HA-mRNA vaccine for preventing influenza A virus infection
A technology of influenza A virus and influenza virus, which is applied in the field of medicine, can solve the problems of long development time, strict requirements, unsuitable use of influenza emergency, etc., and achieve good humoral immunity and good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
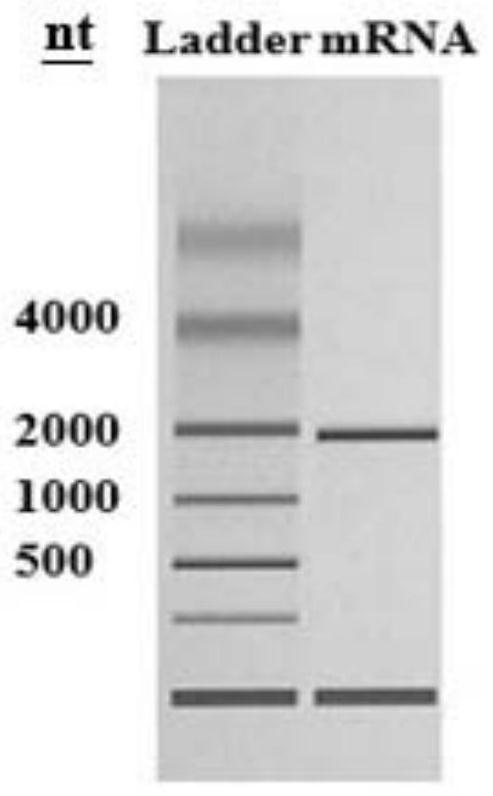
[0028] Design, preparation and in vitro expression detection of embodiment 1, HA-mRNA
[0029] 1. Design and preparation of HA-mRNA
[0030] 1. A template sequence for artificially synthesizing HA-mRNA, as shown in sequence 1 of the sequence listing.
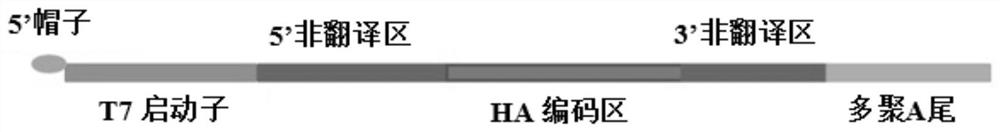
[0031] In sequence 1 of the sequence listing, the 1st to 72nd from the 5' end is 5'-UTR, the 73rd to 1773rd is CDS, and the 1774th to 1990th is 3'-UTR.
[0032] The schematic diagram of the template of HA-mRNA is as follows figure 1 shown.
[0033] 2. Insert the template sequence artificially synthesized in step 1 between the NheI and XhoI sites of the pcDNA3.1(+) vector to obtain a recombinant plasmid (sequenced and verified).
[0034] 3. Perform plasmid extraction on the recombinant plasmid obtained in step 2.
[0035] 4. Use XbaI and PmeI to double digest the recombinant plasmid extracted in step 3, recover and purify the linearized vector fragment containing HA with a size of about 7319bp.
[0036] 5. Configure the reac...
Embodiment 2
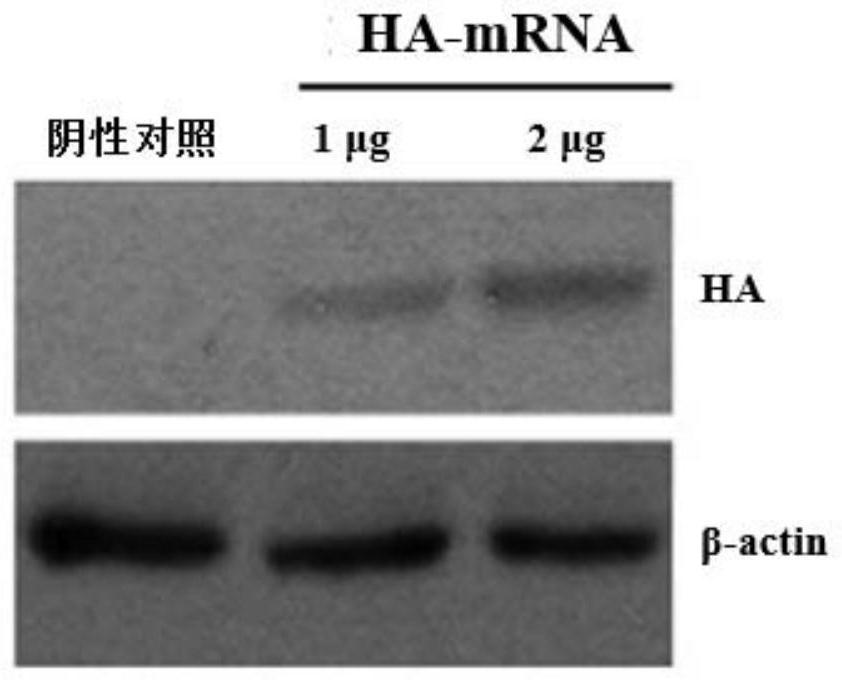
[0102] Embodiment 2, the situation of antibody production after HAmRNA immunization mice and the preventive and protective effect to influenza A virus infection
[0103] 1. Solution preparation
[0104] 1. Sodium pentobarbital solution (prepared at a dose of 0.18mL / 20g)
[0105] Weigh 60 mg of pentobarbital sodium, and prepare 6 mL of pentobarbital sodium solution with a concentration of 10 mg / mL with physiological saline (aqueous NaCl solution with a concentration of 0.9%).
[0106] 2. Protamine solution
[0107] Weigh 1 mg of protamine (Sigma Company), prepare 1 mL of protamine solution with a concentration of 1 mg / mL with normal saline, and place it on ice for use.
[0108] 3. HA-mRNA+protamine vaccine complex
[0109] Prepare 80 μg HA-mRNA per mouse and operate on ice; the concentration of HA-mRNA stock solution is 17.65 μg / μL.
[0110] HA-mRNA+protamine vaccine complex: Take out the frozen HA-mRNA from the -70°C refrigerator, transfer 15.9 μL of HA-mRNA solution (280 μg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com