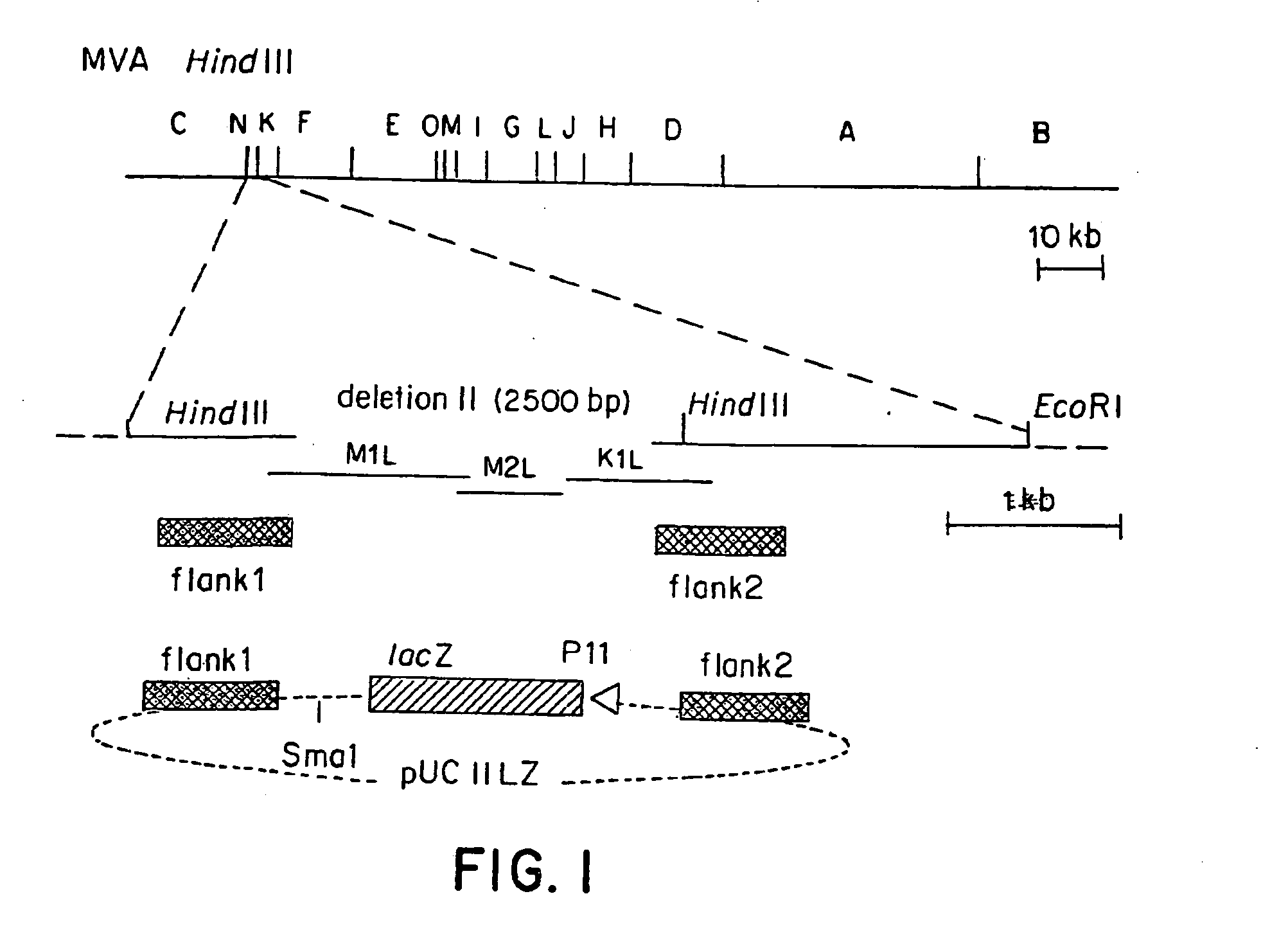
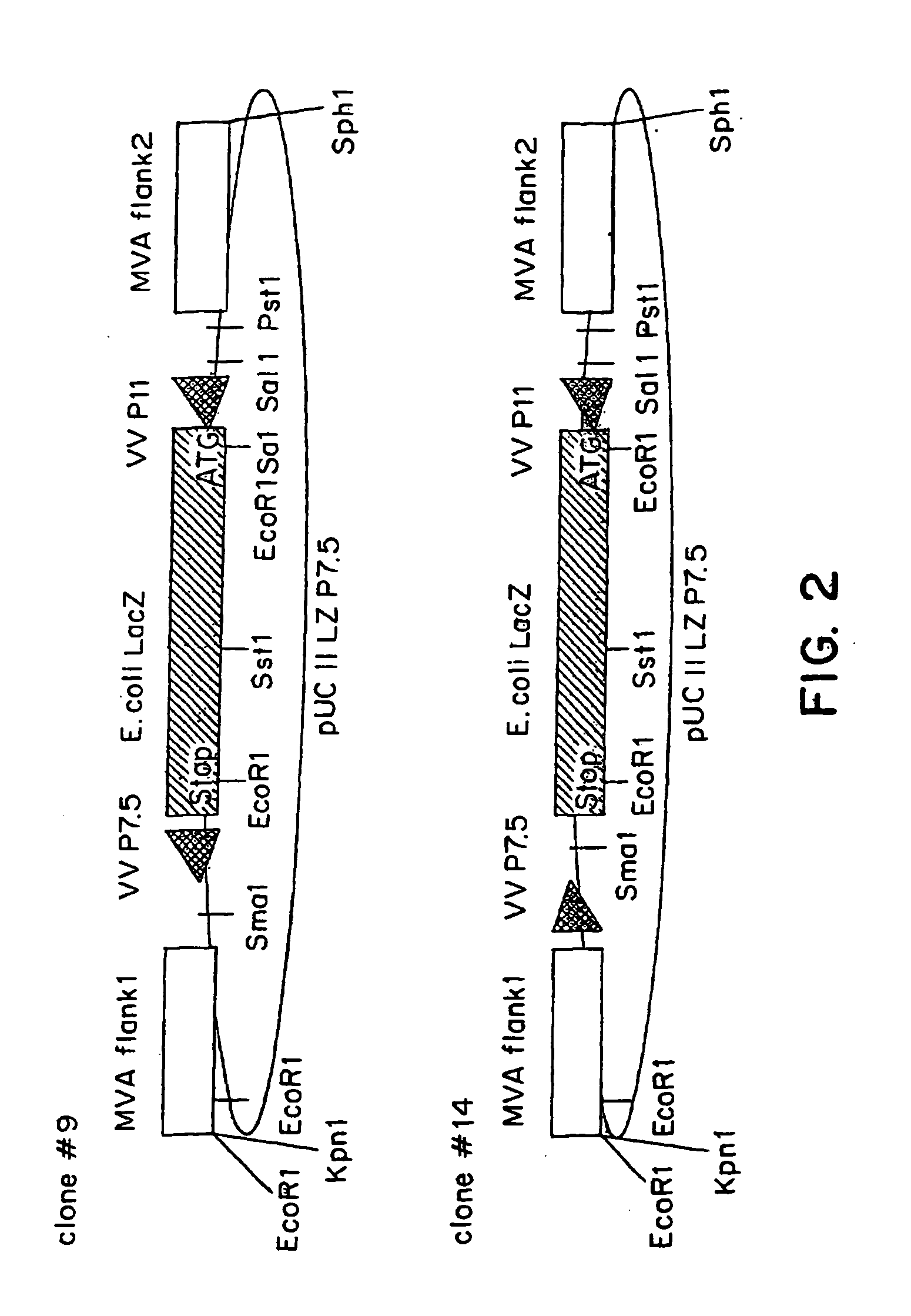
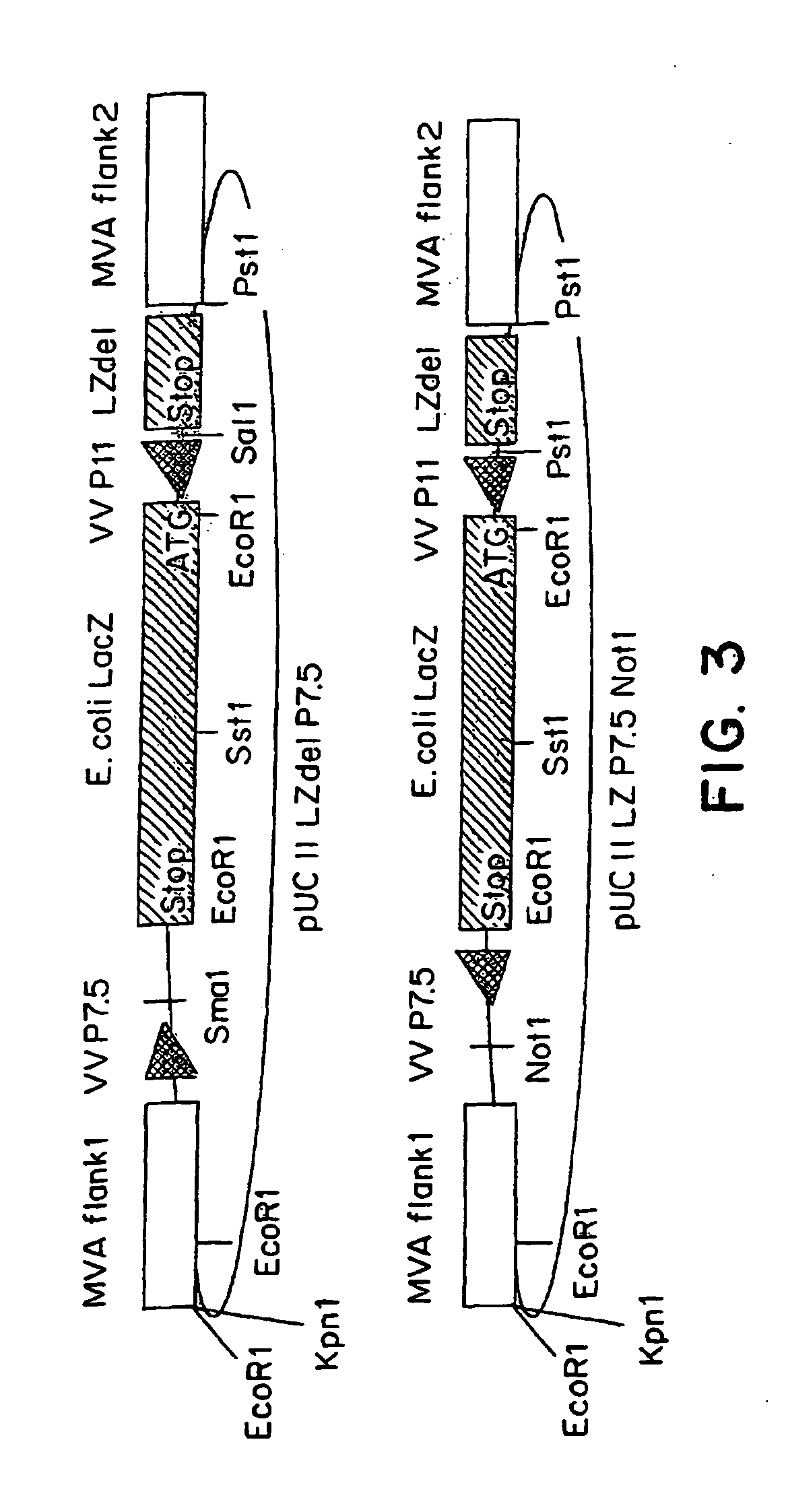
Recombinant MVA virus, and the use thereof
a technology of recombinant mva virus and recombinant mva, which is applied in the manufacture of dna/rna fragments, viruses, genetic therapy compositions, etc., can solve the problems of affecting the development of recombinant vaccinia virus as vector for the development of recombinant live vaccines, the impact of recombinant vaccinia virus, and the productive replication of recombinant vaccini
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Growing and Purification of the Viruses
1.1 Growing of the MVA Virus
[0087] The MVA virus is a highly attenuated vaccinia virus derived from the vaccinia virus strain Ankara (CVA) by long-term serial passages on primary chicken embryo fibroblast (CEF) cultures. For a general rewiew of the history of the production, the properties and the use of MVA strain, reference may be made to the summary published by Mayr et al., in Infection, 3:6-14 (1975). Due to the attenuation in CEF, the MVA virus replicates to high titers in this avain host cell. In mammalian cells, however, MVA is severely growth restricted, and typical plaque formation by the virus is not detectable. Therefore, MVA virus was grown on CEF cells. To prepare CEF cells, 1-day-old embryos were isolated from incubated chicken eggs, the extremities are removed, and the embryos are minced and dissociated in a solution composed of 0.25% trypsin at 37° C. for 20 minutes. The resulting cell suspension was filtered and cells w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com