Anti-COVID-19 virus neutralizing antibody mhC3 as well as humanized antibody and application thereof
An antibody, consistent technology, applied in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problem of no new coronary pneumonia-specific treatment drugs have been approved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, the discovery of antibody
[0058] 1. Mouse immunization and preparation of immune antibody library
[0059] Take 6-8 week-old Balb / C mice, and collect blood from the tail vein of the mice before immunization to keep the background serum. For the first immunization, the recombinant protein of the RBD domain of the new coronavirus S protein (Shenzhou Yiqiao, 40592-V08H5) was emulsified with complete Freund's adjuvant, and 100 μg of the recombinant protein was injected intraperitoneally into each mouse. Boost immunization at intervals of 1 week, take recombinant RBD protein (Shenzhou Yiqiao, 40592-V08H5) and emulsify it with Fischer’s incomplete adjuvant, inject 100 μg of recombinant protein intraperitoneally into each mouse, and collect blood by docking the tail before injection, and perform two booster immunizations in total . The fourth immunization was changed to impulse immunization, and the recombinant RBD protein without adjuvant (prepared in step 1...
Embodiment 2
[0063] Embodiment 2, preparation of anti-new coronavirus chimeric antibody
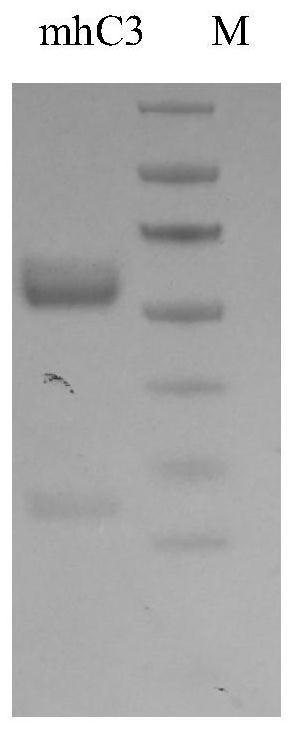
[0064] The mouse single-chain antibody clone C3 was picked to prepare a human-mouse chimeric whole antibody, named mhC3 (the constant region of the heavy chain is IgG1, and the light chain is Kappa). The preparation process is as follows:
[0065] 1. Construction of recombinant plasmids
[0066] 1. The sequence of SEQ ID No.11 is cloned into pcDNA3.1 (+) (Invitrogen, V79020) using conventional molecular biology techniques, and inserted between HindIII and BamH I restriction sites, and the resulting recombinant plasmid is passed through After sequencing and verification, it was named pcDNA3.1-mhC3H, which is the heavy chain expression vector of antibody mhC3.
[0067] 2. The sequence of SEQ ID No.12 is cloned into pcDNA3.1 (+) (Invitrogen, V79020) using conventional molecular biology techniques, inserted between the two restriction sites of Hind III and BamH I, and the resulting recombinant plasmid ...
Embodiment 3
[0083] Example 3, Detection of specific binding ability of mhC3 antibody
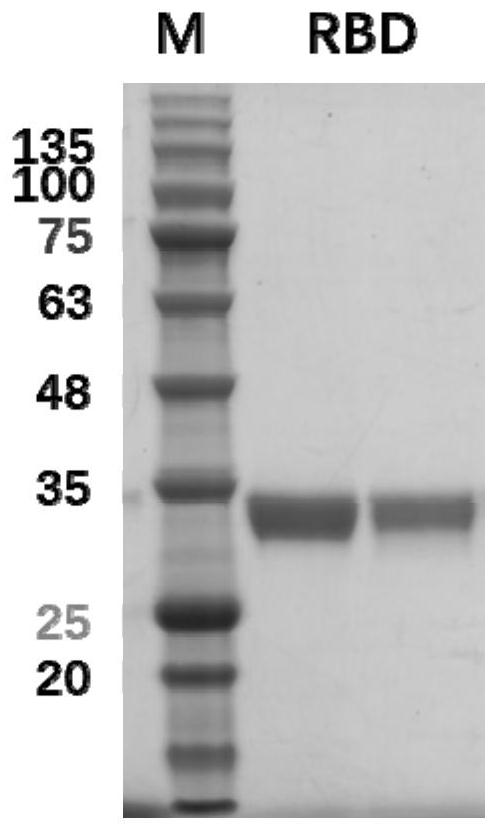
[0084] 1. Preparation of RBD recombinant protein
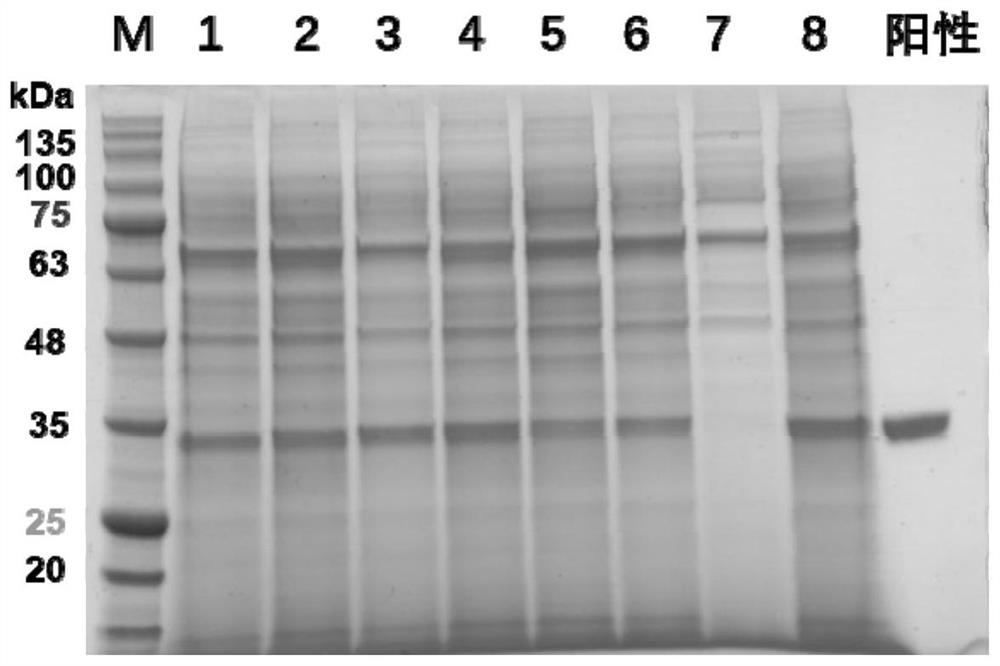
[0085] 1. Yeast expression and preparation of recombinant RBD glycoprotein
[0086] 1.1 Construction of recombinant protein expression vector
[0087] According to the SARS-CoV-2 gene sequence (NC_045512.2) published by GENEBANK, the S protein RBD region (SEQID No.15) was determined, and Shanghai Sangon Bioengineering Co., Ltd. was entrusted to carry out the whole gene synthesis, and enzyme cleavage sites were added at the upper and lower ends. XhoI and NotI were digested and inserted into the yeast expression vector pPICZαA (Invitrogen) which was digested with the same restriction enzyme, and named as pPICZαA-RBD.
[0088] 1.2 Yeast Competent Cell Preparation
[0089] Pick Pichia pastoris GJK0601 bacteria (the basic bacterial strain used in the present invention is the GJK0601 bacterial strain constructed earlier, the preservation number is CGMCC No...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com