Inactivated vaccine for CH-JL5 strain of Feline calicirus
A CH-JL5, virus inactivation technology, applied in the direction of vaccines, viruses, antiviral agents, etc., can solve the problems of inability to eliminate, reduce the immune protection effect of vaccines, and no vaccine virus isolates, and achieve the effect of convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Isolation and Identification of Feline Calicivirus CH-JL5 Strain
[0024] 1. Isolation of feline calicivirus CH-JL5 strain
[0025] 1. In May 2013, the oral saliva of clinically suspected cats in Jilin Province was collected with oral cotton swabs;
[0026] 2. Sample processing: Add 500 μL PBS buffer solution to the cat’s oral cotton swab for 5 minutes, centrifuge at 3000 r / min for 10 minutes, discard the cotton swab, mix and separate out 250 μL for later use;
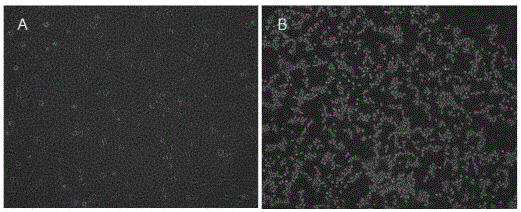
[0027] 3. Isolation and culture of virus: centrifuge the sample at 3000r / min for 15min, take the supernatant and add the same amount of DMEM nutrient solution, filter and sterilize with a 0.22μm filter, inoculate F81 monolayer cells, and place at 37°C in 5% CO 2 When cultured in an incubator, obvious lesions appeared in the second passage after blind passage, and the cytopathic changes were stable at the fifth passage, showing that the cells shrunk round and looked like grape bunches, and finally fell...
Embodiment 2
[0036] Embodiment 2 The preparation method of feline calicivirus inactivated vaccine
[0037] 1. F81 cell recovery: Take out the ampoule or cryovial from the liquid nitrogen tank, put it into a 37°C water bath quickly, and shake it from time to time to completely melt it within 1-2 minutes;
[0038] 2. F81 cell culture: Take out the cells under sterile conditions, centrifuge at 1000r / min for 5min, discard the supernatant, add 1ml 10%DMEM along the tube wall, do not blow up the cells; then add 1ml 10%DMEM, blow evenly cell. Add the cell solution to a T25 cell culture flask, add 5-6ml 10% DMEM to the cell culture flask, mix gently and store at 37°C, 5% CO 2 Cultivate in the incubator and observe twice a day;
[0039] 3. FCV virus amplification: The multiplicity of infection (MOI) was used to determine the amount of virus inoculation, and the FCV was diluted to 0.1, 0.01, and 0.001 respectively, and then inoculated into F81 cells. The virus titers of several infectious amounts ...
Embodiment 3
[0044] Embodiment 3, sterility test and safety experiment
[0045] Take a small amount of above-mentioned inactivated FCV seedlings and inoculate them in dextrose-peptone broth (GP), thioglycolate medium (TG), and casein agar (GA), and detect whether there is bacterial growth according to conventional methods. If there is no bacterial growth, add thimerosal sodium in the sterile room at a rate of 1 / 10,000 of the total amount, then divide into 10 mL per bottle, stopper, seal, label and store in a 4°C refrigerator for later use. Fifteen experimental cats aged 45-60 days were randomly divided into 3 groups, and inoculated with FPV inactivated vaccine 0.5ml, 1ml, 2ml respectively, and 5 negative control cats were not injected with the vaccine, but were raised in the same room as the injected cats, and the vaccine was injected within 15 days Clinical manifestations were observed daily.
[0046] The results showed that the prepared cell cultured inactivated vaccine had no bacterial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com