Neutralizing antibody high-sensitivity detection method and product
A detection method and a technology for detecting samples, which can be used in material inspection products, measuring devices, biological tests, etc., and can solve problems such as low neutralization ability, high mutation frequency, positive conversion rate and low titer of neutralizing antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
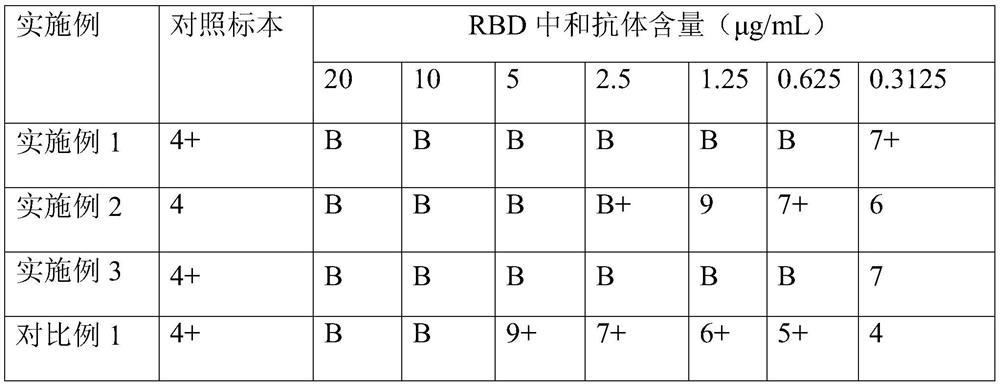
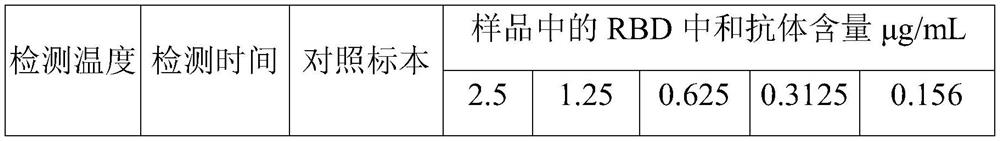
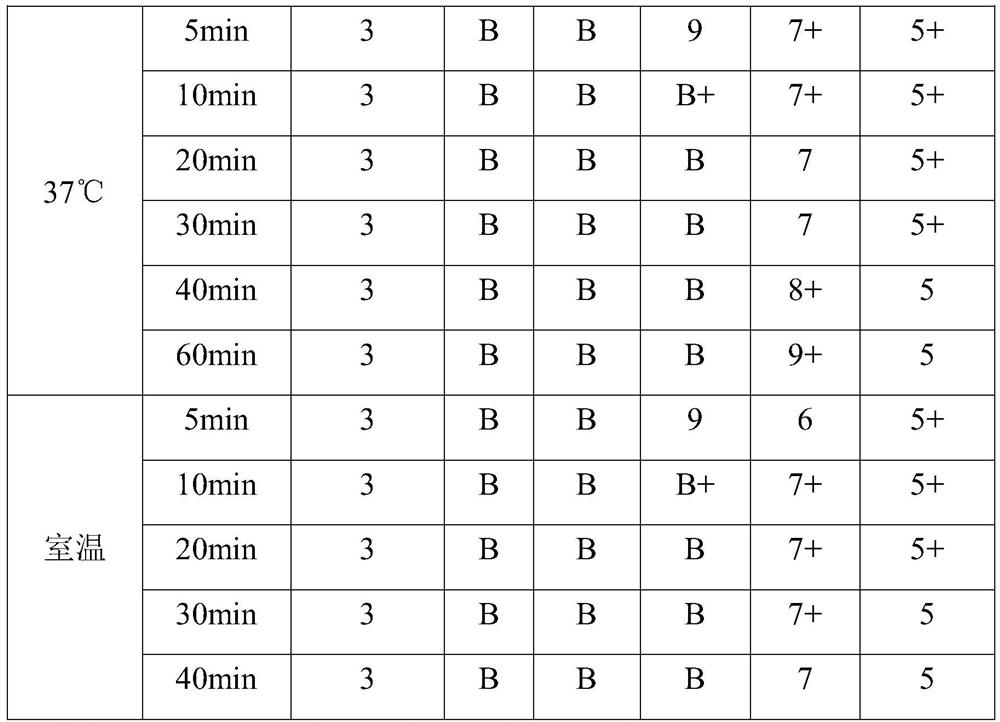
Embodiment 1
[0092] 1. Preparation of colloidal gold
[0093]The colloidal gold solution is prepared by the following method: chloroauric acid is formulated into a 1% solution, and then the above-mentioned solution with a final concentration of 4 / 10,000 is added to the boiled purified water, and the boiling is continued for 3 minutes, and 0.1M sodium citrate is added according to Add 580 μL per 100 mL of chloroauric acid solution, continue stirring and heating for 10 minutes, and cool to room temperature to prepare a colloidal gold solution, which is stored at 2-8°C for later use.
[0094] 2. Colloidal gold labeling
[0095] Using the SARS-CoV-2S protein RBD antibody (No. G3, available from Guangdong Feipeng Biological Co., Ltd.), which can compete with human ACE2, the steps of connecting colloidal gold are as follows: add K to the colloidal gold solution 2 CO 3 , adjust the pH to 8.0, add G3 to the adjusted pH solution to make the protein content 10μg / mL, couple for 5min, add 10% BSA to...
Embodiment 2
[0110] 1. Preparation of colloidal gold
[0111] The colloidal gold solution is prepared by the following method: chloroauric acid is formulated into a 1% solution, and then the above-mentioned solution with a final concentration of 4 / 10,000 is added to the boiled purified water, and the boiling is continued for 3 minutes, and 0.1M sodium citrate is added according to Add 580 μL per 100 mL of chloroauric acid solution, continue stirring and heating for 10 minutes, and cool to room temperature to prepare a colloidal gold solution, which is stored at 2-8°C for later use.
[0112] 2. Colloidal gold labeling
[0113] The SARS-CoV-2S protein RBD antibody G3 used, the steps of connecting colloidal gold are as follows: add K to the colloidal gold solution 2 CO 3 , adjust the pH to 8.0, add G3 to the adjusted pH solution to make the protein content 10μg / mL, couple for 5min, add 10% BSA to it to terminate the coupling reaction; discard the supernatant after centrifugation, and rediss...
Embodiment 3
[0130] 1. Preparation of colloidal gold
[0131] The colloidal gold solution is prepared by the following method: chloroauric acid is formulated into a 1% solution, and then the above-mentioned solution with a final concentration of 4 / 10,000 is added to the boiled purified water, and the boiling is continued for 3 minutes, and 0.1M sodium citrate is added according to Add 580 μL per 100 mL of chloroauric acid solution, continue stirring and heating for 10 minutes, and cool to room temperature to prepare a colloidal gold solution, which is stored at 2-8°C for later use.
[0132] 2. Colloidal gold labeling
[0133] Using human ACE2 protein Ag2, the steps of connecting colloidal gold are as follows: add K to the colloidal gold solution 2 CO 3 , adjust the pH to 8.0, add Ag2 to the adjusted pH solution to make the protein content 10 μg / mL, couple for 5 minutes, add 10% BSA to it to terminate the coupling reaction; discard the supernatant after centrifugation, and redissolve the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com