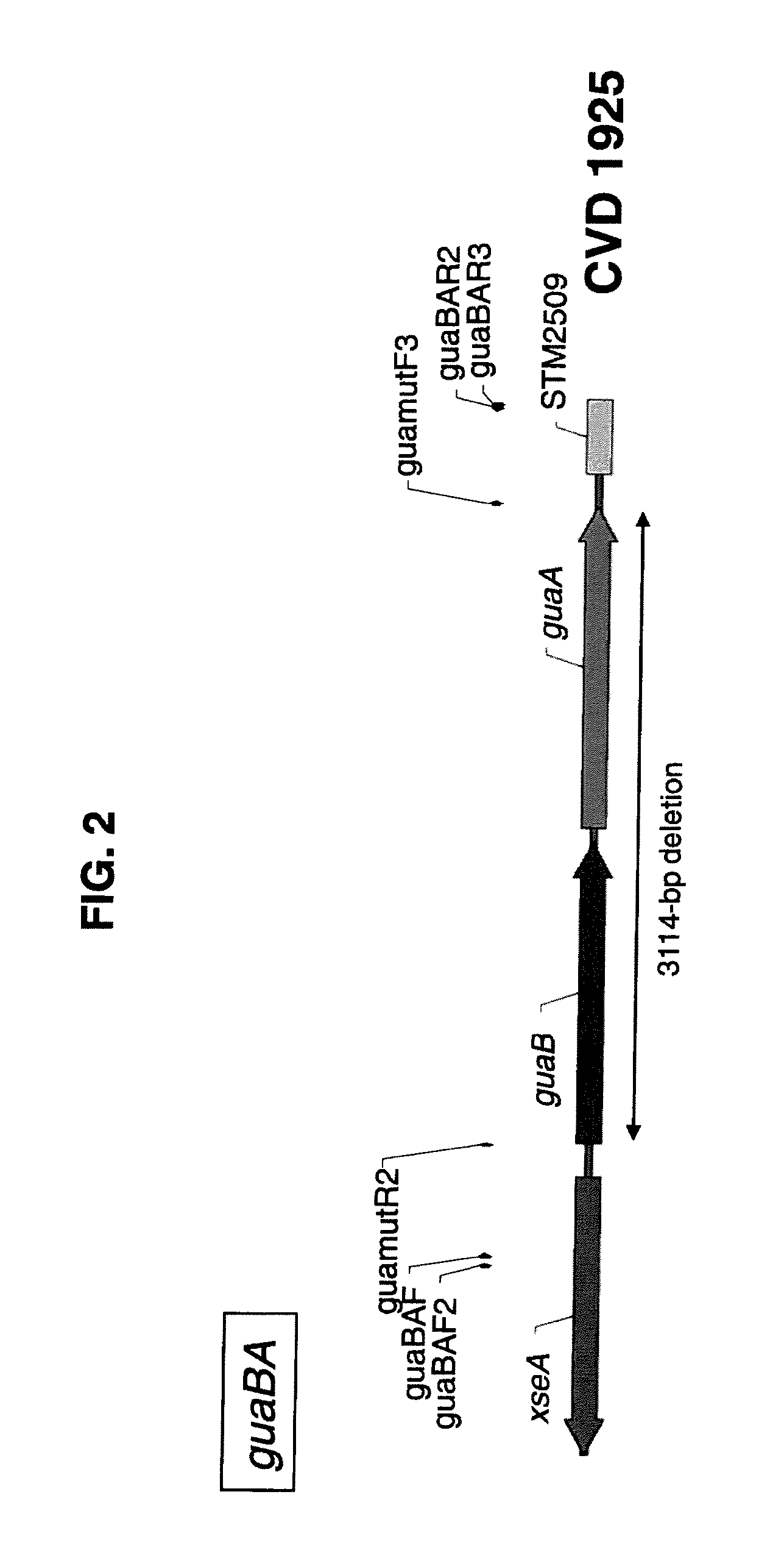
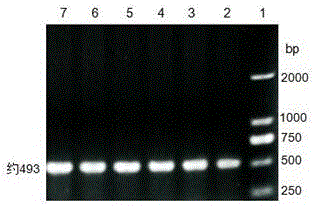
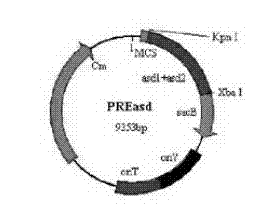
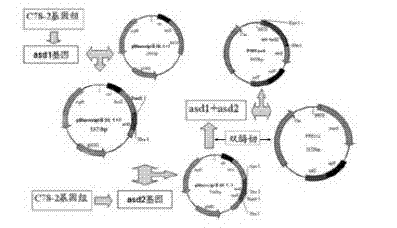
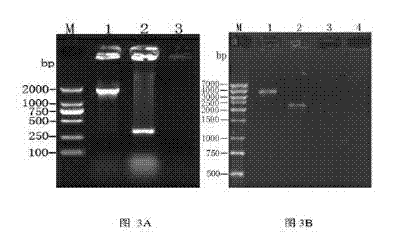
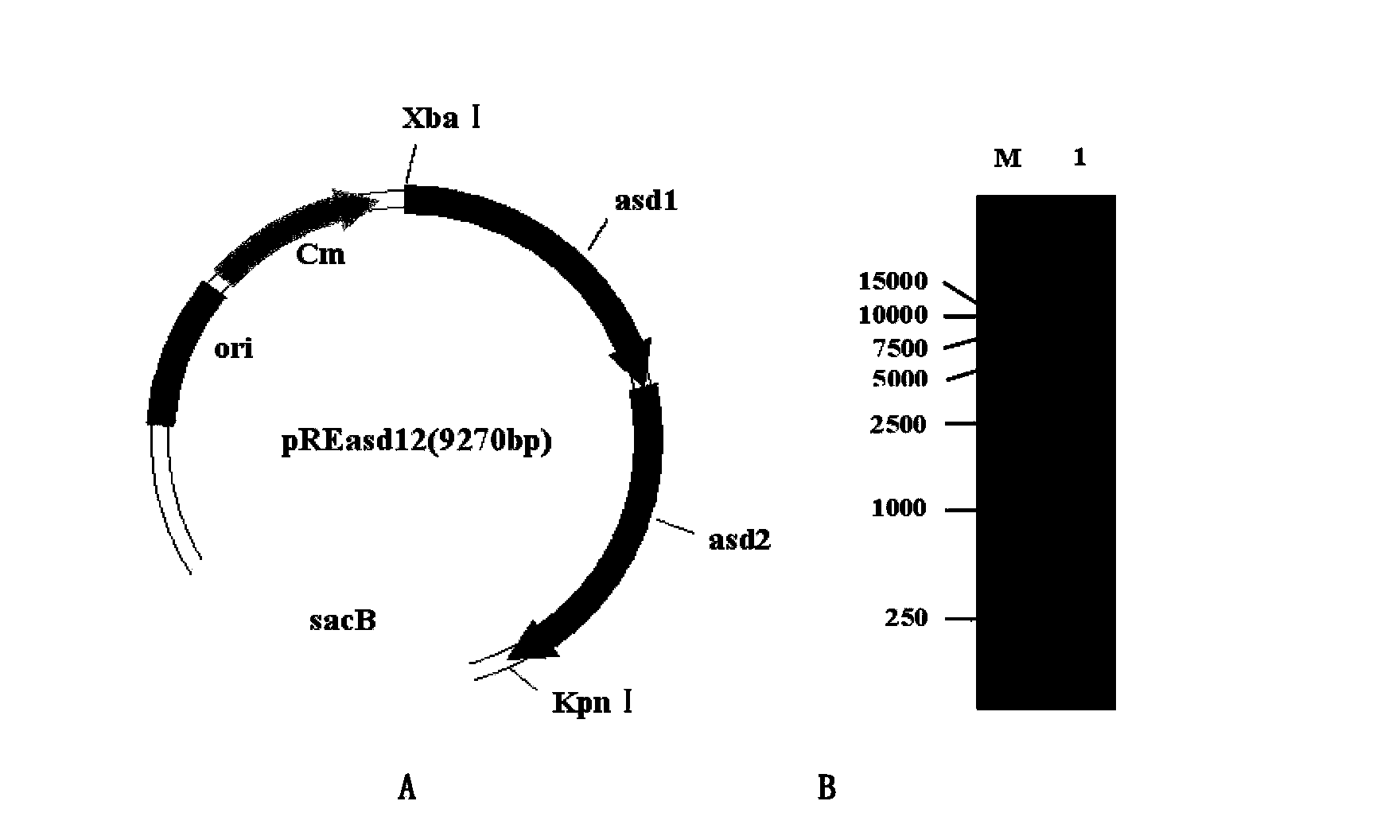
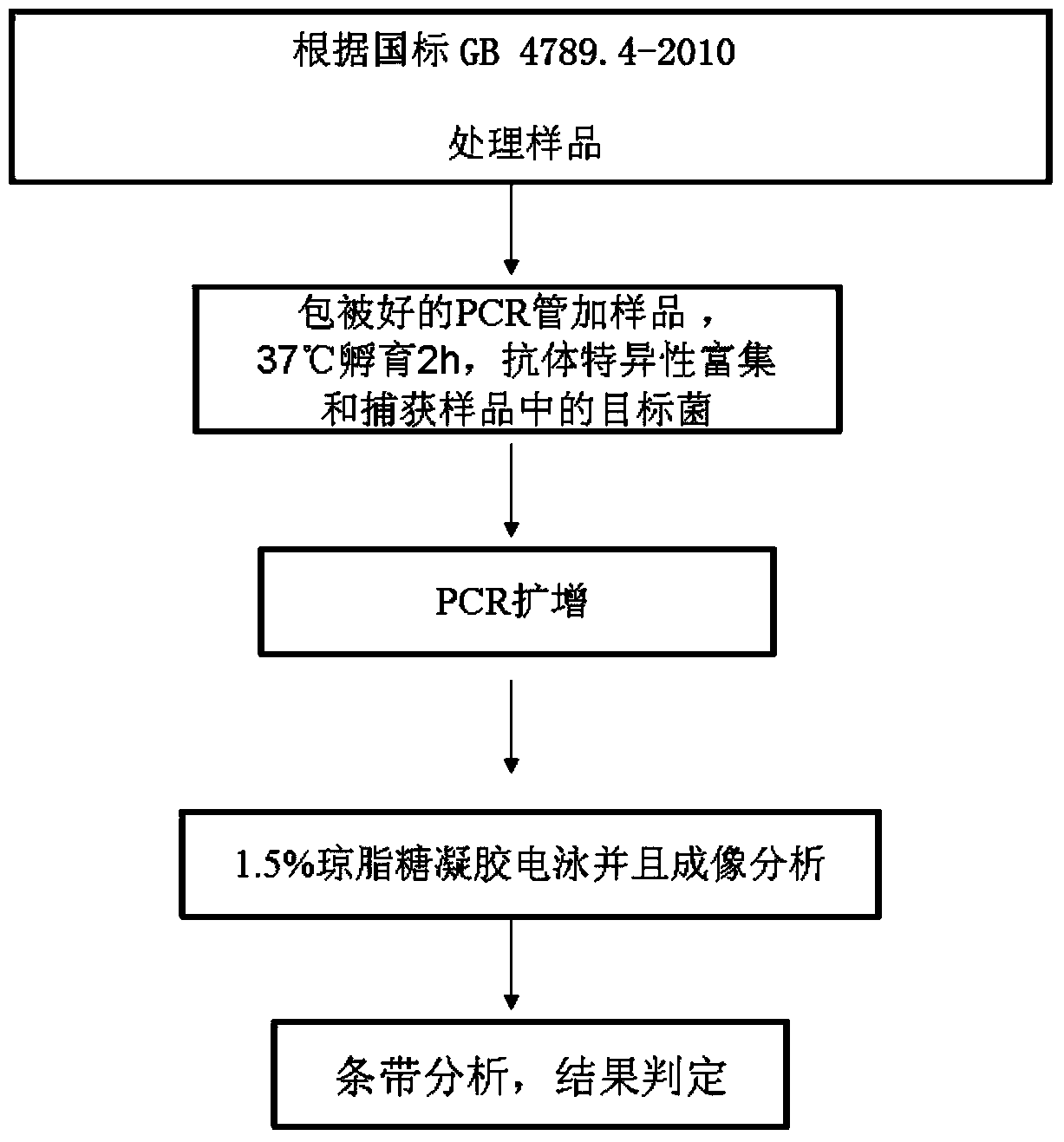
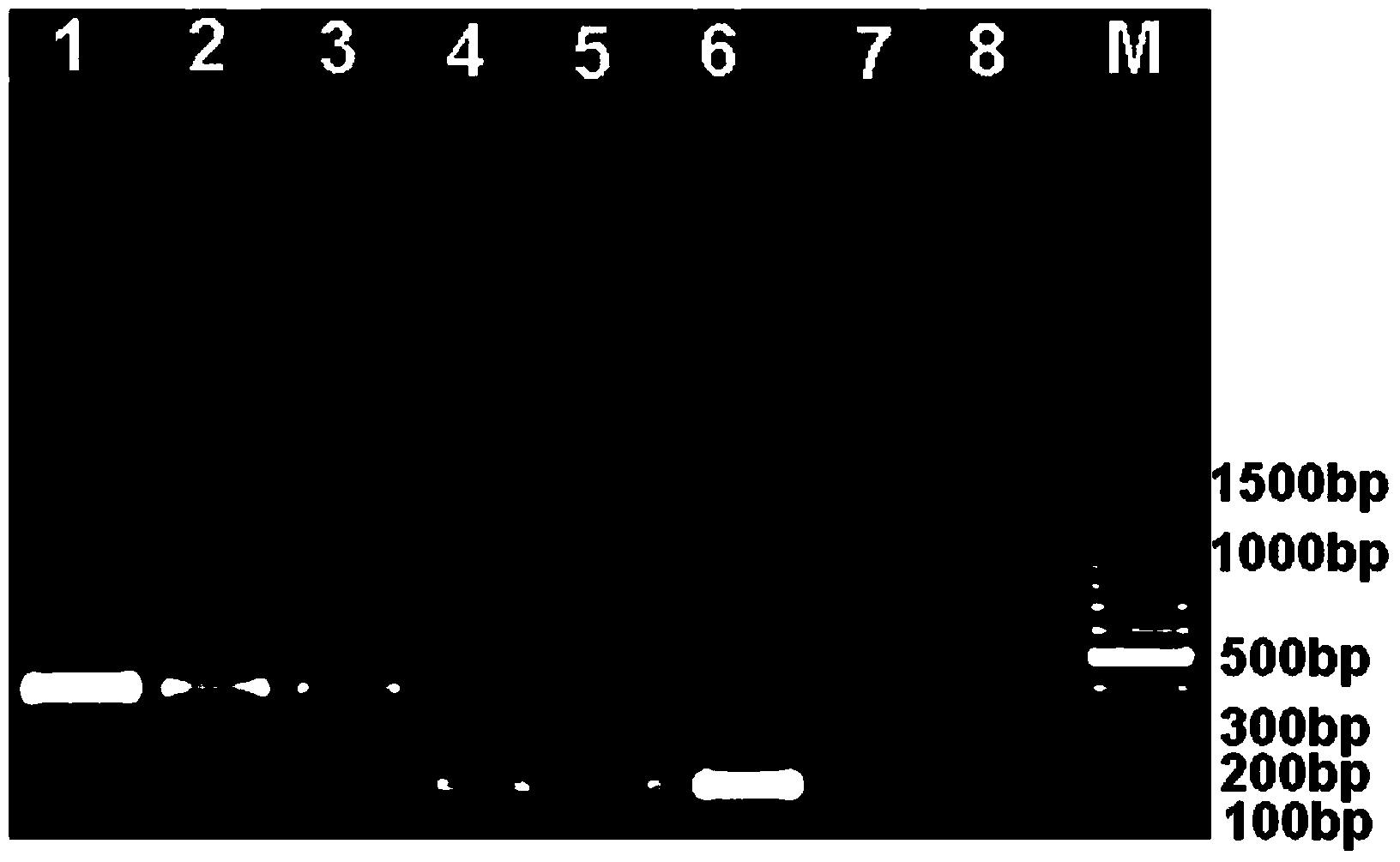
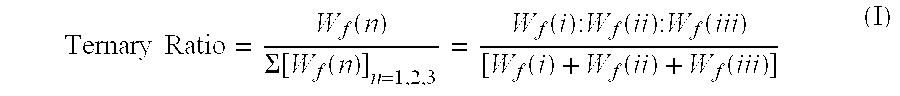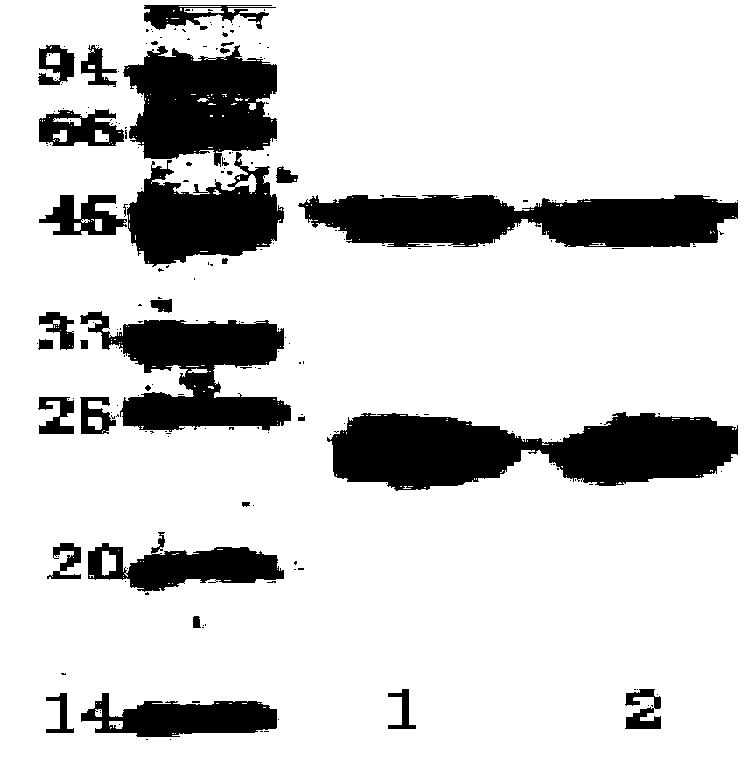Patents
Literature
63 results about "Salmonella cholerae-suis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synergistic acidic ternary biocidal compositions
InactiveUS20060293214A1Great anti-microbial activitySurprising synergistic antimicrobial efficacyBiocideAnionic surface-active compoundsBiotechnologySalmonella kiel
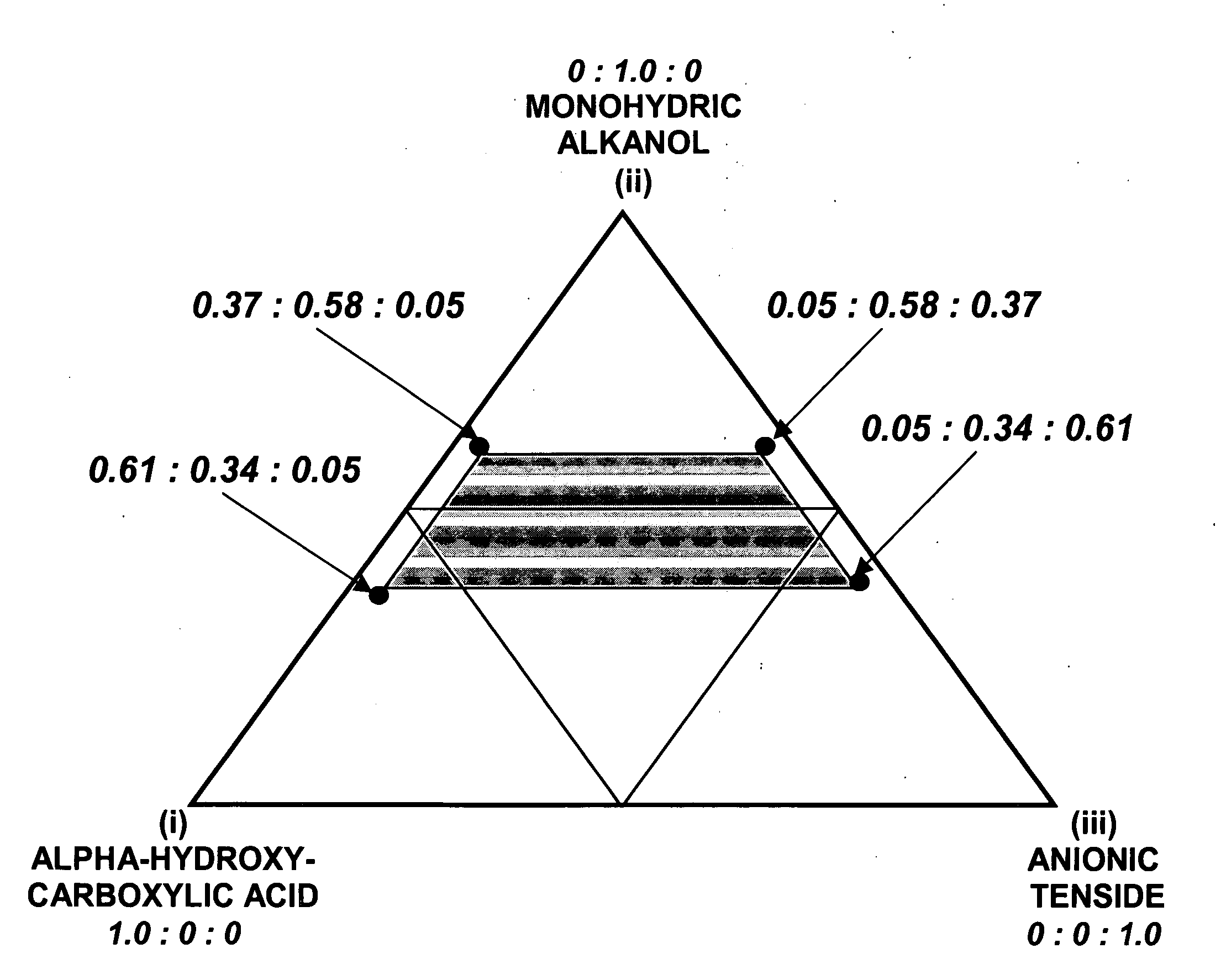
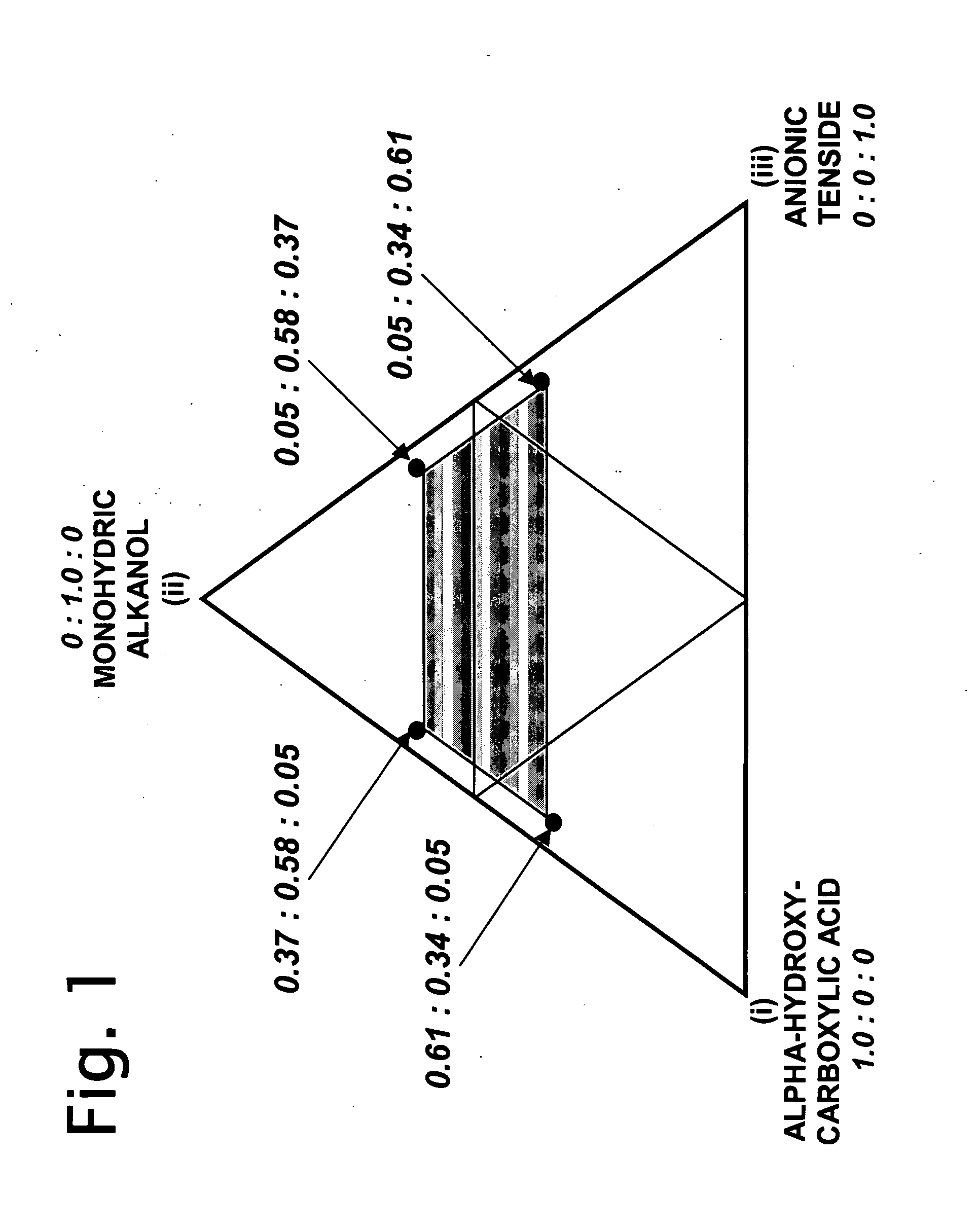
Aqueous dilutions of acidic ternary compositions having unusually synergistic antimicrobial activity against Staphylococcus aureus and Salmonella choleraesuis microorganisms are disclosed. The compositions encompass ternary component mixtures of an alpha-hydroxycarboxylic acid, a monohydric water soluble alkanol and an anionic tenside having a Ternary Ratio residing within a defined phase region wherein synergistic antimicrobial activity is observed; whereas mixtures outside of the defined phase region and those lacking any one of the components do not exhibit synergistic activity. Compositions, methods and articles employing the inventive ternary synergistic compositions, and their utility in sanitizing and disinfecting hard surfaces are described.
Owner:THE CLOROX CO
Cross-protective salmonella vaccines
The present invention relates to a method of protecting pigs against disease caused by infection by heterologous serotypes of Salmonella including but not limited to S. typhimurium comprising administering to the pigs a modified live vaccine incorporating S. cholerasuis.
Owner:INTERVET INT BV
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
Genetic engineering live vaccine of recombinant Salmonella choleraesuis and Porcine epidemic diarrhea virus, preparation and application
ActiveCN103013895APreserve immune efficiencyHigh biosecurityAntibacterial agentsBacteriaBacteroidesBacterial genetics
The invention belongs to the technical field of animal bacterial genetic engineering, and concretely relates to a construction of recombinant Salmonella choleraesuis strains C501-Coe and C501-SD with no resistance marker and expressing main antigenic sites of porcine epidemic diarrhea virus, a preparation of a vaccine and an application. The invention obtains the recombinant Salmonella choleraesuis strains C501-Coe and C501-SD with no resistance marker and expressing the main antigenic sites of the porcine epidemic diarrhea virus, and the strains have the following accession number respectively: CCTCC NO: M2011296 and CCTCC NO: M2011297. The two recombinant strains are deleted with an asd gene necessary for growth of the S. choleraesuis, and contain plasmids which can express the asd gene, as well as a COE gene fragment and a SD gene fragment of the Porcine epidemic diarrhea virus in the strains. <{EN3}>The invention further discloses a method and an application by using the recombinant strain to prepare the vaccine of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. <{EN4}>The vaccine provided by the invention can stimulate swine to generate a protective immunization reaction for resisting the Salmonella choleraesuis and the Porcine epidemic diarrhea virus, and can effectively prevent infection of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. The invention further discloses a method and an application by using the recombinant strain to prepare the vaccine of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. The vaccine provided by the invention can stimulate swine to generate a protective immunization reaction for resisting the Salmonella choleraesuis and the Porcine epidemic diarrhea virus, and can effectively prevent infection of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus.
Owner:HUAZHONG AGRI UNIV +1
Recombinant salmonella choleraesuis, bivalent genetic engineering vaccine and application
InactiveCN101880647AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacteriaBacteroidesRecombinant vaccines
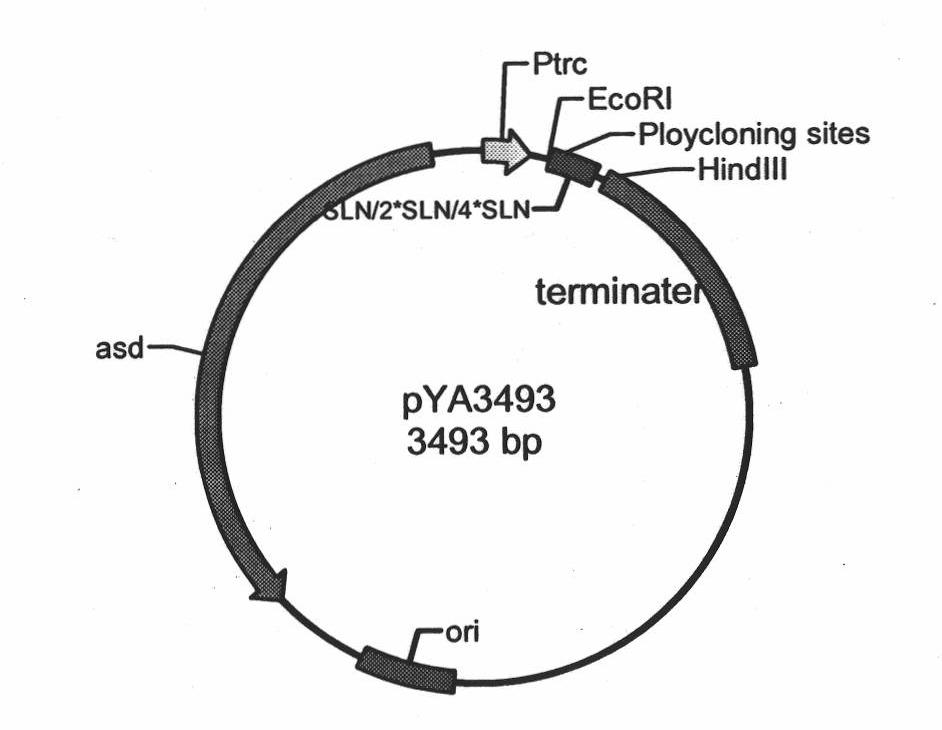
The invention belongs to the technical field of bacterial gene engineering of animals, and in particular relates to the construction of a recombinant salmonella choleraesuis strain which does not contain resistance markers and expresses major antigenic loci of porcine transmissible gastroenteritis virus, vaccine preparation and application. In the recombinant salmonella choleraesuis strain C500 (pYA-2SLN) which does not contain the resistance markers and expresses the major antigenic loci of the porcine transmissible gastroenteritis virus, the preservation No. is CCTCC NO: M 209189, and the strain misses asd genes which are necessary for the growth of salmonella choleraesuis and contains plasmid capable of expressing the asd genes, genes of an antigenic locus A, an antigenic locus D and an antigenic locus N321 of the porcine transmissible gastroenteritis virus in the strain. The invention also discloses a method for preparing the salmonella choleraesuis and porcine transmissible gastroenteritis vaccine by utilizing the recombinant strain and application thereof. The prepared recombinant vaccine can stimulate pigs to generate protective immune response for resisting the salmonella choleraesuis and the porcine transmissible gastroenteritis virus, and prevent the infection of the salmonella choleraesuis and the porcine transmissible gastroenteritis virus effectively.
Owner:HUAZHONG AGRI UNIV
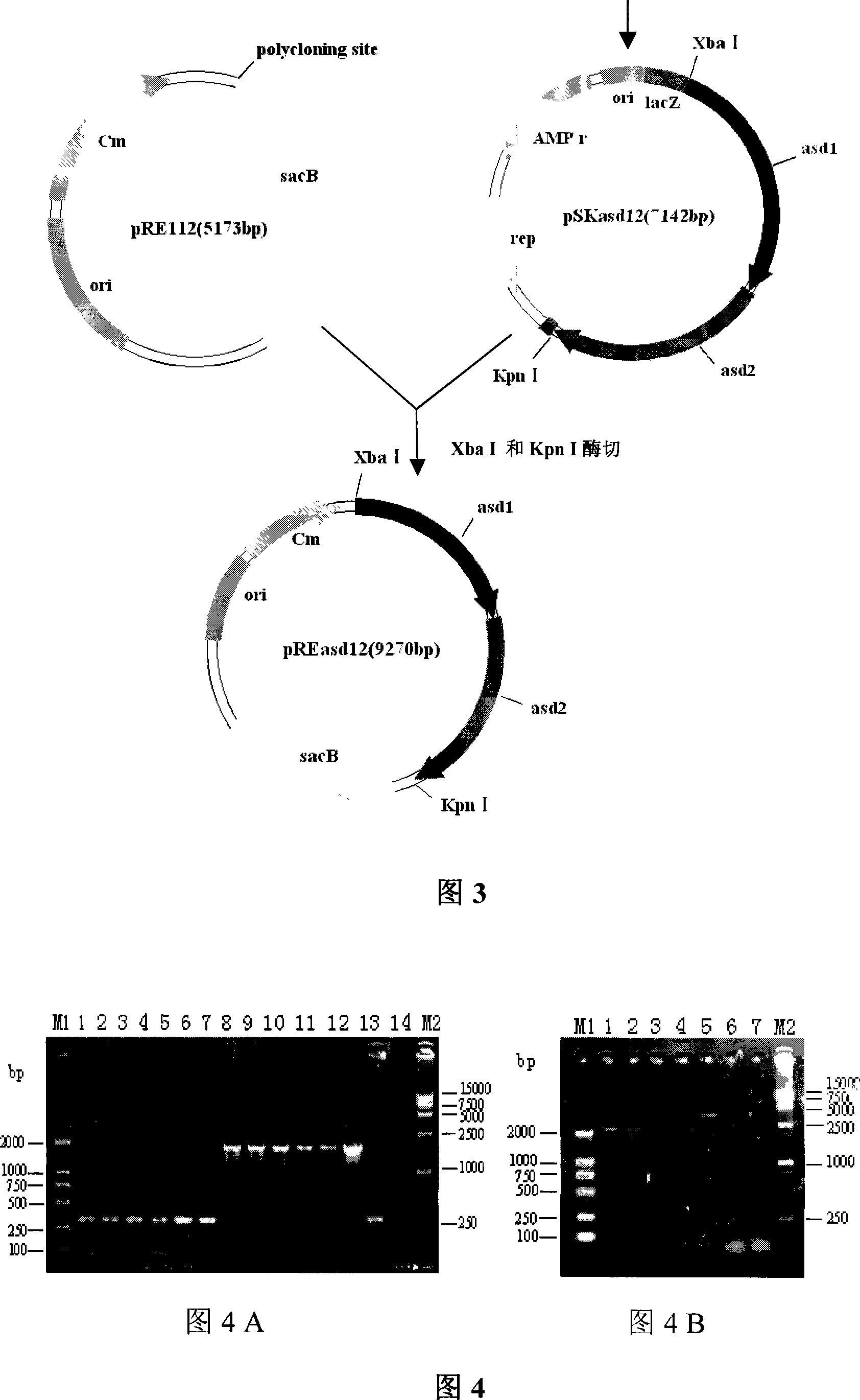
Recombinant salmonella choleraesuis strain for expression of pig origin bordetella bronchisepatica fhaB and prn gene segment, bacterin and uses thereof
InactiveCN101157907AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesBordetella
Owner:WUHAN KEQIAN BIOLOGY CO LTD
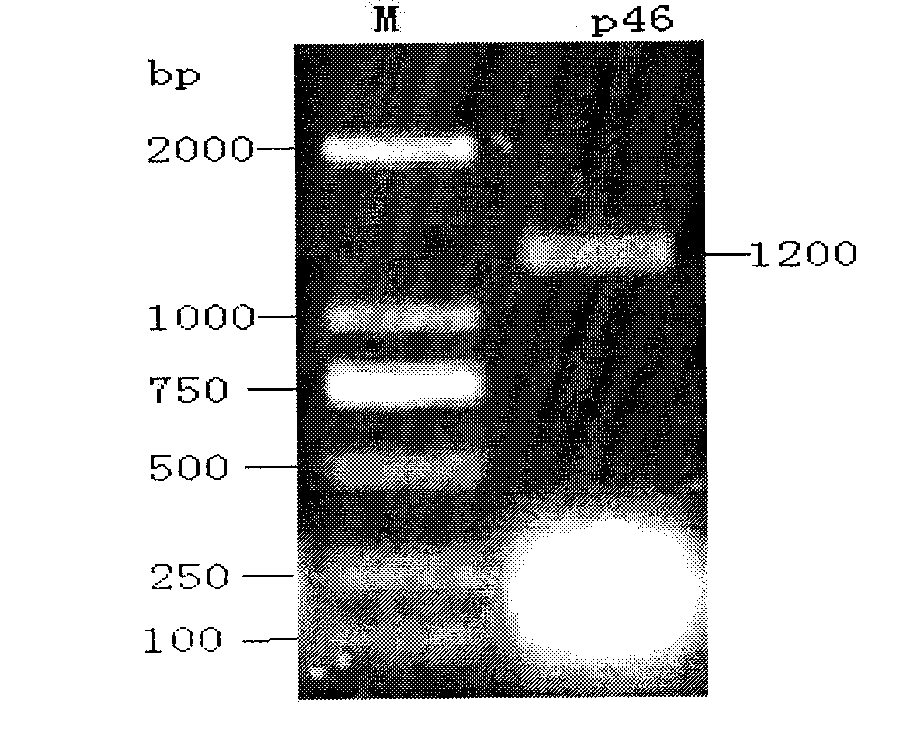
Recombinant salmonella choleraesuis expressing mycoplasma hyopneumoniae p46 protein, preparation method and application thereof
InactiveCN102732473AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesGenetic engineering
The invention belongs to the genetic engineering technology field of animal bacteria, especially relates to construction, vaccine preparation and application of recombinant salmonella choleraesuis expressing a main immunogenic membrane protein of mycoplasma hyopneumoniae and the expression contains no resistance markers. A strain of salmonella choleraesuis C500 (pYA-46) expressing mycoplasma hyopneumoniae p46 protein from the expression which contains no resistance markers is provided by the invention, and the preservation number is: CCTCC NO: M2011106. The recombinant strain lacks of an asd gene which is essential for the growth of samonella choleraesuis, and contains plasmid which can express asd in the strain and mycoplasma hyopneumoniae p46 protein. The invention also discloses the preparation method and the application of salmonella choleraesuis and pig mycoplasma pneumonia vaccine prepared by the recombination stain. The bivalent vaccine prepared in the invention can stimulate porcine to produce an immune reaction to protect porcine from the salmonella choleraesuis and the porcine pneumonia mycoplasma and can effectively prevent from being infected by the salmonella choleraesuis and the porcine pneumonia mycoplasma.
Owner:HUAZHONG AGRI UNIV +1
Construction method for delaying attenuation and increasing expression exogenous antigen salmonella suipestifer carrier through regulation and control of gene
ActiveCN104498418ALarge market applicabilityImmunogenicBacteriaMicroorganism based processesBacteroidesEscherichia coli
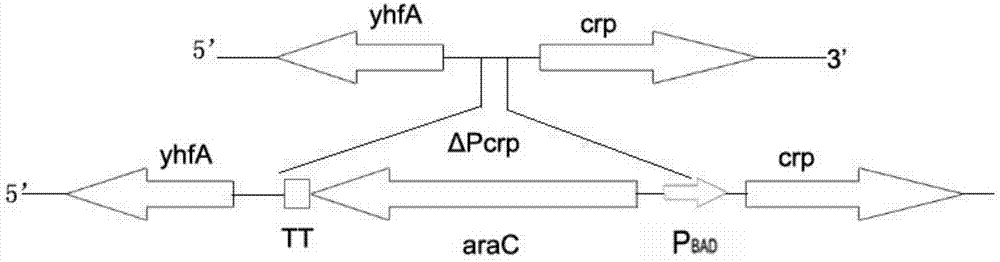
The invention provides a construction method for delaying attenuation and increasing an expression exogenous antigen salmonella suipestifer carrier through regulation and control of gene, which is characterized in that receptor bacterium C78-3 is combined with manA, crp, relA and asd -containing deleted suicide carrier escherichia coli donor bacterium, mutants delta manA, delta Pcrp: : TT ara C PBAD crp, delta relA: : araC PBAD lacI TT and delta asd A are introduced in wild-type salmonella suipestifer C78-3, the introduced salmonella suipestifer after mutation can be called x0011; and four types of mutation enable phenotype identification. The method of the invention makes salmonella suipestifer to become safe and effective vaccine carrier of many exogenous antigens; and provides the delaying attenuation and increasing expression exogenous antigen salmonella suipestifer carrier through regulation and control of gene for various pig diseases, especially many bacteria diseases of pig, and has great market applicability.
Owner:YANGZHOU UNIV
Broad Spectrum Vaccine Against Typhoidal and Non-typhoidal Salmonella Disease
ActiveUS20130129776A1Carrier-bound antigen/hapten ingredientsAgainst vector-borne diseasesAntigenConjugate vaccine
The present invention is drawn to multivalent Salmonella enterica serovar conjugate vaccines comprising conjugates of S. Typhimurium, S. Enteritidis, S. Choleraesuis, S. Typhi, S. Paratyphi A and optionally S. Paratyphi B, wherein the conjugates comprise a hapten antigen and a carrier antigen, wherein at least one of the hapten antigens or carrier antigens is characteristic of the Salmonella enterica serovar. The present invention also provides Salmonella enterica serovar reagent strains to produce the multivalent conjugate vaccines and attenuated Salmonella enterica serovars for use as vaccines.
Owner:UNIV OF MARYLAND BALTIMORE
Attenuated vector bacterium of salmonella choleraesuls and construction method of attenuated vector bacterium
InactiveCN106754594AReduce usageHas attenuated safety featuresBacteriaMicroorganism based processesBacteroidesAntibiotic Y
The invention provides an attenuated vector bacterium of salmonella choleraesuls and a construction method of the attenuated vector bacterium and belongs to the technical field of animal bacterium genetic engineering. The attenuated vector bacterium is C78-3 salmonella choleraesuls without deltamanA, deltacrp::TT araC PBAD, deltarelA:: araC PBAD lacI TT, deltasoB and deltaasdA genes and is named as rSC0016. The method for taking a suicide vector without a resistance marker as a salmonella choleraesuls constructing vector is adopted and the utilization of the vector marked with antibiotics can be avoided; the vector can be safely used in clinical practice; furthermore, a balanced lethal system carries an exogenous antigen and can guarantee that only vaccine strains carrying the exogenous antigen can be used for producing vaccines and enter a host and survive, so that the immunity efficiency of the vaccines is improved.
Owner:YANGZHOU UNIV
Porcine interferon gene family and application thereof in blue-ear porcine disease resistant medicament and vaccine adjuvant
InactiveCN101955941AGood immune protectionEasy gene insertionPeptide/protein ingredientsGenetic material ingredientsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of genetic engineering, and particularly relates to a porcine interferon family gene and application thereof in a blue-ear porcine disease resistant medicament and a vaccine adjuvant. The porcine interferon gene is taken from a home pig genome and has anti-virus effect, and the amino acid sequence of the porcine interferon gene is SEQ ID No. 1, SEQ ID No. 3, SEQ ID No. 5, SEQ ID No. 7 or SEQ ID No. 9; and the medicament and the vaccine adjuvant are a recombinant plasmid obtained by cloning the home pig interferon gene to an eukaryotic expression vector through molecular biology technology and a live bacterial vaccine obtained by transferring the plasmid to a low virulent strain of Salmonella choleraesuls. Animal experiments prove that the medicament and the adjuvant have good safety and remarkable effect. After a home pig is directly injected, the toxicity is removed on the same day, and the protective rate can reach 80 percent. When the adjuvant is used with a blue-ear disease inactivated vaccine and after the home pig is subjected one-time immune injection, the toxicity is removed in 28 days, and the protective rate can reach 80 percent.
Owner:FUDAN UNIV
Salmonella choleraesuis double-gene-deletion strain free of resistance marker and application thereof
InactiveCN102732442AImprove securityClear genetic backgroundBacteriaMicroorganism based processesVirulent characteristicsExogenous antigen
The invention relates to a Salmonella choleraesuis double-gene-deletion strain free of a resistance marker, Salmonella choleraesuis C7822 (with an accession number of CCTCC NO: M2011402 ). The strain is obtained by carrying out deletion of the asd gene, one of the important nutrition and metabolism genes of Salmonella choleraesuis, on Salmonella choleraesuis C7821 (with an accession number of CCTCC NO: M2010102 ) which has undergone deletion of the crp virulence gene. The gene-deletion strain C7822 loses the capability of synthesizing diaminopimelic acid (DAP), so the strain cannot survive in a DAP negative environment. After plasmids containing the asd gene are transformed into C7822, the asd gene in the plasmids can form a complementary gene with C7822, which enables C7822 to regain the capability of surviving in a DAP negative environment. The double-gene-deletion strain C7822 obtained in the invention has distinct genetic background, strong security and no resistance marker and can stably carry and express exogenous antigens; the double-gene-deletion strain is a good carrying vector and expression strain for exogenous antigens and has a wide application prospect.
Owner:HENAN UNIV OF SCI & TECH
Shigella boydii enzyme-linked immunosorbent assay kit
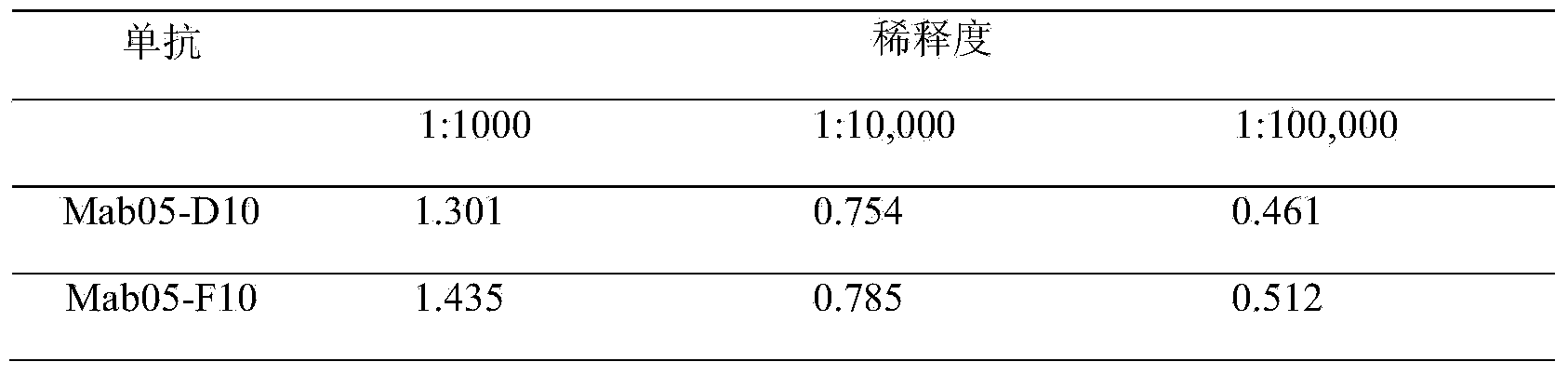
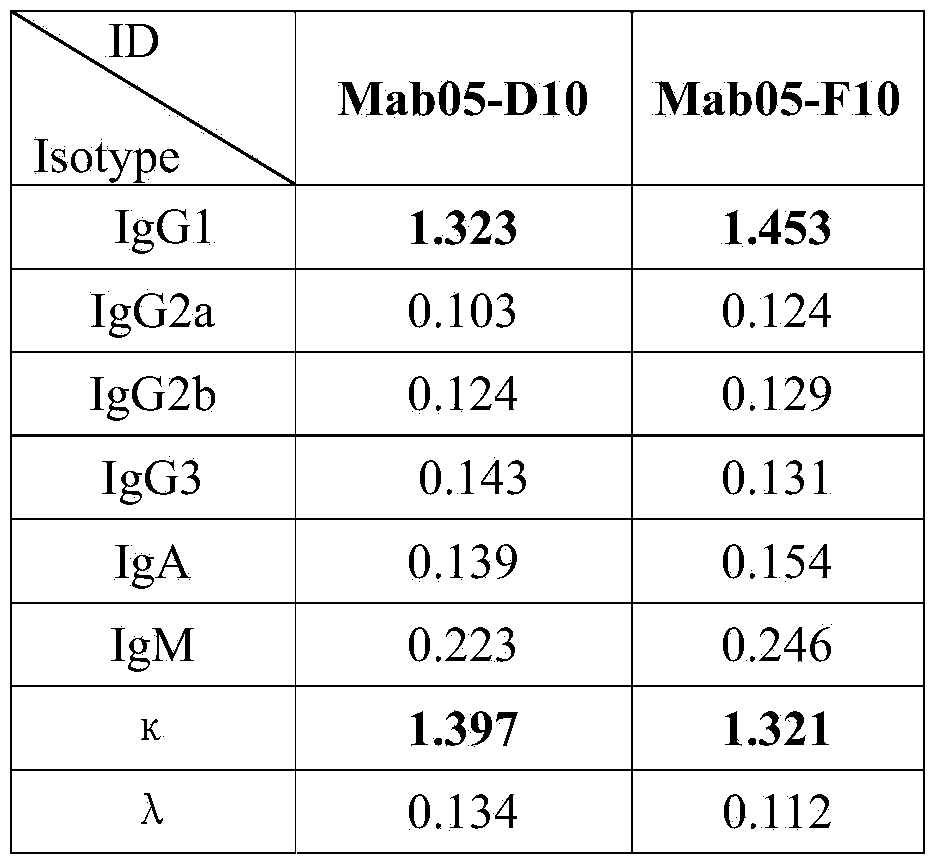
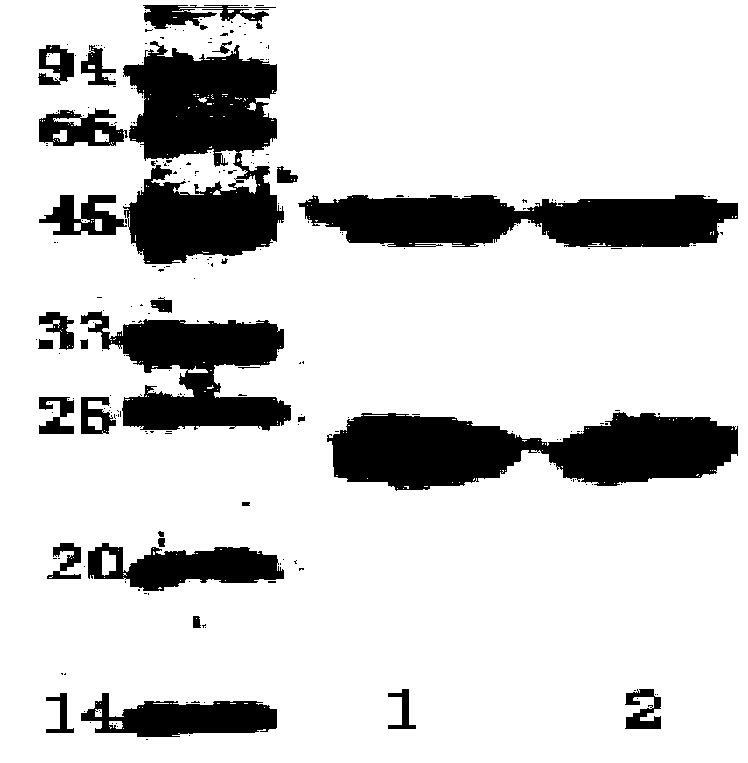
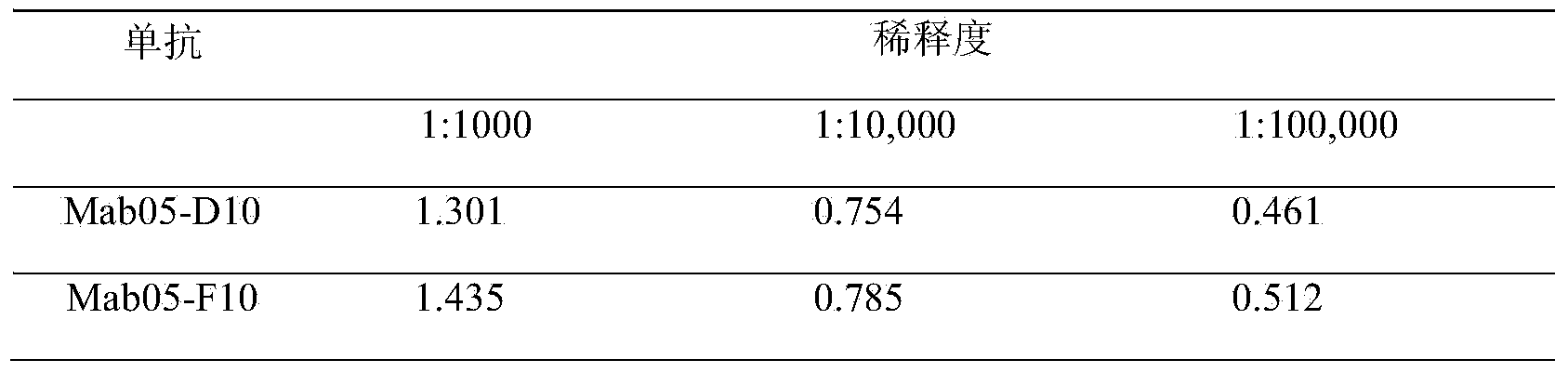
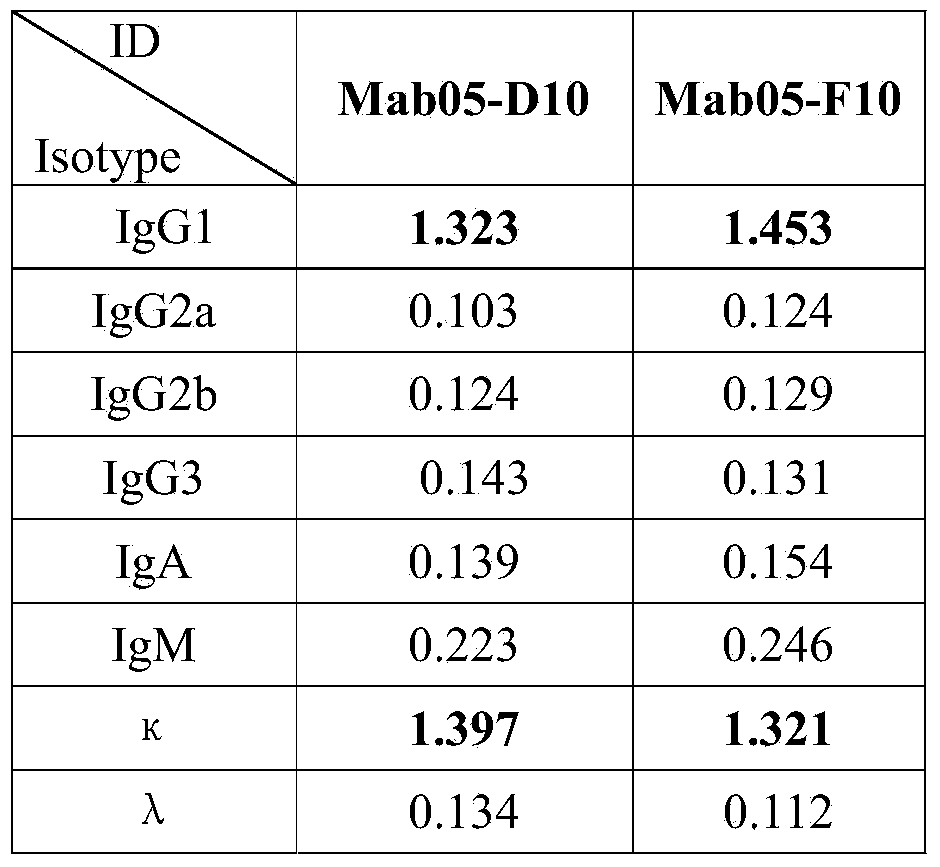
The invention discloses an enzyme-linked immunosorbent assay kit for detecting shigella boydii. The kit contains two strains of monoclonal antibodies which can be specifically combined in shigella boydii, one strain is a monoclonal antibody Mab05-F10 specialized as a capture antibody, and the other strain is a monoclonal antibody Mab05-D10 as a detection antibody. A large number of tests confirm that the kit can be specifically and efficiently detect shigella boydii, moreover, has no cross reaction with other 77 kinds of common pathogenic bacteria, and is a pathogenic bacteria detection product with excellent performance.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Foot and mouth disease virus inhibitor and preparation method and application thereof
InactiveCN101732710AQuick solveImprove the immunityAntiviralsAntibody medical ingredientsIntramuscular injectionSalmonella Be
The invention belongs to the genetic engineering field, in particular relates to a foot and mouth disease virus inhibitor which is designed by adopting RNAi technology and a preparation method and application thereof. The inhibitor is a recombinant live bacterial vaccine containing plasmid for expressing siRNA of foot and mouth disease virus and uses attenuated salmonella suipestifer as recipientbacterium. The recombinant live bacterial vaccine of the invention is convenient to store and use. The inhibitor can be given by injection as well as by mouth. The attenuated salmonella is used as carrier to transfer the expression plasmid of siRNA so that the organism can resist the targeting virus of siRNA and can also resist the infection of salmonella.
Owner:FUDAN UNIV
Primers, probes, a test kit and a test method for triple real-time fluorescence PCR detection of four bacteria
ActiveCN103060447AMicrobiological testing/measurementFluorescence/phosphorescenceSalmonella GallinarumFluorescence
The invention discloses primers, probes, a test kit and a test method for triple real-time fluorescence PCR detection of Salmonella choleraesuis, Salmonella paratyphi, Salmonella typhi, and Salmonella gallinarum. According to the present invention, a triple real-time PCR method is used, wherein a consensus sequence of Salmonella choleraesuis and Salmonella paratyphi is used for the detection of Salmonella choleraesuis and Salmonella paratyphi, a specific sequence of Salmonella typhi is used for the detection of Salmonella typhi, and a specific sequence of Salmonella gallinarum is used for the detection of Salmonella gallinarum. Whether a sample is contaminated by Salmonella choleraesuis, Salmonella paratyphi, Salmonella typhi, and Salmonella gallinarum is determined via a real-time fluorescence PCR amplification, and then Salmonella choleraesuis and Salmonella paratyphi are distinguished via a single fluorescent PCR amplification; the detection is rapid, the process of preparing sample and issuing test results can be finished in 31hours, the results are reliable, and sensitivity and specificity are high.
Owner:许龙岩 +5
Fast check reagent of Salmonella causing food poisoning
InactiveCN101565737AFull of nutritionEasy to prepareMicrobiological testing/measurementAgainst vector-borne diseasesSource typeFood poisoning
The invention relates to a fast check reagent of Salmonella causing food poisoning, in particular to a fast check reagent of food source type Salmonella aertrycke, Salmonella suipestifer, Salmonella enteritidis, TAB vaccine and salmonella B and a preparation method thereof. The fast check reagent has the characteristics of high speed, high accuracy, high sensitivity, and the like. Compared with a traditional method, the fast check reagent has an application value and has an important meaning for guiding clinical diagnosis and treatment and avoiding abusing antibacterial medicines. Specific Salmonella polyvalent and monovalent serum are added in enriched liquid, the check diagnosis process is shortened to 2-6 hours, the serum agglutination reaction of specific serum can be utilized at the time of enriching, and the identification accuracy is larger than 97 percent. The fast check reagent has little time consumption, high sensitivity and strong accuracy when checking pathogenic bacteria.
Owner:于维森
Gene recombined swine cholera salmonella choleraesuis vaccine for blue-ear disease and application thereof
ActiveCN103421729AImprove protectionPreserve immune efficiencyAntibacterial agentsBacteriaBacteroidesDisease
The invention relates to a gene recombined swine cholera salmonella choleraesuis vaccine for a blue-ear disease and application thereof, belongs to the technical field of animal bacteria genetic engineering, and particularly relates to construction of recombined swine cholera salmonella choloraesuis without resistance maker and used for expressing main immunogenicity membrane protein of porcine reproductive and respiratory syndrome virus(PRRSV) and preparation and application of a live vaccine. Recombined swine cholera salmonella choleraesuis C501-GP5m expressing the swine PRRSV GP5m protein is gained and preserved in the China model culture preservation centre, and the preservation number is CCTCC NO: M2012275. The invention further discloses a method for preparing swine cholera salmonella choleraesuis and the porcine reproductive and respiratory syndrome virus vaccine from the recombination strain and application thereof. The prepared recombined live vaccine can stimulate swine to develop the immunity to resist swine cholera salmonella choloraesuis and PRRSV main immunogenicity membrane protein GP5m, and can effectively prevent the infection of paratyphus suum and PRRSV.
Owner:HUAZHONG AGRI UNIV
Uses of Phyllanthus emblica, and Phyllanthus emblica composition and uses thereof
InactiveCN109276602AExpand the scope of antibacterial applicationGrowth inhibitionAntibacterial agentsDigestive systemEscherichia coliBacteroides
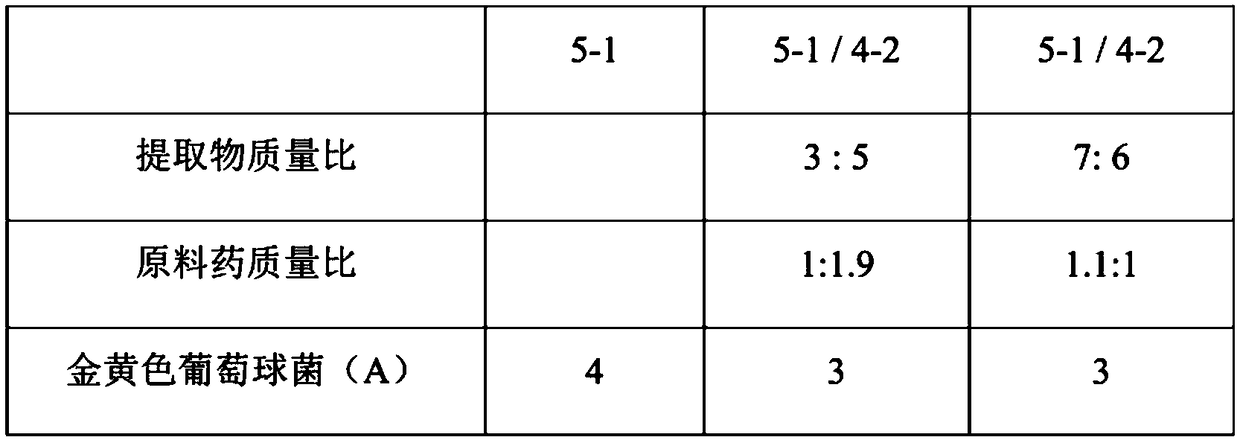
The invention discloses applications of Phyllanthus emblica or Phyllanthus emblica and a Phyllanthus emblica composition in preparation of products for preventing and / or treating at least one of mastitis, diarrhea and porcine respiratory diseases, and further provides antibacterial effects of Phyllanthus emblica or Phyllanthus emblica extract and the Phyllanthus emblica composition, wherein the Phyllanthus emblica or Phyllanthus emblica extract and the Phyllanthus emblica composition can effectively inhibit the growth of one or a variety of bacteria selected from Staphylococcus aureus, Streptococcus agalactiae, Pseudomonas, Salmonella choleraesuis, Escherichia coli, Group A beta-hemolytic streptococcus, Streptococcus suis, Bordetella, Pasteurella multocida, Salmonella enterica, Staphylococcus haemolyticus, Enterococcus faecalis and Erysipelothrix rhusiopathiae, and the effectiveness of the uses are proved through experiments so as to expanded the applications of Phyllanthus emblica andthe Phyllanthus emblica composition.
Owner:SICHUAN ANIMAL SCI ACAD
Attenuated vaccine of salmonella choleraesuis capable of expressing surface antigen of haemophilus parasuis
ActiveCN103421732AGood immune protectionEasy to operateAntibacterial agentsBacterial antigen ingredientsAntigenBacterial disease
The invention belong to the field of gene engineering vaccines for animal bacterial diseases, and particularly relates to construction, vaccine preparation and application of an attenuated vaccine strain of recombined salmonella choleraesuis capable of expressing a surface antigen gene HbpB of haemophilus parasuis without resistance markers. The recombined salmonella choleraesuis asd-C500 / pYA-HbpB capable of the expressing surface antigen gene HbpB of hemophilus parasuis without resistance markers is obtained (the preservation number is CCTCC No: M2013052); the asd gene on the genome of the salmonella choleraesuis is lost by the recombined strain, so that the asd gene and the recombinant plasmid pYA-HbpB of an outer membrane antigen of haemophilus parasuis can both be expressed in the strain. The invention further discloses a construction method of the recombination strain asd-C500 / pYA-HbpB and a corresponding manufacturing method of the attenuated vaccine of the recombination strain asd-C500 / pYA-HbpB, and application to the preparation of vaccines of salmonella choleraesuis and haemophilus parasuis.
Owner:HUAZHONG AGRI UNIV
Salmonella choleraesuis immune PCR detection kit
ActiveCN103820549AMicrobiological testing/measurementAgainst vector-borne diseasesSalmonella diarizonaeImmuno detection
The invention discloses a PCR reaction tube coated with a specific antibody and used for detecting salmonella choleraesuis and an immune PCR detection kit. The PCR reaction tube is pre-coated with the monoclonal antibody which can specifically enrich salmonella choleraesuis in samples. The detection kit can fast, accurately and sensitively detect salmonella choleraesuis from food and other samples, and the sensitivity can reach 103-104 cfu / ml and is increased by 10-100 times than the sensitivity of a conventional PCR. An immunology method and a molecular biology method are organically combined into a whole, enrichment and detection of salmonella choleraesuis in the samples can be achieved in one PCR tube, the operation is simple, the cost is low, the detection is fast, and results are accurate.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Food-borne pathogenic bacterium hybridoma cell rapid screening antigen macro array
ActiveCN104034899AShort manufacturing cycleSolve the costMaterial analysisAgainst vector-borne diseasesAntigenFood borne
The invention discloses a food-borne pathogenic bacterium hybridoma cell rapid screening antigen macro array. The food-borne pathogenic bacterium hybridoma cell rapid screening antigen macro array comprises a substrate, and a pathogenic bacterium, a positive control and a negative control are fixed on the substrate. The pathogenic bacterium is one or more of staphylococcus aureus, hemorrhagic escherichia coli O157: H7, Listeria monocytogenes, salmonella cholerae suis, salmonella typhimurium, salmonella enteritidis, Shigella flexneri, Shigella sonnei, shigella dysenteriae, Shigella boydii, vibrio parahaemolyticus, enterobacter sakazakii, yersinia enterocolitica, campylobacter jejuni, salmonella typhi, Salmonella paratyphi A and Salmonella paratyphi B. According to the food-borne pathogenic bacterium hybridoma cell rapid screening antigen macro array, multi-target screening can be carried out, dozens of hybridoma cells can be screened within 2.5 hours, the preparing period of food-borne pathogenic bacterium monoclonal antibody is greatly shortened, and the production cost is reduced.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Inhibin DNA vaccine capable of improving animal fertility, and preparation and use thereof
InactiveCN101407781AImprove securitySimple preparation processBacteriaMicroorganism based processesWhole bodyAnimals vaccines
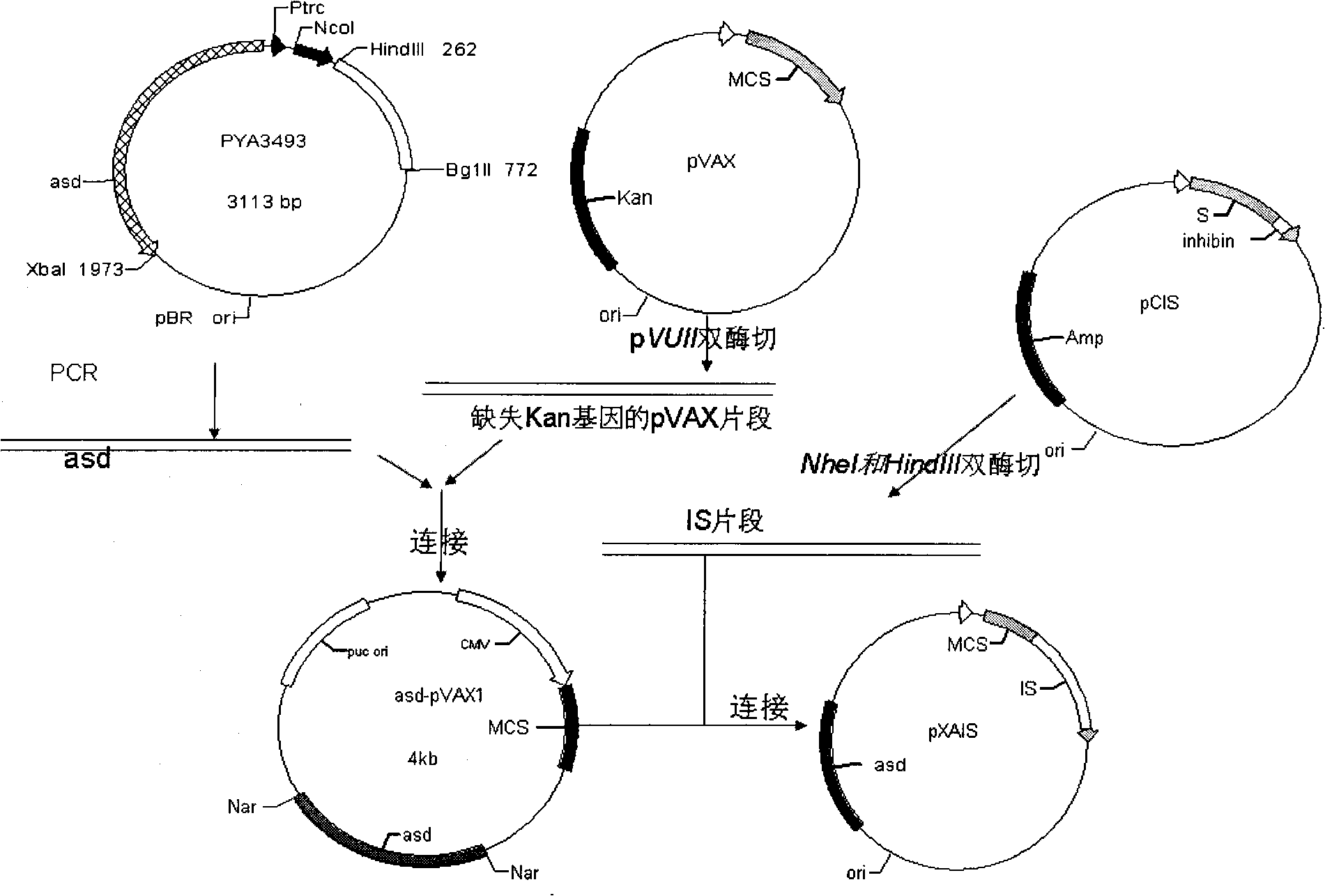
The invention belongs to the preparation technical field of animal vaccines, and particularly relates to a pig cholera salmonella inhibin DNA vaccine which does not contain the residue of antibiotics genes and can improve the propagating fecundity of the animals as well as the preparation and applications thereof. A pig inhibin Alpha (1-32) gene and a hepatitis b surface antigen fusion gene are inserted into a plasmid vector pVAX1 which is lack of a kanamycin resistance gene for obtaining the eukaryotic expression plasmid pXAIS of the inhibin; and the eukaryotic expression plasmid pXAIS of the inhibin is transfected into the pig cholera salmonella C500 for constructing the pig cholera salmonella inhibin DNA vaccine. Compared with the prior art, the vaccine does not contain the residue of antibiotics genes and has the advantages of high moving efficiency, simple immune method and good immune effect. The vector bacteria self can be used as immune adjuvant which can simultaneously activate mucosal immunity and general immunity, and can obtain the immunity to the vector bacteria, and the like.
Owner:HUAZHONG AGRI UNIV
Salmonella choleraesuis immune colloidal gold rapid testing strip
ActiveCN103941006AImprove featuresIncreased sensitivityBiological material analysisGold labellingBiochemistry
The invention discloses a salmonella choleraesuis immune colloidal gold rapid testing strip. The salmonella choleraesuis immune colloidal gold rapid testing strip comprises a sample pad, a colloidal gold pad, a NC membrane, and an absorbent pad which are connected successively; monoclonal antibody Mab05-D10, which is generated by a hybridoma cell strain with a preservation number of CGMCC No.7710, is used as gold-labeled antibody coupled colloidal gold, and is sprayed onto the colloidal gold pad; and monoclonal antibody Mab05-F10, which is generated by a hybridoma cell strain with a preservation number of CGMCC No.7712, is used as a detection antibody, and is sprayed onto the NC membrane so as to form detection lines. In addition, the invention also provides applications of the salmonella choleraesuis immune colloidal gold rapid testing strip in detecting food salmonella choleraesuis. It is shown by experiments that detection specificity and sensitivity of the salmonella choleraesuis immune colloidal gold rapid testing strip on salmonella choleraesuis are both relatively high.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
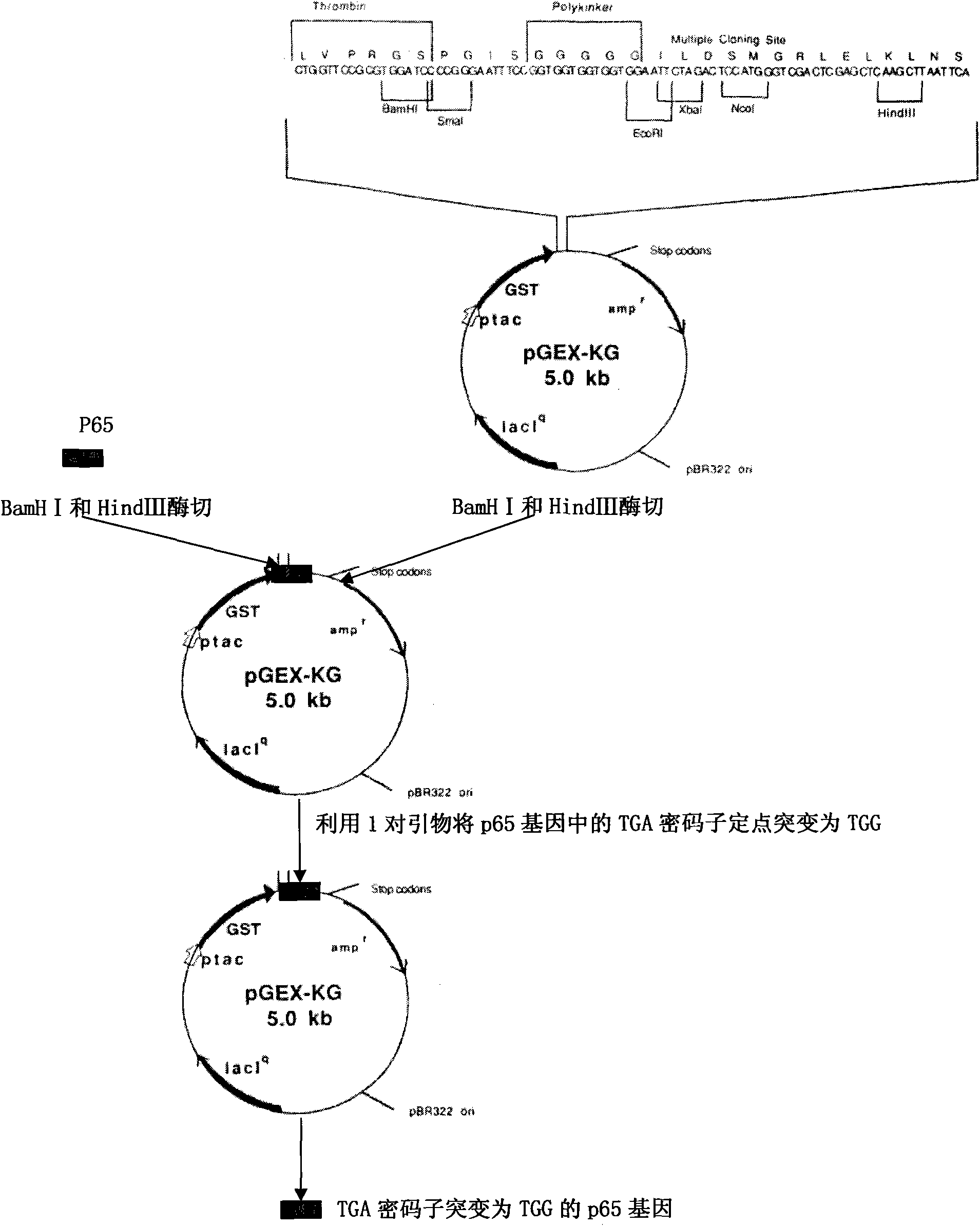
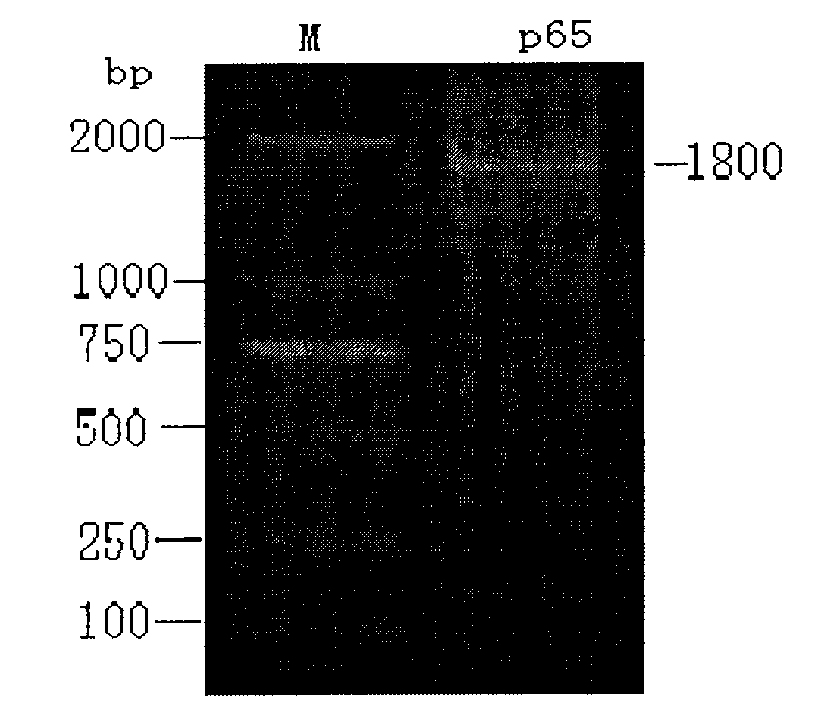
Construction and application of attenuated Salmonella choleraesuis for expressing mycoplasma hyopneumoniae p65 protein
InactiveCN102732472AGood immune protectionWeak toxicityAntibacterial agentsBacterial antigen ingredientsBacteroidesImmunogenicity
The invention belongs to the field of animal bacteria engineering technology, and specially relates to a construction of a resistance marker-free recombinant attenuated Salmonella choleraesuis for expressing a main immunogenic membrane protein of mycoplasma hyopneumoniae, a vaccine preparation and an application thereof. The resistance marker-free attenuated Salmonella choleraesuis C500(pYA-65) for expressing mycoplasma hyopneumoniae p65 protein is obtained, and the collection number is CCTCC NO: M2011107. An asd gene necessary for the growth of Salmonella choleraesuis is deleted in the attenuated strain, and the attenuated strain contains a plasmid capable of expressing the asd gene and the p65 gene of mycoplasma hyopneumoniae. The invention further discloses a Salmonella choleraesuis and mycoplasma hyopneumoniae vaccine prepared from the attenuated strain, a preparation method and an application thereof. The bivalent vaccine of the invention can stimulate a swine to generate a protective immune response resisting the Salmonella choleraesuis and mycoplasma hyopneumoniae, and effectively prevent infection of the Salmonella choleraesuis and mycoplasma hyopneumoniae.
Owner:HUAZHONG AGRI UNIV +1
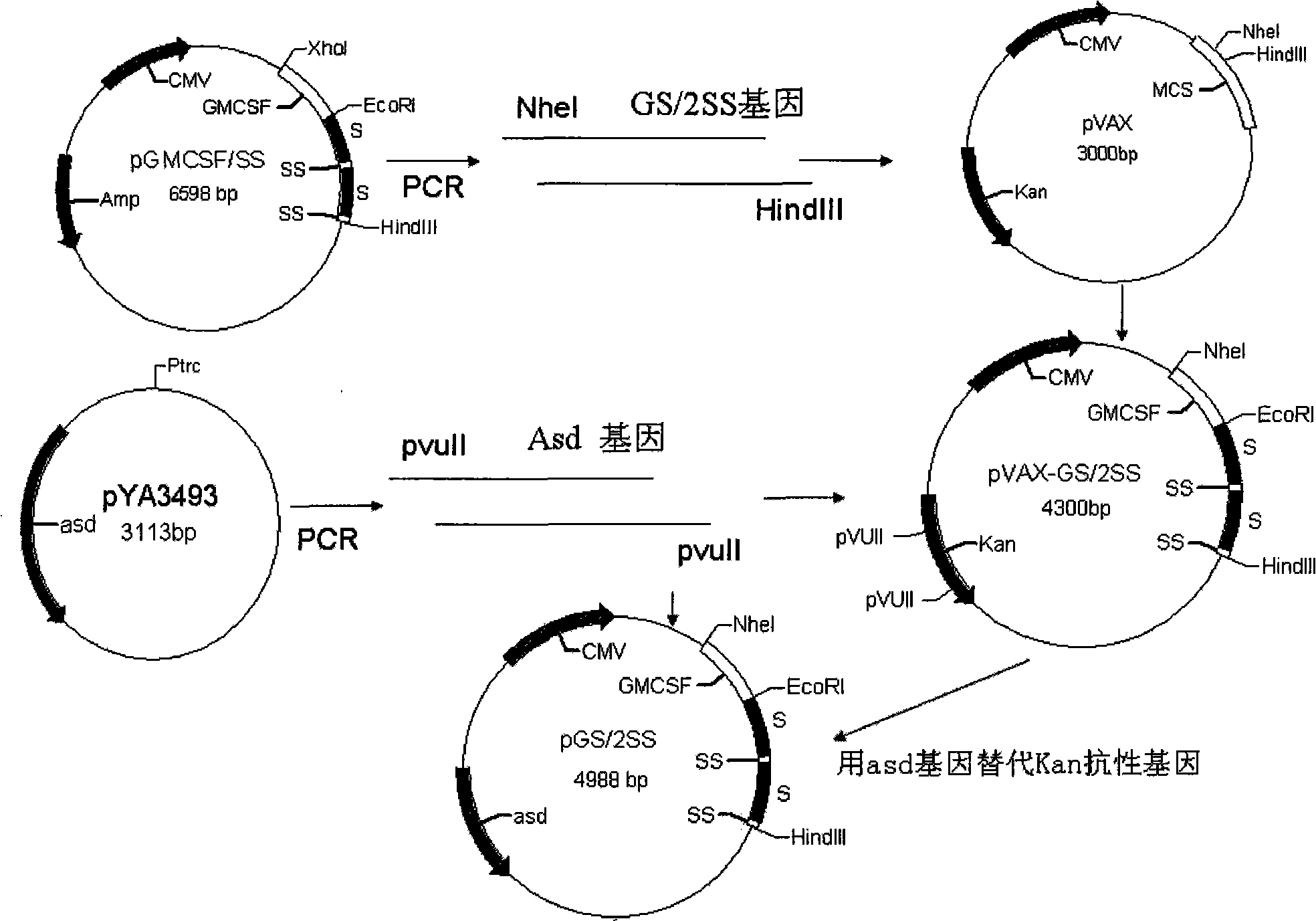
Oral DNA vaccine for promoting growth of animal by non-antibiotics resistance gene screening, and preparation and use thereof
InactiveCN101407782AWon't spreadEasy to useBacteriaMicroorganism based processesSalmonella entericaKanamycin
The invention belongs to the preparation field of animal vaccines, and particularly relates to a non-antibiotically screened animal growth promotion oral DNA vaccine as well as a preparation method and applications thereof. A somatostatin with a GMCSF cell gene and a hepatitis b surface antigen fusion gene GS / 2SS are cloned to a plasmid vector pVAX1 for obtaining a middle plasmid pVAX-GS / 2SS; an aspartic acid Beta-galactose dehydrogenase gene (asd) is used for replacing a kanamycin (Kan) gene in the middle plasmid pVAX-GS / 2SS to obtain an eukaryotic expression plasmid pGS / 2SS of the non-antibiotically screened somatostatin; and then the plasmid pGS / 2SS is converted into a pig cholera salmonella C500 lack of asd and crp genes for obtaining the non-antibiotically screened animal growth promotion oral DNA vaccine. The pig cholera salmonella (Salmonella enterica sv. Choleraesuis) C500 / pGS / 2SS which comprises the eukaryotic expression plasmid of the somatostatin is preserved in the China Center for Type culture collection (CCTCC) and the preservation number thereof is CCTCC NO: M208194. The method also discloses the preparation method of the vaccine and the applications thereof in the aspect of promoting the growth of the animals.
Owner:HUAZHONG AGRI UNIV
Application of oligonucleotide aptamer capable of identifying salmonella specifically
InactiveCN102808022AShort detection timeShort development cycleMicrobiological testing/measurementFluorescence/phosphorescenceEscherichia coliAptamer
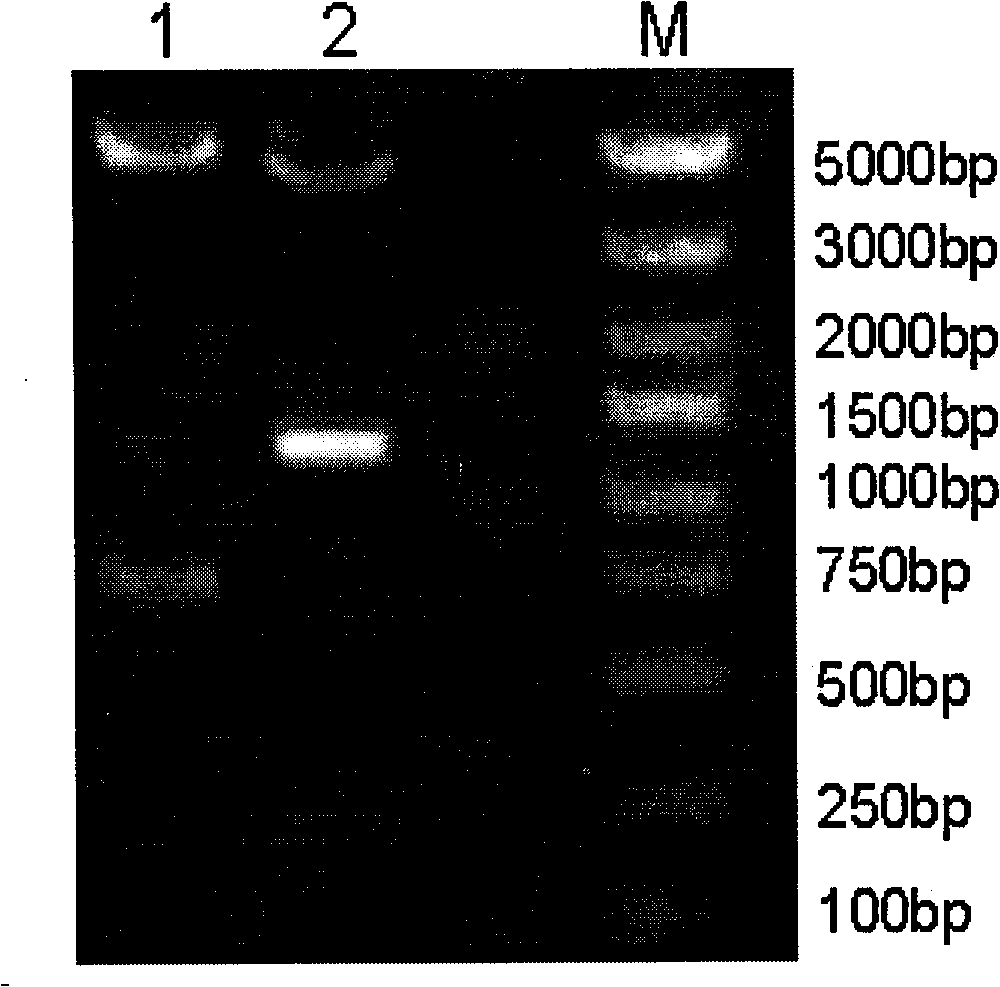
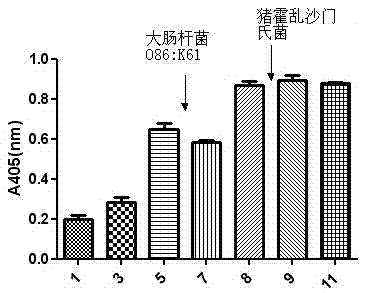
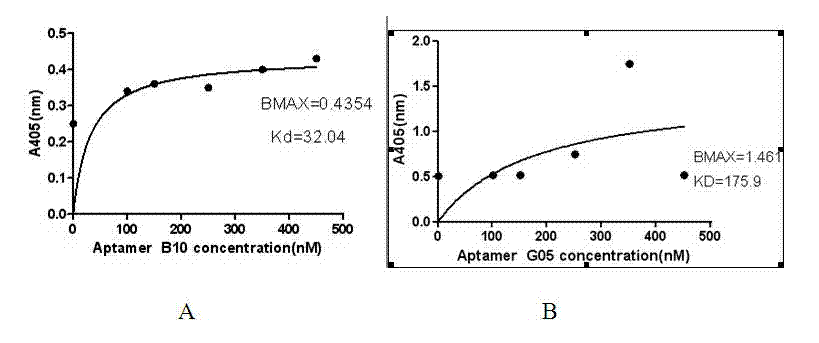
The invention relates to application of an oligonucleotide aptamer capable of identifying salmonella specifically. The oligonucleotide aptamer is a single-stranded deoxyribonucleic acid (DNA) which has 78 bases in length, and is applied to the detection of the salmonella 08. Food-borne salmonella 08 is used as a target molecule, and escherichia coli 086: K61 and salmonella choleraesuis are used as reverse screening bacteria, so that a specific aptamer of the salmonella 08 is obtained. The method has the advantages of short detection time, short development period, stable quality, simplicity of operation and the like, and is widely applied to the detection of food and sanitary safety.
Owner:安徽出入境检验检疫局检验检疫技术中心
Salmonella cholerae enzyme-linked immunosorbent assay kit
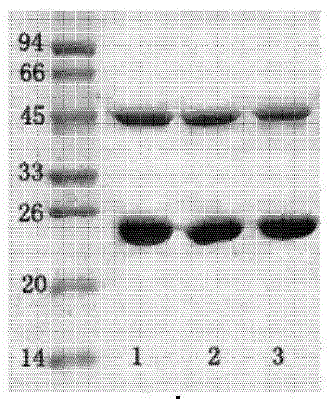
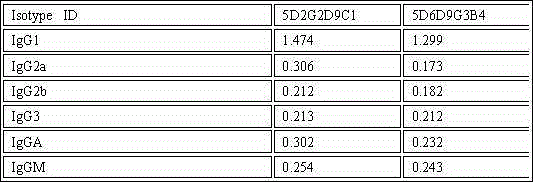
The invention discloses an enzyme-linked immunosorbent assay kit for detecting salmonella cholerae. The kit comprises two monoclonal antibodies which can be specifically combined with salmonella cholerae, wherein one is a monoclonal antibody 5D6D9G3B4 exclusively serving as a capture antibody, and the other one is a monoclonal antibody 5D2G2D9C1 serving as a detection antibody. A large number of tests prove that the kit can specifically and efficiently detect salmonella cholerae and does not carry out cross reaction with other 74 common pathogenic bacteria, and is a pathogenic bacteria detection product with perfect performance.
Owner:JIANGSU WISE SCI & TECH DEV
Construction method of broad-spectrum antiviral recombinant salmonella
InactiveCN113046384AAchieve antiviral effectBacteriaPhosphorus-oxygen lyasesAnimal virusHost immunity
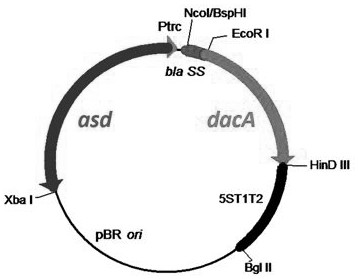
The invention relates to a construction method of broad-spectrum antiviral recombinant salmonella. The construction method comprises the following steps: constructing a recombinant plasmid pS-DacA carrying adenylate cyclase, and introducing the plasmid into a salmonella choleraesuis cracking vector rSC0120 to form a recombinant salmonella choleraesuis cracking vector rSC0120 (pS-DacA). When the DacA is expressed in the salmonella choleraesuis cracking vector, the ATP is catalyzed to form c-di-AMP. After oral administration, the recombinant salmonella choleraesuis cracking vector rSC0120 (pS-DacA) enters host immune cells, c-di-AMP is released, and a host STING pathway is activated to achieve a broad-spectrum antiviral effect. The recombinant strain rSC0120 (pS-DacA) is proved to be capable of activating the STING pathway in macrophages and mice, causing the antiviral state of the macrophages and mice, and resisting the attack of PRRSV and PRV. The recombinant strain has broad-spectrum antiviral efficacy and is suitable for preventing and treating various animal viruses clinically.
Owner:YANGZHOU UNIV
Salmonella choleraesuis gene deletion mutant without resistant marker and vaccine thereof
InactiveCN101962625AAvoid infectionPreserve immune protection potencyAntibacterial agentsBacteriaAnimal testingVeterinary Drugs
Owner:HENAN UNIV OF SCI & TECH
Method for detection of shigella boydii and monoclonal antibodies
ActiveCN103823063AImmunoglobulins against bacteriaBiological material analysisMicroorganismShigella boydii 8
The invention belongs to the field of microbial detection. Specifically, the invention relates to a method for detection of shigella boydii, and two strains of anti-shigella boydii monoclonal antibodies used in the method and produced in a same-time monoclonal antibody preparation process. The monoclonal antibodies can be specifically combined with shigella boydii, and are produced from a mouse hybridoma cell line Mab05-D10 with CGMCC No.7710 or Mab05-F10 with CGMCC No.7712. The invention also provides a use of the anti-shigella boydii monoclonal antibodies and hybridoma cells for producing the antibodies.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com