Anti-Henipavirus monoclonal antibody with broad-spectrum neutralizing activity and application
A monoclonal antibody and virus technology, applied in the fields of immunology and microbiology, to achieve good binding activity and excellent broad-spectrum effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Screening and Preparation of Antibodies
[0045] 1. Blood Sample Collection
[0046] Female rhesus macaques were immunized three times, that is, on days 0, 28, and 49, intramuscular injection was used to immunize the adenovirus vector Nipah virus candidate vaccine, recombinant NiV G protein, and recombinant HeV G protein, respectively. Finally, blood samples from rhesus monkeys were collected on day 77.
[0047] 2. FITC-labeled NiV-BD G protein
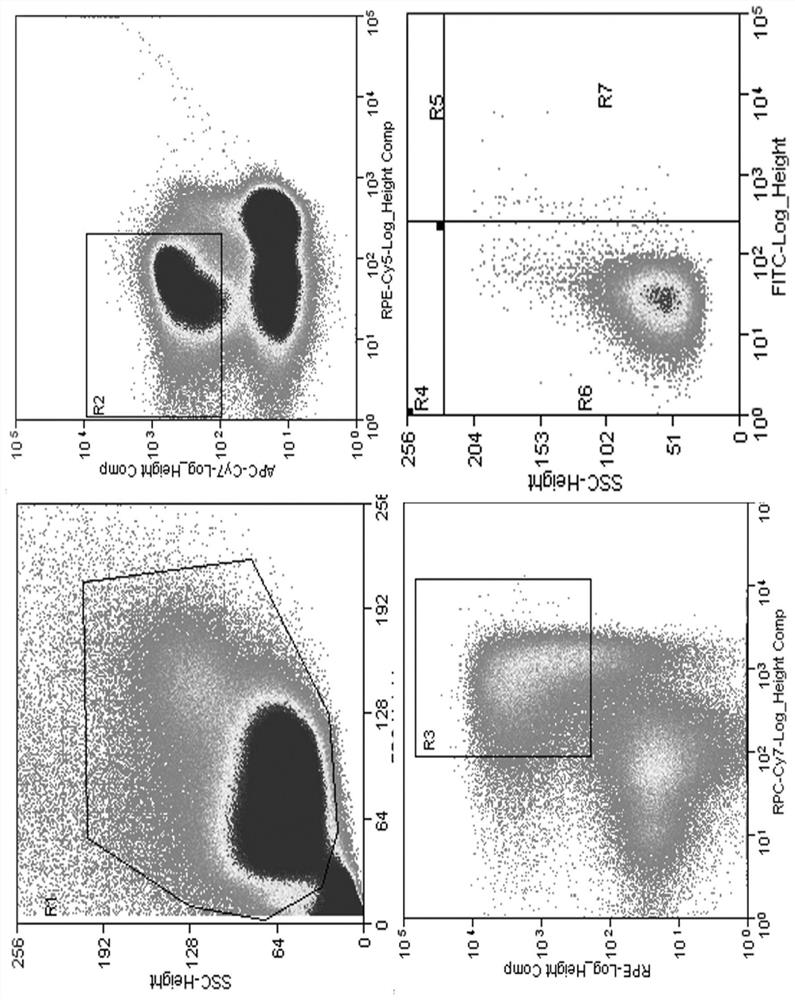
[0048] To sort antigen-specific memory B cells, FITC-labeled antigen protein NiV-BD G was used. Methods as below:
[0049] 1) Fluorescein Isothiocyanate FITC (SIGMA, F4274) was dissolved in DMSO at a concentration of 20 mg / mL. Take 100μL NiV-BD G protein (3.3mg / mL), add buffer (pH 9.6 carbonate buffer) to 400μL.
[0050] 2) Add 8 μL FITC to the NiV-BD G protein solution, and incubate at 4° C. in the dark for 3 hours.
[0051] 3) Use a 30kDa ultrafiltration tube to replace the solution with PBS until the filtrate...
Embodiment 2
[0114] Example 2.ELISA detection of antibody binding activity
[0115] 1) One day before the experiment, the 96-well ELISA plate was coated with 1 μg / mL NiV-BD / MY or HeV G protein (NiV-BD G Genbank accession number: AY988601.1, NiV-MY G Genbank accession number: FN869553.1, HeV G Genbank accession number: NC_001906.3), 100 μL of coating per well, overnight at 4°C.
[0116] 2) On the day of the experiment, wash 5 times with a plate washer, add 100 μL of blocking solution to each well, and place at room temperature for 1 hour.
[0117] 3) Wash the plate, add 150 μL of monoclonal antibody with a concentration of 20 μg / mL to the first well, and add 100 μL of diluent to the remaining wells. Aspirate 50 μL from the first well and add to the second well, and so on, dilute the solution in a 1:3 gradient to a final volume of 100 μL in each well. Let stand at room temperature for 1 hour.
[0118] 4) Wash the plate, dilute HPR-labeled goat anti-human IgG secondary antibody at 1:10000 ...
Embodiment 31E5
[0127] Example 3.1E5 Affinity Determination
[0128] 1) Dilute 1E5 to concentrations of 100nM, 50nM, 25nM, 12.5nM, 6.25nM, 3.13nM, 1.56nM.
[0129] 2) Prepare the Octet RED instrument (fortéBIO, Pall Corp, USA), and set the kinetic detection method in the supporting software DataAnalysis Software v9.0. The method includes 5 steps: Baseline, Loading, Baseline, Association and Dissociation, and the duration of each step is set to 100s, 180s, 60s, 300s and 600s respectively.
[0130] 3) Place the antibody, antigen and PBS buffer solution to start detection. After the experiment, the software DataAnalysis was used for data processing, and the equilibrium dissociation constant KD value and the binding-dissociation curve of 1E5 and Henipa virus G protein were calculated and fitted. like Figure 5 Shown, wherein, A, B, C are the G protein binding dissociation curves of 1E5 and HeV, NiV-BD and NiV-MY, respectively. The calculation results of KD value are shown in the table below. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com