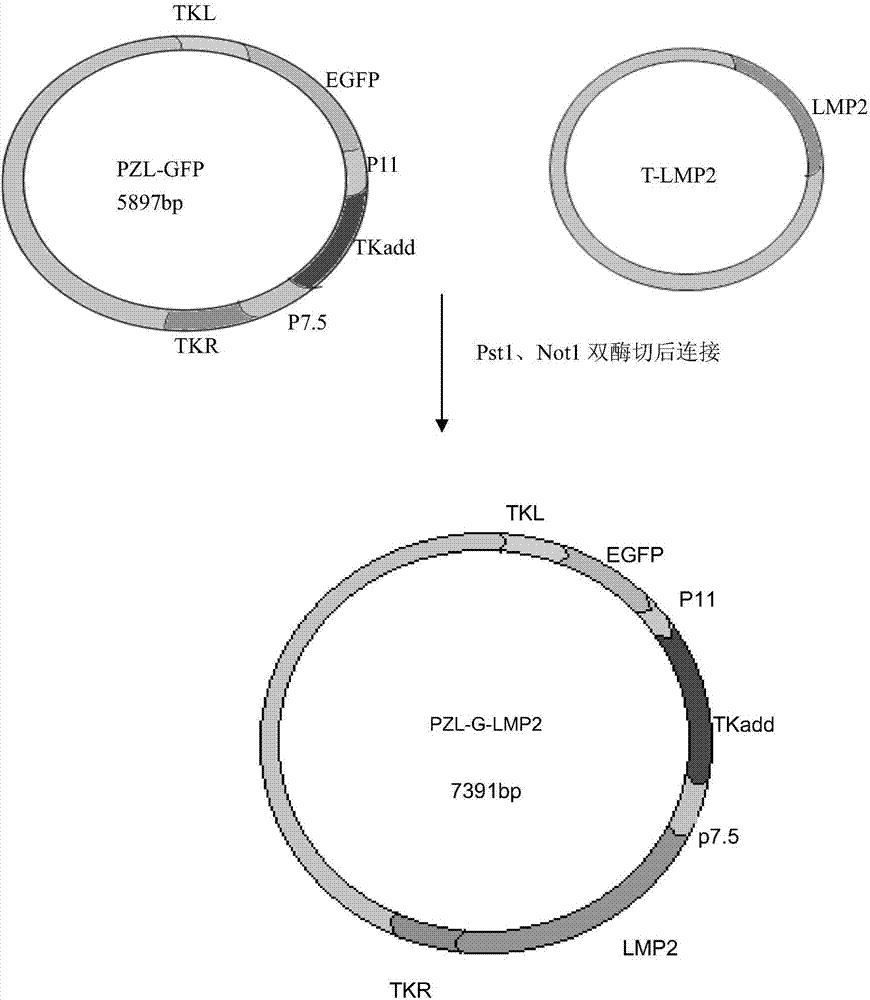
Recombinant vaccinia virus carrying EB virus latent membrane antigen 2 gene and application of recombinant vaccinia virus
A technology of vaccinia virus and Epstein-Barr virus, applied in application, antiviral agent, genetic engineering, etc., to achieve rapid detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Example 1: Construction and Identification of PZL-GFP-LMP2 Recombinant Plasmid
[0045] 1.1 Acquisition of EBV LMP2 gene fragment
[0046] Pst1 and Not1 double digestion T-LMP2 plasmid (Zhang Lixia. EBV-LMP2 recombinant vaccinia virus immune effect research. Beijing University of Technology), the enzyme digestion system is 20ul (DNA plasmid 2μL; NEBuffer3.1 1μL; BSA: 2μL; Pst1 0.5μL ; Not 10.5 μL; ddH 2 O 14 μL), digestion at 37°C for 2 hours. Load 10 μL of the digested product, electrophoresis on 1% agarose gel at 110V, observe whether the target band appears under ultraviolet light, and recover the target fragment using Qiagen’s gel cutting recovery kit. The specific operation is as follows: according to 0.1 Add 300 μL of QG to g gel, mix 2-3 times intermittently at 50°C for 10 min; transfer supernatant to QIA quick spin column, centrifuge at 12000 rpm for 1 min; add 700 μL of PE Buffer, centrifuge at 12000 rpm for 1 min; centrifuge again at 12000 rpm for 1 min; ...
Embodiment 2
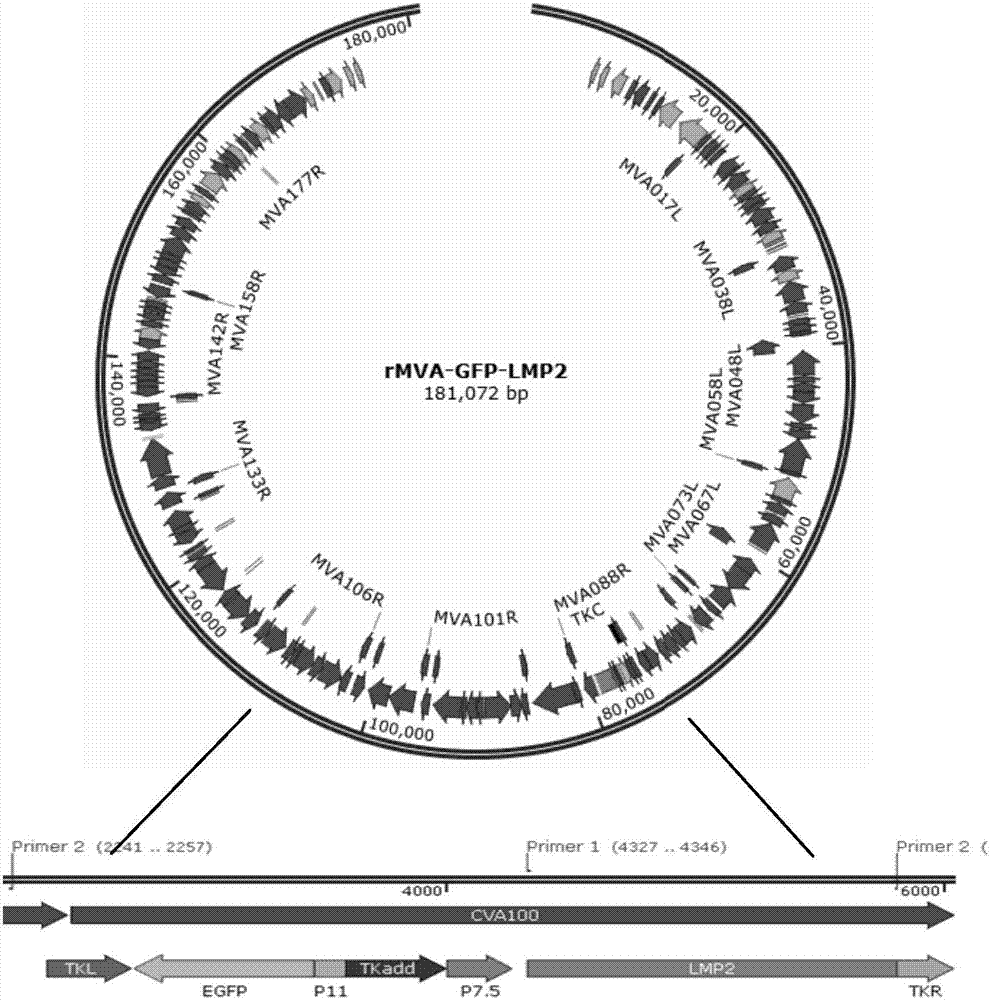
[0053] Embodiment 2: MVA-GFP-LMP2A recombinant virus construction
[0054] 2.1 Optimum conditions for the construction of MVA-GFP-LMP2A
[0055] Table 1 MVA-GFP-LMP2A Construction Conditions
[0056]
[0057] Note: "+" in the table represents the expression intensity of GFP under the fluorescence microscope.
[0058] rMVA (Sutter, G. and C. Staib, Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. CurrDrug Targets Infect Disord, 2003.3 (3): p.263-71.) according to 10 -1 , 10 -2 , 10 -3 Infect the BHK-21 cells in the 6-well plate, infect two wells for each dilution, wash the infected cells twice with PBS after 2 hours, and use 4ug and 6ug of PZL-GFP-LMP2 plasmid content for each dilution For transfection (see Table 1), refer to the manual of Roche reagent (X-tremeGENE HP DNA Transfection Reagent manual) for specific operation steps. Place the infected cells in a cell incubator at 37°C, observe GFP expression u...
Embodiment 3
[0070] Embodiment 3: Obtaining and identification of MVA-LMP2A recombinant virus
[0071] 3.1 MVA-LMP2A recombinant virus harvest and morphology observation
[0072] Homologous recombination to obtain MVA-GFP-LMP2A, because the probability of homologous recombination is low, the virus is screened by the same extreme dilution method as above to obtain the MVA-LMP2A recombinant virus, that is, the GFP gene is removed, and only the white color carrying the EBV LMP2 foreign gene is obtained. For the recombinant virus, the virus was stored at -80°C. Observed under a fluorescent microscope, although the infected cells were diseased, there was no green fluorescent expression ( Figure 6 ). Freezing and thawing at -80°C and 37°C were repeated three times (to break the cells and release the virus), centrifuge at 3000rpm / min for 10min, absorb the supernatant, that is, MVA-LMP2A recombinant virus, and store at -80°C.
[0073] 3.2 PCR identification of EBV LMP2 gene expression
[0074...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com