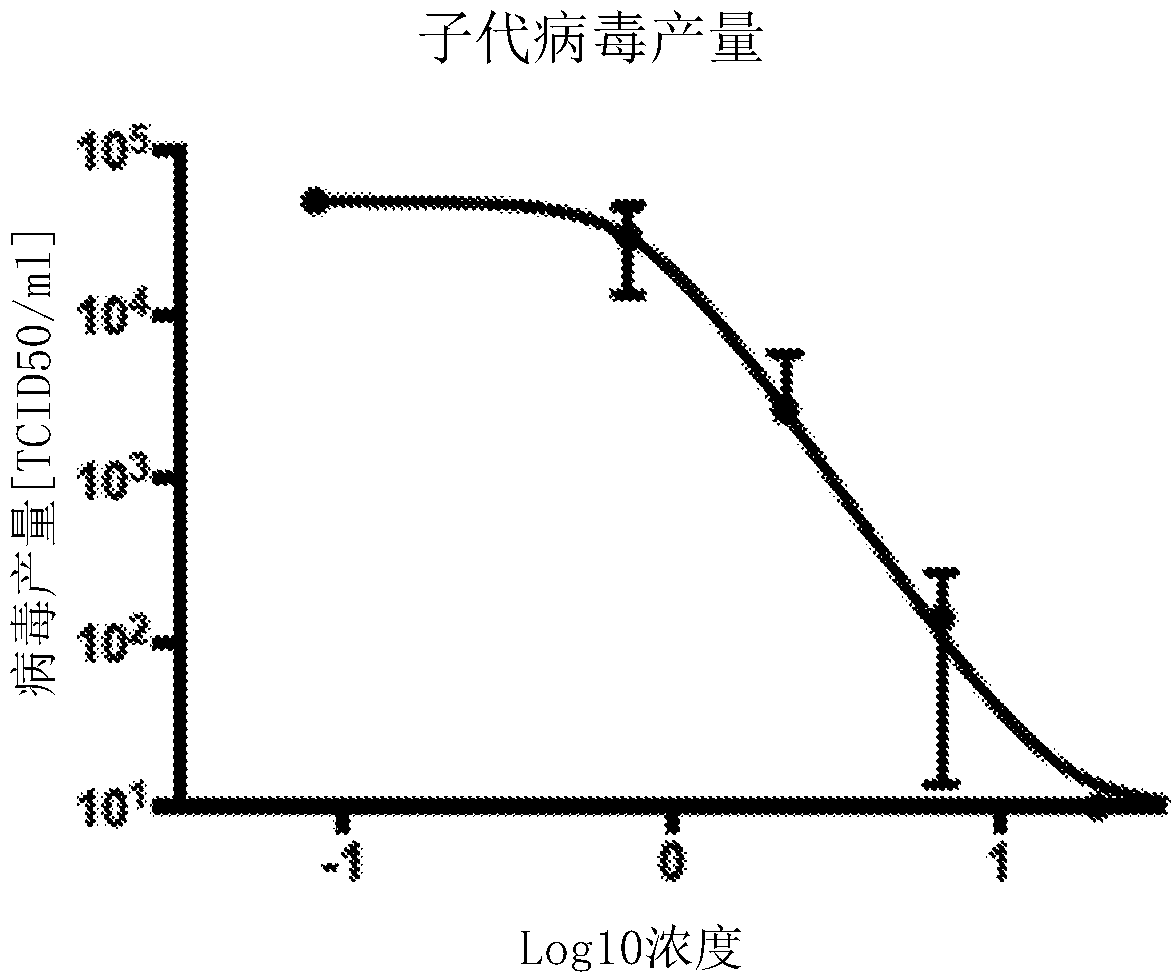
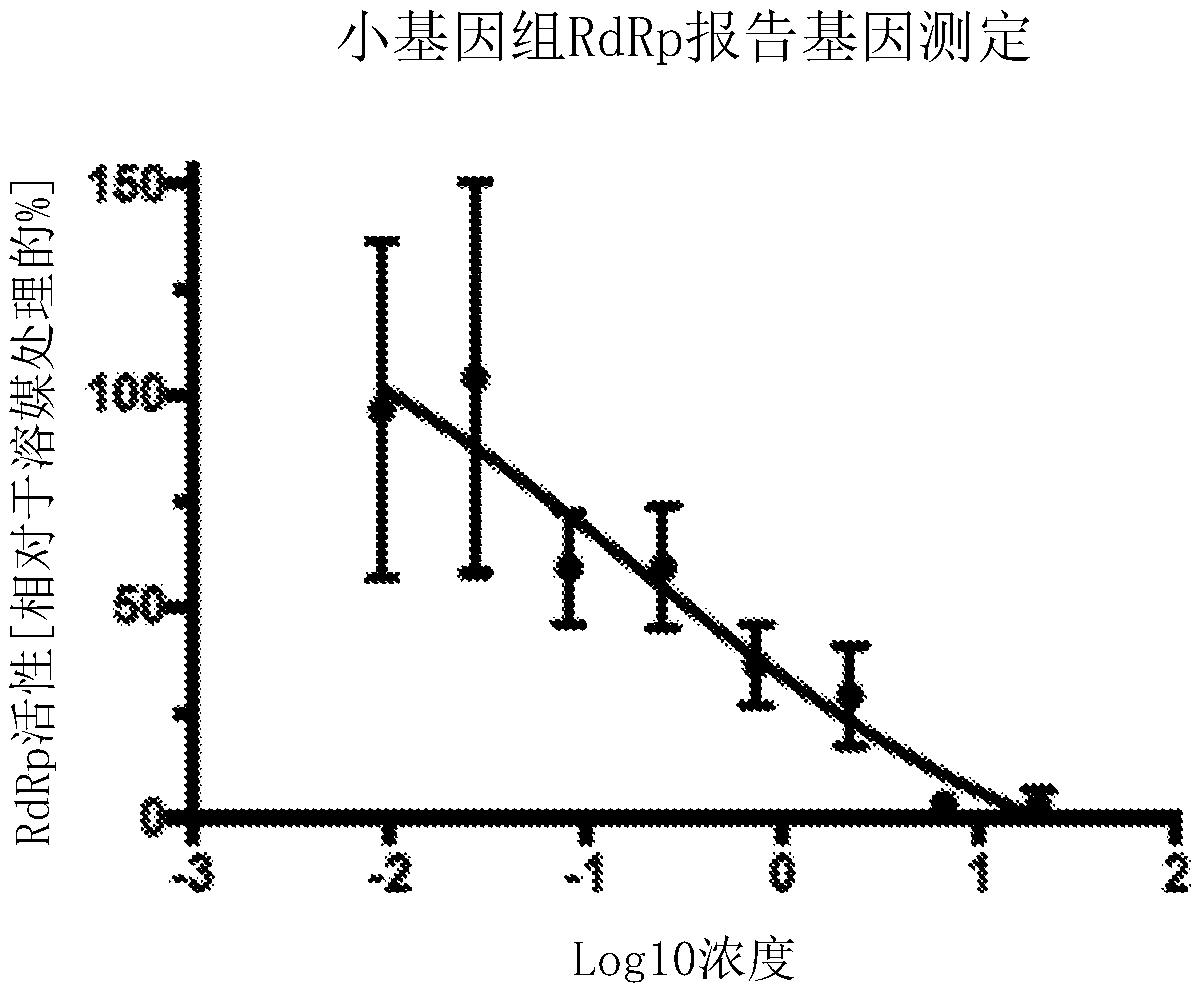
Bicyclic fused pyrazole derivatives for the treatment of rsv
A compound, -NR0 technology, applied in the fields of microorganisms, biochemical equipment and methods, antibody medical components, etc., can solve the problems of unmet, high cost, preventing large-scale implementation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] The method preparation of series A compound
[0195] In all experimental data reported below, the following abbreviations are used:
[0196] rt: room temperature
[0197] UV: Ultraviolet
[0198] HPLC: High Pressure Liquid Chromatography
[0199] Rt: retention time
[0200] LCMS: Liquid Chromatography Mass Spectrometry
[0201] NMR: nuclear magnetic resonance spectroscopy
[0202] CC: column chromatography
[0203] TLC: Thin Layer Chromatography
[0204] sat: saturated
[0205] aq: water-based
[0206] DCM: dichloromethane
[0207] DCE: dichloroethane
[0208] DMF: Dimethylformamide
[0209] DIPEA: Diisopropylethylamine
[0210] EtOAc: ethyl acetate
[0211] TEA: Triethylamine
[0212] THF: Tetrahydrofuran
[0213] TFA: Trifluoroacetic acid
[0214] t-BuOK: potassium tert-butoxide
[0215] n-BuOH: n-Butanol
[0216] EtOH: ethanol
[0217] HOAc: acetic acid
[0218] o / n: Overnight
[0219] MW: microwave
[0220] h: hours
[0221] min: minute
[02...
Embodiment 1A
[0224] Embodiment 1A: conventional synthesis method A1
[0225]
[0226] Exemplary Experimental Procedure for General Method A1
[0227] 1.4-(3-(cyclopentyloxy)-4-methoxyphenyl)-1-phenyl-4,5-dihydro-1H-pyrazolo[3,4-b]pyridine-6(7H )-ketone (AVG-065) was synthesized as follows:
[0228]
[0229] Compound M2 (200mg, 1.3mmol), 2,2-dimethyl-1,3-dioxane-4,6-dione (272mg, 1.9mmol) and 3-(cyclopentyloxy)-4- A mixture of methoxybenzaldehyde (415 mg, 1.9 mmol) in EtOH (10 mL) was refluxed for 16 h. After cooling to room temperature, the mixture was concentrated in vacuo. The residue was diluted with water and extracted with EtOAc (30 mL x 2). The organic layer was washed with water and brine, washed with Na 2 SO 4 Drying, filtration and concentration afforded crude product, which was purified by preparative TLC to afford compound AVG-065 (50 mg, 10% yield) as a white solid.
[0230]Agilent LCMS1200-6110, column: Waters X-Bridge C18 (50mm*4.6mm*3.5μm); column temperature: 4...
Embodiment 1B
[0239] Embodiment 1B: conventional synthesis method A2
[0240]
[0241] Exemplary Experimental Procedure for General Method A2
[0242] 1. Synthesis of 3-(2-(2,4-dimethoxybenzylidene)hydrazino)propionitrile (F2-2)
[0243]
[0244] Hydrazine hydrate (80%, 5.0 g, 124 mmol) was added to a stirred solution of acrylonitrile (6.0 g, 113 mmol) in THF (50 mL) cooled to 0 °C over a period of about 20 min, then the reaction mixture was stirred at room temperature 2h. To the reaction mixture was added 2,4-dimethoxybenzaldehyde (19.7 g, 119 mmol) over a period of about 15 min, and the reaction mixture was stirred at room temperature for 4 h. Subsequent concentration gave a crude oil which was used directly in the next step.
[0245] Agilent LCMS1200-6110, column: Waters X-Bridge C18 (50mm*4.6mm*3.5μm); column temperature: 40°C; flow rate: 2.0mL / min; mobile phase: within 1.6min from 95% [water+0.05% TFA] and 5% [CH 3 CN+0.05%TFA] to 0%[Water+0.05%TFA] and 100%[CH 3 CN+0.05%TF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com