Oral DNA vaccine for promoting growth of animal by non-antibiotics resistance gene screening, and preparation and use thereof
A DNA vaccine and resistance screening technology, applied in the direction of recombinant DNA technology, DNA/RNA fragments, and microbial-based methods, can solve the problems of clinical drug-resistant strains spreading, increasing production costs, unfavorable vaccine promotion and application, etc., to achieve It is safe to use, promotes animal growth, and has good growth-promoting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
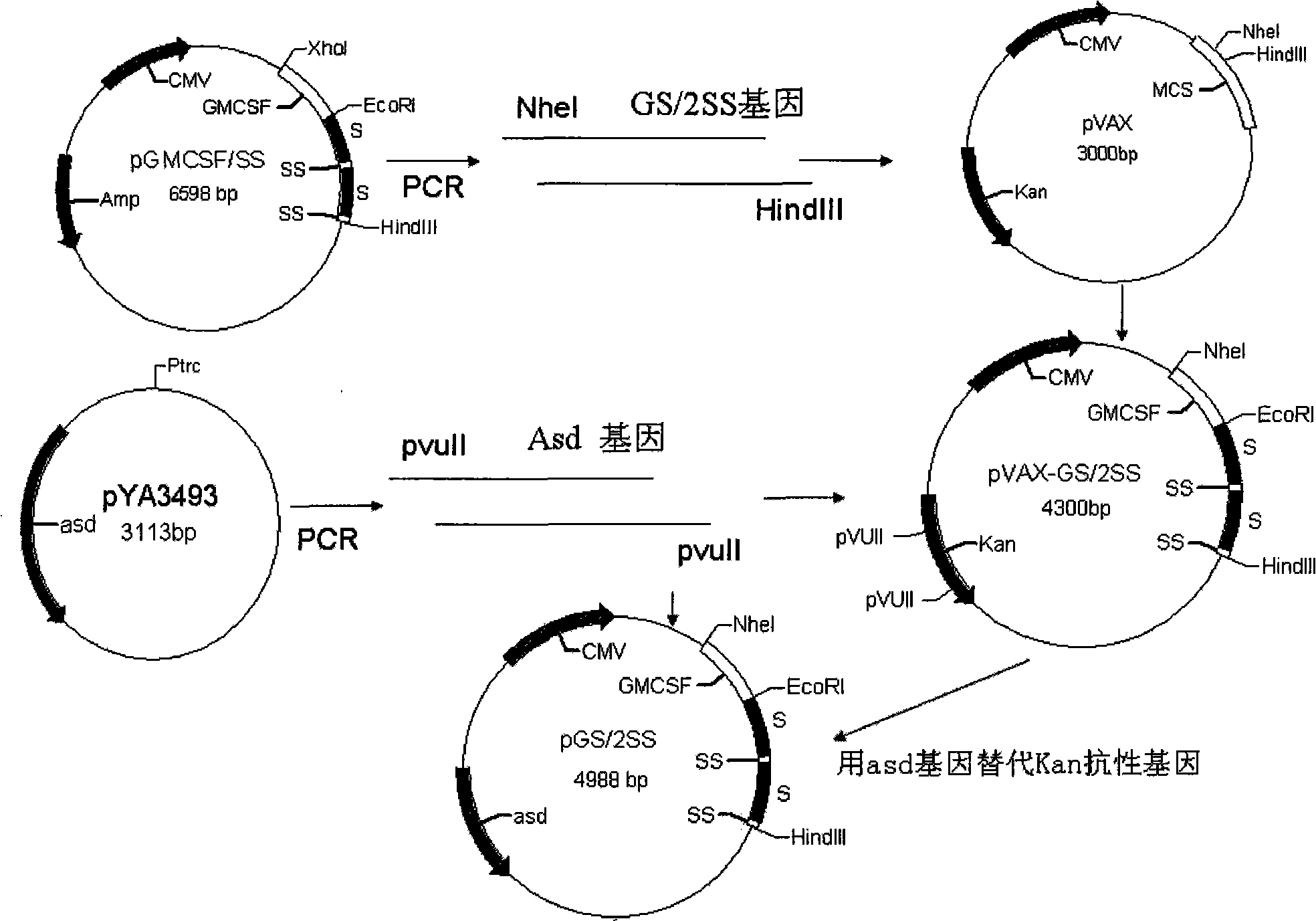
[0026] Example 1: Construction of non-resistance screening somatostatin eukaryotic expression plasmid pGS / 2SS
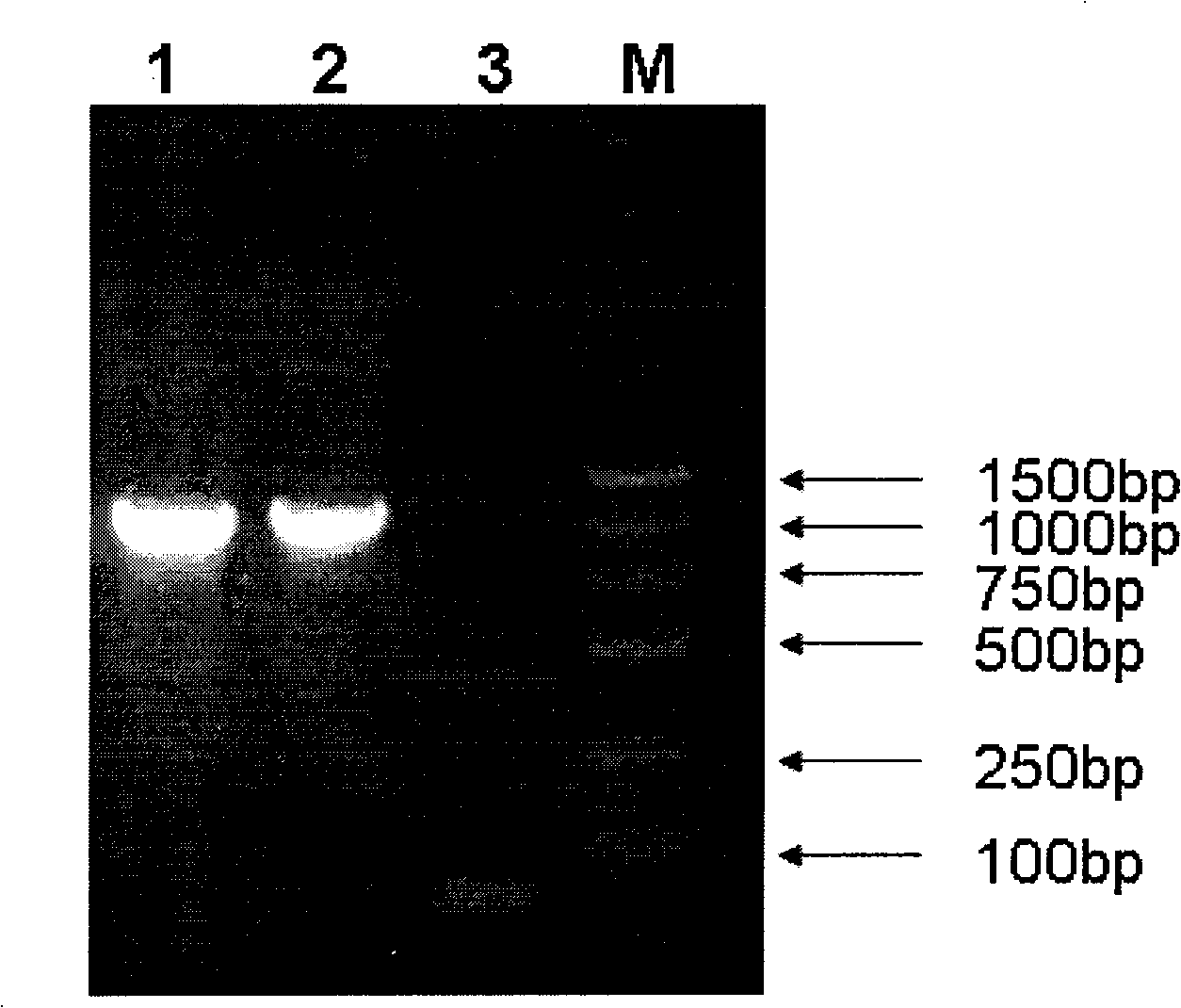
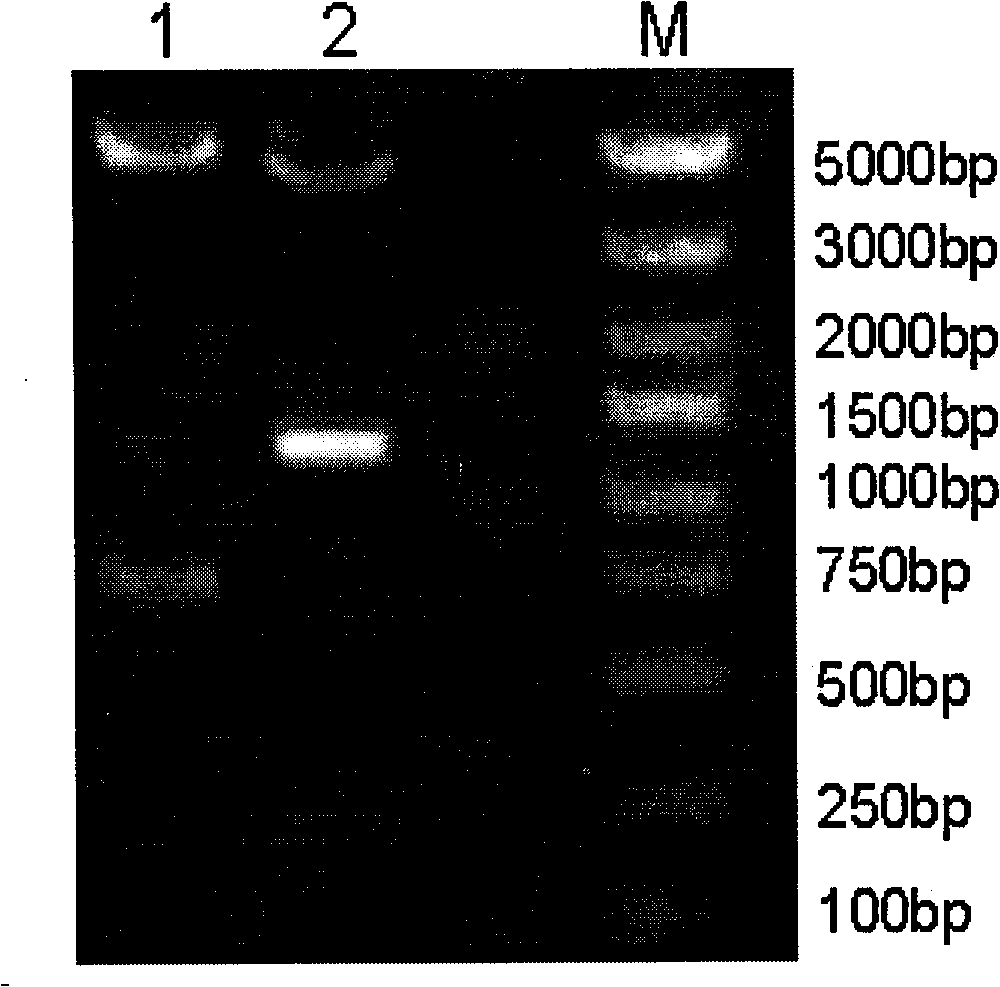
[0027] Using the original plasmid pGM-CSF / SS (patent application number: 200610125580.2, strain deposit number CCTCC NO: M206141) as a template, use primer 5.0 software to design primer pairs (synthesized by Shanghai Sangon Bioengineering Co., Ltd.), forward primer P1: 5'-CTCTAGA GCTAGC CTCAGAAGGA-3' (the underline is NheI enzyme); reverse primer: P2: 5'-TTTGTTCTACGT AAGCTT AAC-3' (the underline is HindIII enzyme), amplifies the somatostatin fusion expression gene GS / 2SS, and then ligates the purified and recovered GS / 2SS gene fragment to pMD18T-Simple Vector (purchased from Bao Biological Engineering Co., Ltd. (Dalian) Co., Ltd.), after identification and sequencing by NheI and HindIII double enzyme digestion, it was connected into the eukaryotic expression vector pVAX1 by NheI and HindIII double enzyme digestion to obtain the intermediate plasmid pVAX-GS / 2SS, wh...
Embodiment 2
[0079] Example 2: Preparation of DNA vaccine C500 / pGS / 2SS for non-resistance screening to promote animal growth
[0080] 2.1 pGS / 2SS plasmid extraction and purification
[0081] The plasmid mini-extraction kit (purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd.) was used to extract the pGS / 2SS plasmid, and the specific operation steps refer to the instruction manual of the kit.
[0082] 2.2 Preparation of C500 Competent Cells
[0083] Refer to the literature (J. Sambrook, D.W. Russell, Molecular Cloning Experiment Guide [M]. Third Edition, Beijing: Science Press, 2002, 99-102) to prepare the Salmonella choleraesuis with the deletion of the asd gene by the power supply transformation method C500 competent cells and stored at -70°C.
[0084] 2.3 Electroconversion
[0085] Transfer the plasmid pGS / 2SS purified in 2.1 into C500 competent cells by electroporation, and the specific steps are as follows:
[0086] 1) Thaw electrocompetent cells on ice and incubate o...
Embodiment 3
[0095] Embodiment 3: the application of DNA vaccine of the present invention in promoting the growth of mice
[0096] 3.1 Vaccine immunization and sample collection
[0097] Use a sterile inoculation loop to pick a single colony of Salmonella choleraesuis C500 / pGS / 2SS containing the eukaryotic expression plasmid pGS / 2SS for non-resistance selection and inoculate it in LB liquid medium without any foreign substances, and at the same time inoculate C500 empty bacteria (Diaminopimelic acid (DAP) with a final concentration of 50 μg / mL needs to be added to the LB medium), shake culture at 37°C and 220rpm to OD6000.3-0.4, centrifuge at 3000g at 4°C for 10min, discard the supernatant, and use The method of plate counting uses sterilized PBS phosphate buffer (pH 7.4, the formula of every liter of PBS phosphate buffer is as follows: KH 2 PO 4 -0.2 g, Na 2 HPO 4 12H 2 O-2.9 grams, NaCl-8.0 grams, KCl-0.2 grams, add distilled water to 1L) adjust the bacterial concentration to 10 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com