Salmonella choleraesuis gene deletion mutant without resistant marker and vaccine thereof
A gene deletion vaccine, Salmonella technology, applied in the direction of antibacterial drugs, medical preparations containing active ingredients, bacteria, etc., can solve the problems that do not meet the biological safety requirements, can not be used as vaccine strains, etc., to achieve broad market application prospects, Good immune protection, weak toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
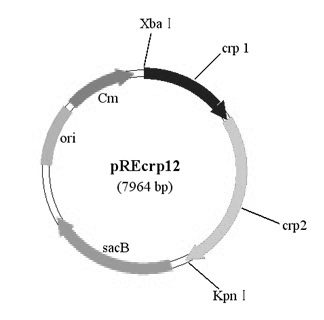
[0031] Example 1 Salmonella choleraesuis C78-2 crp Construction of gene deletion strains
[0032] 1. Primer design (for gene cloning and molecular detection)
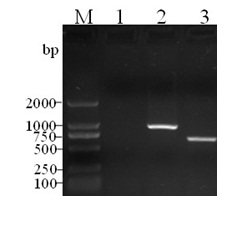
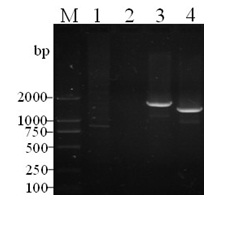
[0033] Refer to literature (Xu Yindi, et al. Construction and identification of a balanced lethal vector system for △crp △asd deletion strain of Salmonella choleraesuis C500 strain. Acta Biological Engineering, 2006, 22(3):366–372) and the reported Salmonella typhi strain Ty2 of crp Gene sequence (GenBank No: AE016847) designed 2 pairs of primers (pr1 / pr2 and pr3 / pr4, see Table 1), from the virulent strain of Salmonella choleraesuis C78-2 (purchased from the National Veterinary Microorganism Collection Center of the China Veterinary Drug Administration). strains) were amplified separately in the genome crp Gene Upstream and Downstream Fragments crp1 (upper arm) and crp2 (lower arm), the sizes of the amplified fragments are 1048 bp and 1743 bp respectively, and the two ends of the upper arm are respectively ...
Embodiment 2
[0044] Example 2 Identification and biological characteristics of Salmonella choleraesuis C78-2 crp gene deletion mutant strain C7821
[0045] 1. Phenotype identification of gene deletion strain C7821
[0046] The gene deletion strain C7821 (experiment number: △crpC78-2) and the parental strain C78-2 were streaked and inoculated on solid LB plates, and then transferred to glucose, maltose, lactose, sucrose, rhamnose, mannose, arabinose, xylose , dulcitol, urea and other carbon sources and H 2 S and other biochemical identification tubes (purchased from Hangzhou Tianhe Company) were used for biochemical reactions. O and H antigens were identified according to the instructions of serum factors (purchased from China Veterinary Drug Administration). The results showed that the biochemical characteristics of the gene deletion strain C7821 were not completely consistent with the parent strain C78-2. Parental strain C78-2 can utilize maltose, and red colonies appear on MacConkey a...
Embodiment 3
[0051] Example 3 Preparation of Salmonella choleraesuis Gene Deletion Vaccine
[0052] The obtained Salmonella choleraesuis gene deletion mutant strain C7821 (experiment number: △ crp C78-2) for identification, each generation was inoculated on 1% maltose MacConkey agar medium to observe the color of the colony, and the use of Salmonella choleraesuis crp Genetic PCR detection was performed to identify the genetic stability of the missing bacteria. After 50 passages, it was found that the colonies of the deletion mutant strain C7821 could not turn red on 1% maltose MacConkey agar medium, and still met crp Phenotypic characterization of gene deletion; and PCR identification crp The gene is still missing 320 bp, and heredity is stable. The Salmonella choleraesuis gene deletion mutant strain C7821 was cultured on LB solid medium, and a single colony was picked and cultured in LB liquid medium until the concentration of viable bacteria reached 1×10 10 CFU / mL. Add gelati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com