Efficiently recombinant vaccinia virus vector without screening markers and establishment method of vaccinia virus vector
A technology for vaccinia virus vectors and recombinant virus vectors, applied in the field of highly efficient recombination and non-screening vaccinia virus vectors and its establishment, can solve problems, generate safety and other issues, and achieve increased possibilities, time savings, and improved recombination efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Obtaining recombinant virus by CRISPR-Cas9 system
[0055] 1. Method
[0056] 1.1. Design of gRNA
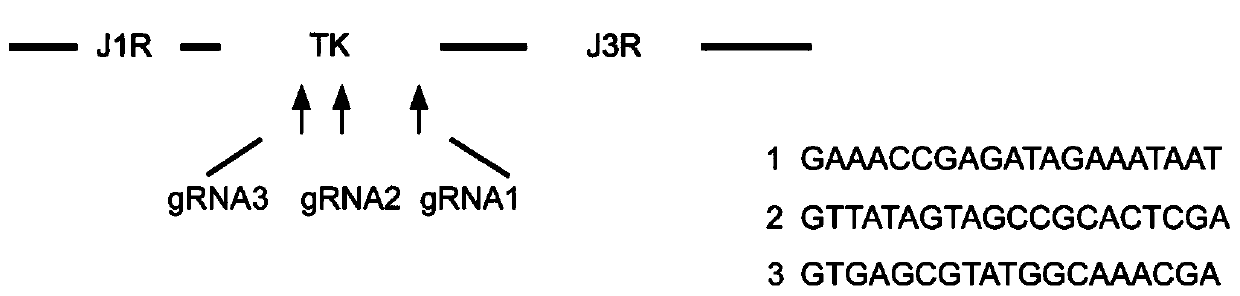
[0057] For the Tiantan strain vaccinia virus TK region, design gRNA sequences through the website (http: / / crispr.mit.edu / , http: / / www.e-crisp.org / E-CRISP / ), including gRNA1, gRNA2 and gRNA3 ( Such as figure 1 shown).
[0058] The sequence of gRNA1 is: GAAACCGAGATAGAAATAAT;
[0059] The sequence of gRNA2 is: GTTATAGTAGCCGCACTCGA;
[0060] The sequence of gRNA3 is: GTGAGCGTATGGCAAACGA.
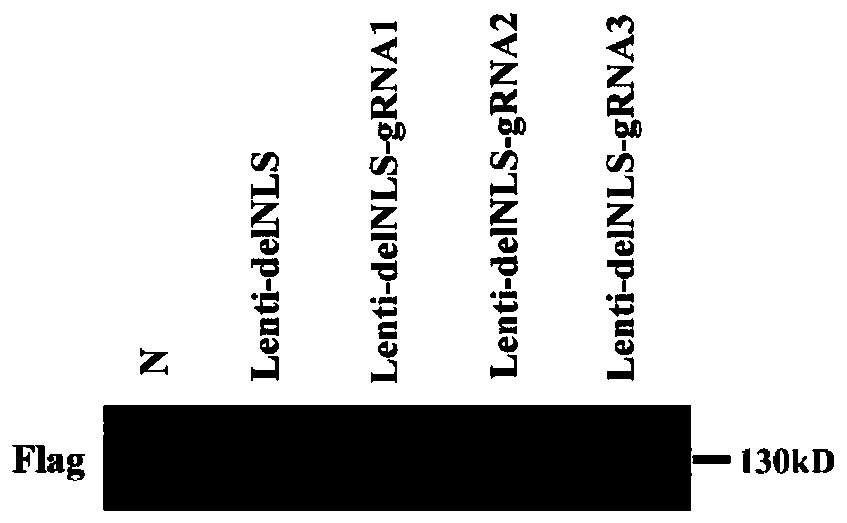
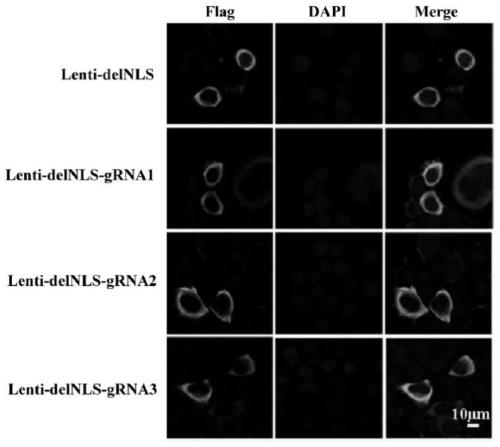
[0061] 1.2. Detection of expression and localization of Cas9 protein in gRNA-Cas9 plasmid
[0062] 1.2.1, protein extraction
[0063] At 293T (5×10 5 ) cells were transfected with Lenti-delNLS, Lenti-delNLS-gRNA1, Lenti-delNLS-gRNA2, Lenti-delNLS-gRNA3, and the cells were harvested after 48 hours. Centrifuge at 3000rpm for 3min, wash once with PBS, add 100μL of cell lysate RIPA, and store at -20°C for later use.
[0064] 1.2.2, Western Blot detection of Cas9 expression ...
Embodiment 2
[0116] Example 2 Delete the screening marker EGFP in the recombinant virus by Cre-LoxP technology
[0117] 1. Method
[0118] 1.1 Construction and identification of pQCXIP-Cre plasmid
[0119] The Cre was constructed into the pQCXIP vector by molecular cloning, and the sequence was confirmed to be correct by sequencing.
[0120] 1.2. Acquisition of recombinant virus VACV-ΔTK
[0121] 1.2.1. Delete EGFP in VACV-ΔTK-EGFP-LoxP virus by transfecting pQCXIP-Cre plasmid
[0122] 293T cells (5×10 5 ) were inoculated in a six-well plate in complete antibiotic-free DMEM medium, cultured overnight, and when the cell growth was 60% to 70%, the control and pQCXIP-Cre plasmids were transfected, and the medium was replaced with 10% FBS after 4 to 6 hours. After 24h, the recombinant virus was diluted with DMEM
[0123] VACV-ΔTK-EGFP-LoxP was used to infect the cells; after 2 hours, the culture medium was replaced with 2% FBS; after 48 hours, 1.2 mL of the supernatant was discarded, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com