Bird flu vaccine by using attenuated vaccinia Tian Tan as vector
A technology of vaccinia virus and avian influenza virus, which is applied in the field of avian influenza vaccine and its preparation, can solve the problems of difficult supply, a large number of vaccines, and stimulation of effective neutralizing antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Construction of a recombinant vaccinia virus transfer vector expressing the HA gene of avian influenza virus
[0038] RNA of H5N1 subtype avian influenza virus A / Bar-headed Goose / Qinghai / 1 / 05 strain (Jinhua Liu#, et al. Highly pathogenic H5N1 influenza virus infection in migra-tory birds. Science, 2005, 309: 1206) As a template, use synthetic primers (primer 1: 5'TTGGATCCATGGAGAAAATAGTGCT3'; primer 2: 5'GGCTCGAGTTAAATGCAAATTCTGC3'; where a BamH I restriction site is added to the 5' end of the primer 1 sequence, and an XhoI restriction site is added to the 5' end of the primer 2 sequence ), the HA gene is amplified by RT-PCR, and the amplified HA gene includes the complete reading frame of the HA gene.
[0039] Subcloning the amplified HA gene into pZC also digested with BamH I and Xho I through BamH I and Xho I restriction sites XZ Among the shuttle vectors (Huang, X., B. Lu, W. Yu, Q. Fang, L. Liu, K. Zhuang, T. Shen, H. Wang, P. Tian, L. Zhang, and Z. Ch...
Embodiment 2
[0040] Example 2: Construction of recombinant vaccinia virus expressing avian influenza virus HA gene
[0041] The prepared chicken embryo fibroblasts were used to inoculate a six-well plate, and after 80% of the cells covered a monolayer, the vaccinia virus Tiantan strain (CCTCC: V200416) was inoculated with an inoculation volume of 0.5 MOI per well. Infect at 37°C for 2 hours, discard the infection solution, and rinse the cells three times with DMEM medium without antibiotics and calf serum; then use liposome Lipofectamin TM 2000 (purchased from Invitrogen Company) instructions, using the liposome method with 4 μg of purified recombinant transfer plasmid pZC XZ -HA transfected chicken embryo fibroblasts that had been infected with vaccinia virus Tiantan strain, infected at 37°C for 1 hour, discarded the transfection solution, and added DMEM culture solution containing 5% calf serum (both DMEM and calf serum were purchased from Invitrogen), 37°C, 5% CO 2 After culturing in...
Embodiment 3
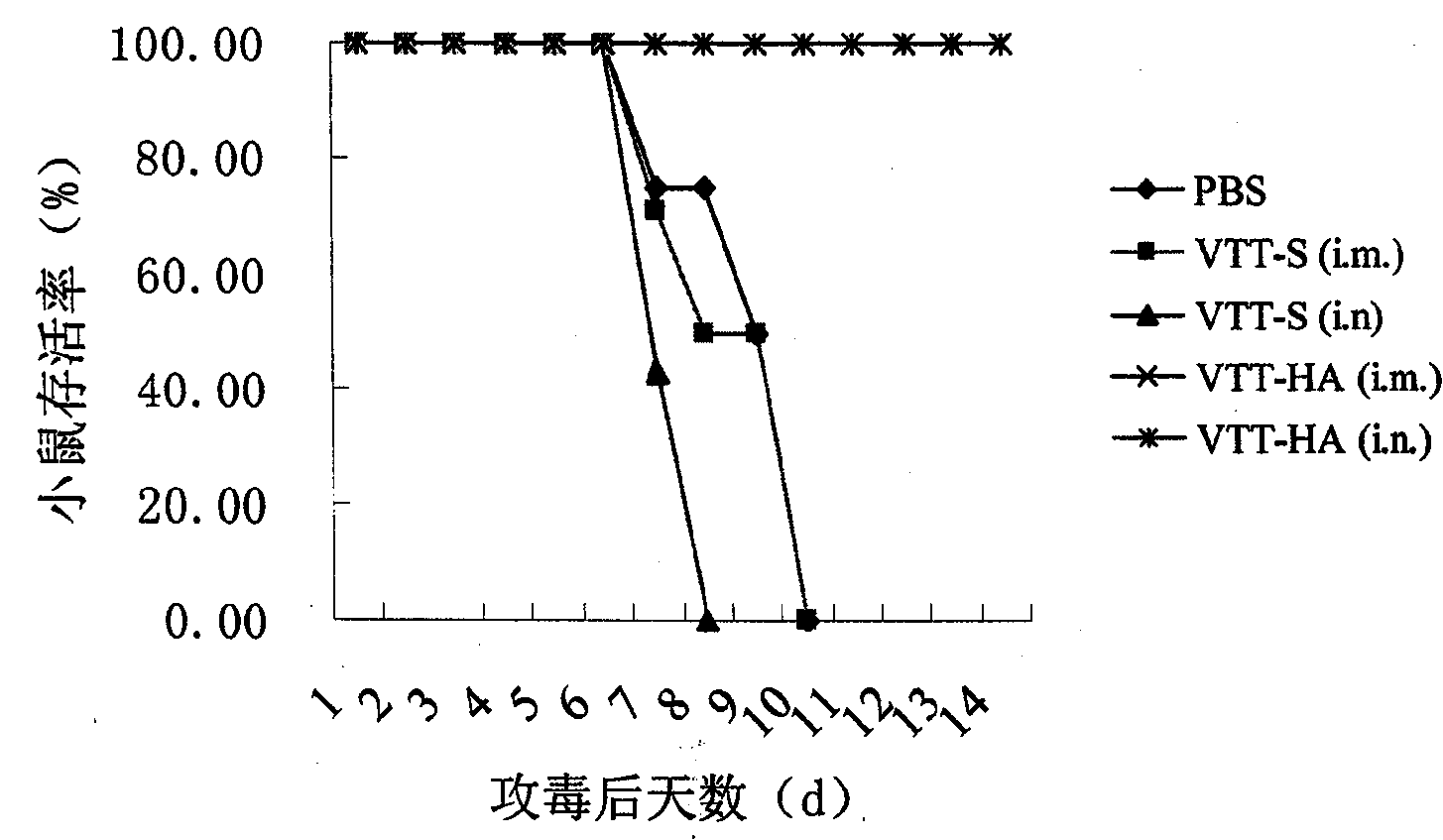
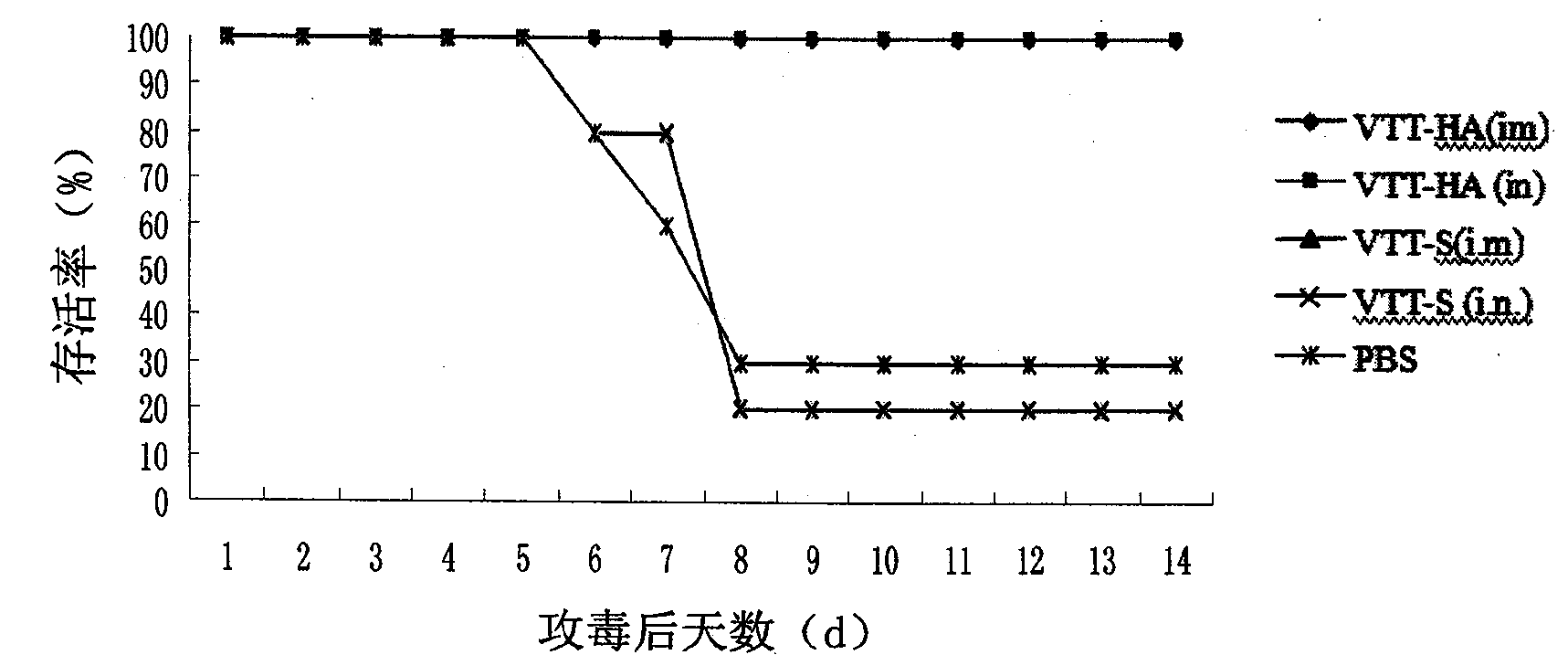
[0044] Example 3: Efficacy of recombinant Tiantan vaccinia virus avian influenza vaccine inducing AIV-specific immunity in animals
[0045] 1. Protective research of different immunization pathways of vaccines
[0046] 1) Determination of antibody levels produced by vaccines
[0047] Six-week-old SPF Balb / c mice (purchased from Beijing Experimental Animal Center) were immunized by intramuscular injection, intranasal drip and oral administration, with a dose of 3.0×10 6 PFU (plaque forming unit, plaque forming unit), a total of two immunizations with an interval of 31 days. Blood was collected on days 0, 28 and 45 after the first immunization, and the levels of hemagglutination-inhibiting antibodies (HI) and trace neutralizing antibodies against H5N1 virus in serum were determined according to the method recommended by the World Organization for Animal Health (http: / / www.oie.int / Eng / Normes / Mmanual / A_00037.htm). It was found that the background serum of the mice (that is, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com