Construction method of broad-spectrum antiviral recombinant salmonella
A Salmonella and construction method technology, applied in antiviral agents, microbial-based methods, recombinant DNA technology, etc., can solve the problems of body cell damage, increased risk of drug administration, and difficulty in being accepted by the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Implementation 1 Construction and identification of rSC0120(pS-DacA) and rSC0119(pS-DacA);
[0031] 1.1 Construction and identification of pS-DacA plasmid;
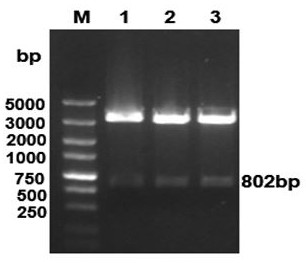
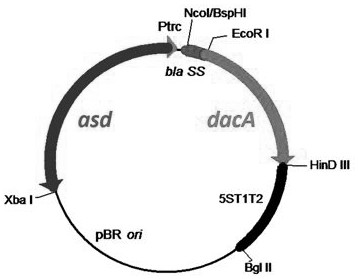
[0032] The complete gene sequence of Listeria monocytogenes NH1 strain (Genbank ID: 1358004970) was used as a template. Design and synthesize DacA base fragments without "ACA" base sequence. The above fragment and pYA3493 plasmid were digested by EcoR I and Hind III at the same time, and the digested products were ligated by T4 ligase. The ligation product was transformed into engineering bacteria Escherichia coli rSC0002, the clone was picked, and identified by double enzyme digestion and sequencing. The double enzyme digestion identification results were as follows: figure 2 .
[0033] The correct clone was identified by double enzyme digestion and sequencing as a positive clone, named rSC0002(pS-DacA), and the pS-DacA plasmid in rSC0002(pS-DacA) was extracted for future use.
[0034] 1.2 Construction and id...
Embodiment 2
[0037] rSC0120(pS-DacA) synthesizes c-di-AMP(CDA) in vitro and releases CDA to the extracellular space The ability to induce the body to produce mucosal immunity, humoral immunity and cellular immunity has been widely used as a carrier to deliver protective antigens and nucleic acid vaccines to prevent certain infectious diseases. However, it is difficult for the protective antigen and nucleic acid recombined into the bacterial vector vaccine to pass through the bacterial cell wall and release into the host cell to play a role. In order to solve this problem, our laboratory developed a Salmonella choleraesuis lysis vector rSC0120 that can lyse and release intracellular substances in a programmed manner. In this part, the in vitro synthesis of the Salmonella choleraesuis lysis vector rSC0120 (pS-DacA) was evaluated by in vitro passage experiments. And unleash the power of CDA.
[0038] to rSC0119(ΔrelA::araC P BAD lacIΔP mazE ::TT araC P BAD mazEΔpmiΔasdA) and rSC0120(ΔrelA...
Embodiment 3
[0041] rSC0120(pS-DacA) activates the STING pathway in 3D4 / 21 cells;
[0042] 3.1 rSC0120(pS-DacA) up-regulates the expression of STING in the cGAS-knockdown porcine lung macrophage cell line 3D4 / 21CDA can activate the STING pathway and up-regulate the expression of STING protein. When Salmonella infects cells, its own DNA may up-regulate the expression of cGAS and activate the subsequent STING pathway. Therefore, in order to prove that it is the influence of the CDA-activated STING pathway catalyzed by the DacA expressed by rSC0120 (pS-DacA), rather than the effect of cGAS, the present invention knocks down the cGAS of the porcine macrophage cell line 3D4 / 21 cells by lentivirus infection , to explore the characteristics of rSC0120(pS-DacA) in activating the STING pathway. 3D4 / 21 cells knocked down cGAS were plated in a 24-well plate, 2.5×10 per well 6 cells. After the cells adhered tightly to the wall, use 1 MOI of recombinant Salmonella choleraesuis rSC0120(pS-DacA) and r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com