New genetically-modified vaccinia virus
A technology of vaccinia virus and composition, which is applied in the field of new genetically recombined vaccinia virus, and can solve the problems such as the revelation that there are no multiple therapeutic gene combinations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] (Example 1: Construction of Transfer Vector Plasmid DNA)
[0165] The transfer vector plasmid DNA used for producing recombinant vaccinia virus by the homologous recombination method was prepared as follows.
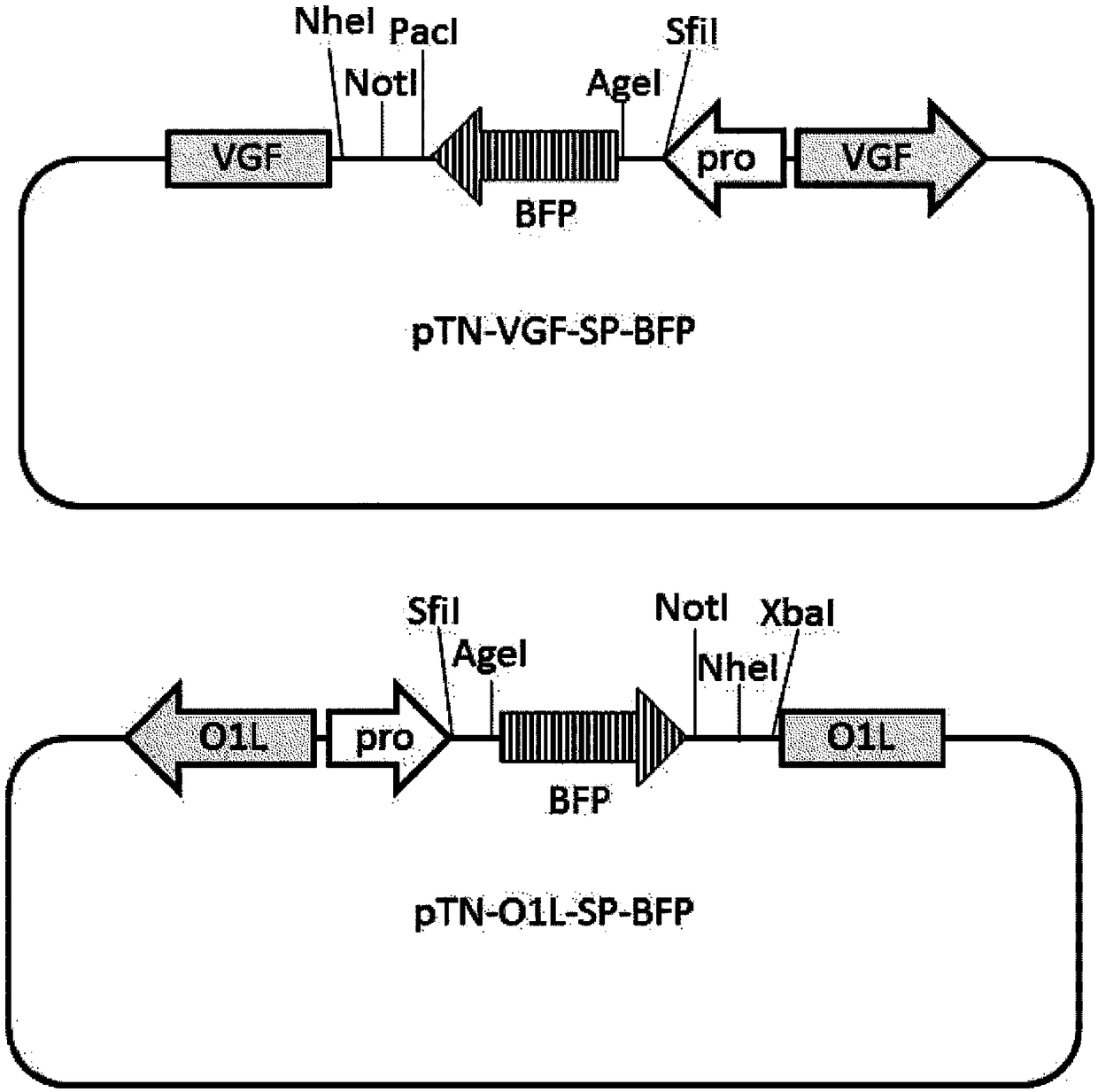
[0166] (1) Construction of pTN-VGF-P-DsRed transfer vector plasmid DNA
[0167] The pUC19-VGF vector was prepared based on International Publication No. 2015 / 076422. More specifically, for the preparation of pUC19-VGF vector, genomic DNA of LC16mO strain (accession number AY678277.1) was used as a template, and pUC19 vector (product code: 54357) of Invitrogen Corporation was used. The prepared pUC19-VGF vector was cleaved with restriction enzyme AccI, and the ends were blunted. A DNA fragment (SEQ ID NO: 22) containing the p7.5k promoter and the DsRed fragment was inserted into the cleavage site, thereby constructing a transfer vector plasmid DNA. The constructed plasmid DNA was called pTN-VGF-P-DsRed.
[0168] (2) Construction of pTN-VGF-SP-IL12 and pTN-VGF...
Embodiment 2
[0174] (Example 2: Construction of genetically recombinant vaccinia virus)
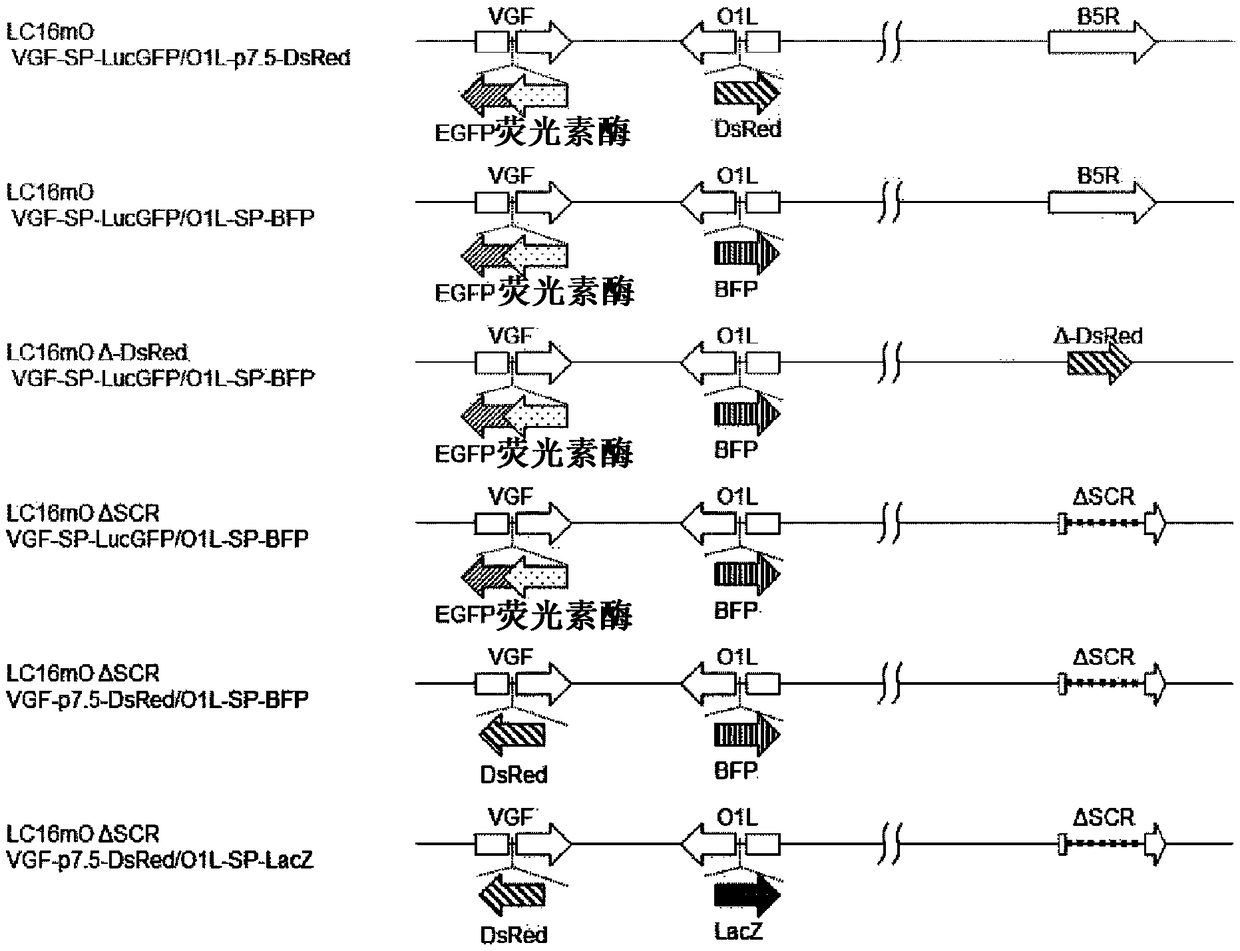
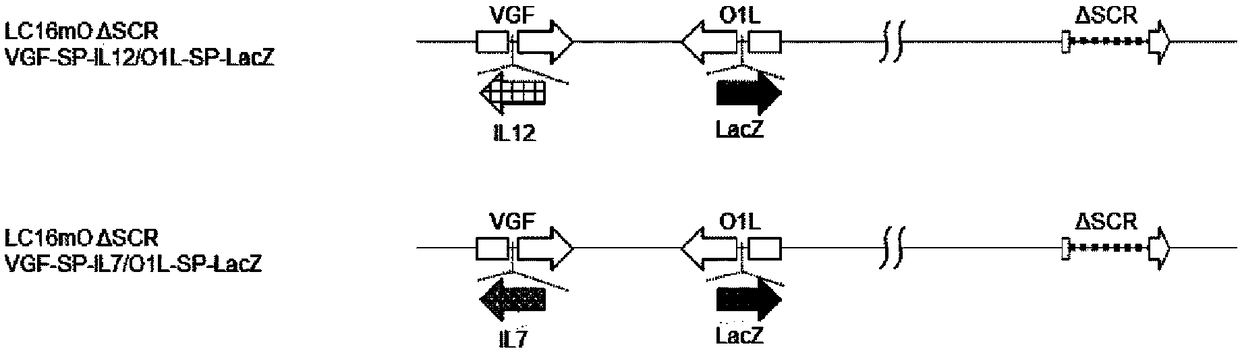
[0175] A recombinant vaccinia virus (called LC16mOVGF-SP-LucGFP / O1L-p7.5-DsRed) deficient in the functions of VGF and O1L was produced from the vaccinia virus LC16mO strain. For this virus, use the next generation sequencer PacBio RSII (Pacific Bioscience Company) to carry out sequencing, use Sprai [BMC GENOMICS.2014 AUG 21; 15:699.] software to carry out the reconstruction of viral genome from the obtained sequence information to determine the base As a result, it has the base sequence represented by SEQ ID NO: 21. In addition, loop sequences are added to both ends of the base sequence, and the loop sequences at both ends are the base sequences represented by SEQ ID NO: 19 or 20.
[0176] (1) Recycling has figure 2 Recombinant vaccinia viruses with viral genomes indicated. Hereinafter, the recovery step will be specifically described. Make LC16mO VGF-SP-LucGFP / O1L-p7.5-DsRed infect CV1 cells (...
Embodiment 3
[0182] (Example 3: Tumor lysis of genetically recombinant vaccinia virus)
[0183] The hIL12- and hIL7-carrying vaccinia viruses prepared in Example 2 were evaluated for their lytic ability (cell death ability) in various human cancer cells. Furthermore, the lytic ability in various human cancer cells was similarly evaluated for the combination mixture of two viruses, hIL12-carrying vaccinia virus and hIL7-carrying vaccinia virus prepared in Example 2.
[0184] Specifically, first, in a 96-well plate (AGC Techno Class company), add the following medium (containing 10% fetal bovine serum (GE Healthcare company) and 1% penicillin streptomycin (Life Technology company) culture medium) to reach 1×10 4 100 μL of each cell suspended in a cell / mL format. After culturing overnight, a mixture of 1) hIL12 and hIL7-carrying vaccinia virus and 2) hIL12-carrying vaccinia virus and hIL7-carrying vaccinia virus at a concentration of 1:1 was mixed using Opti-MEM (Life Technology Co., Ltd....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com