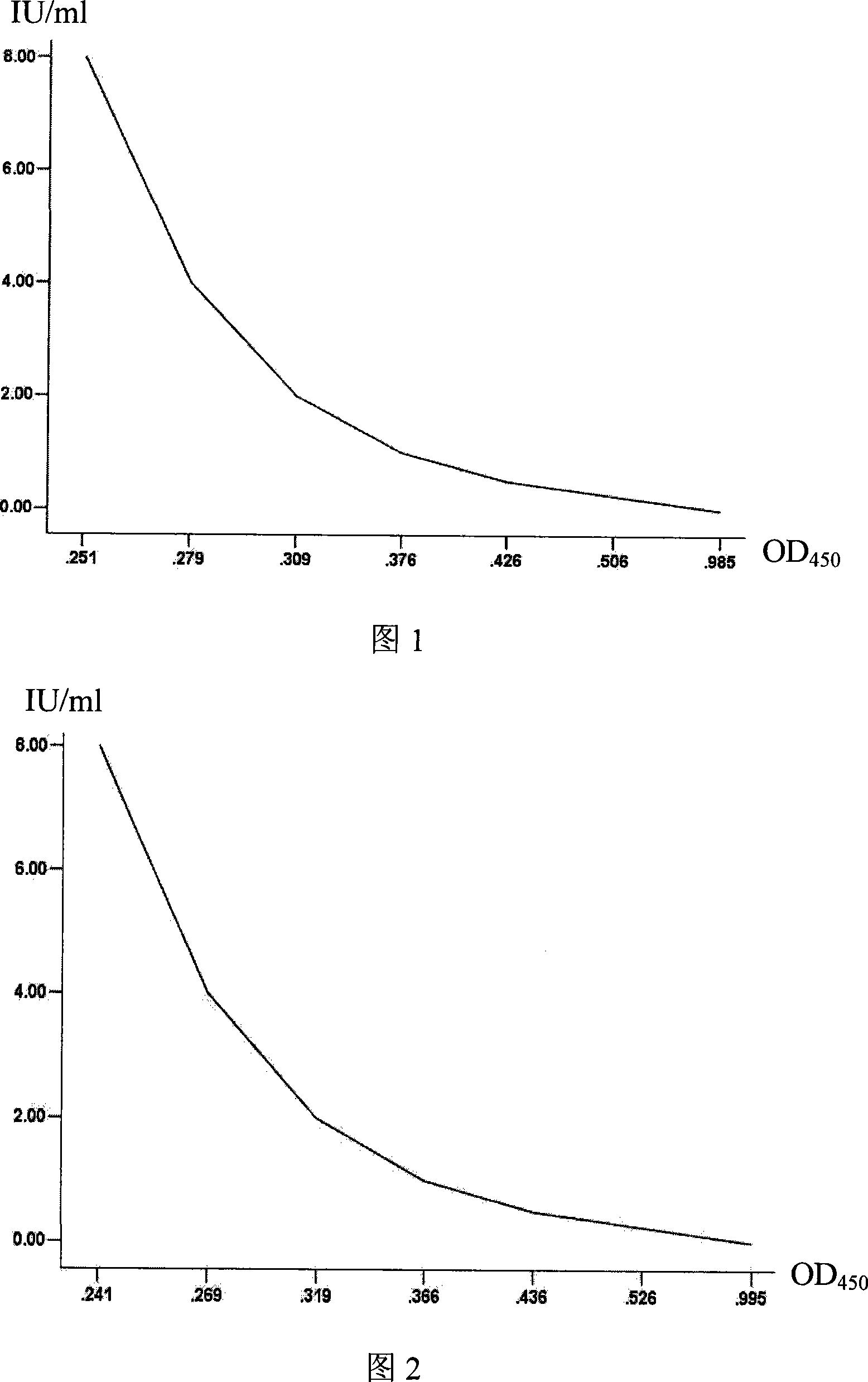
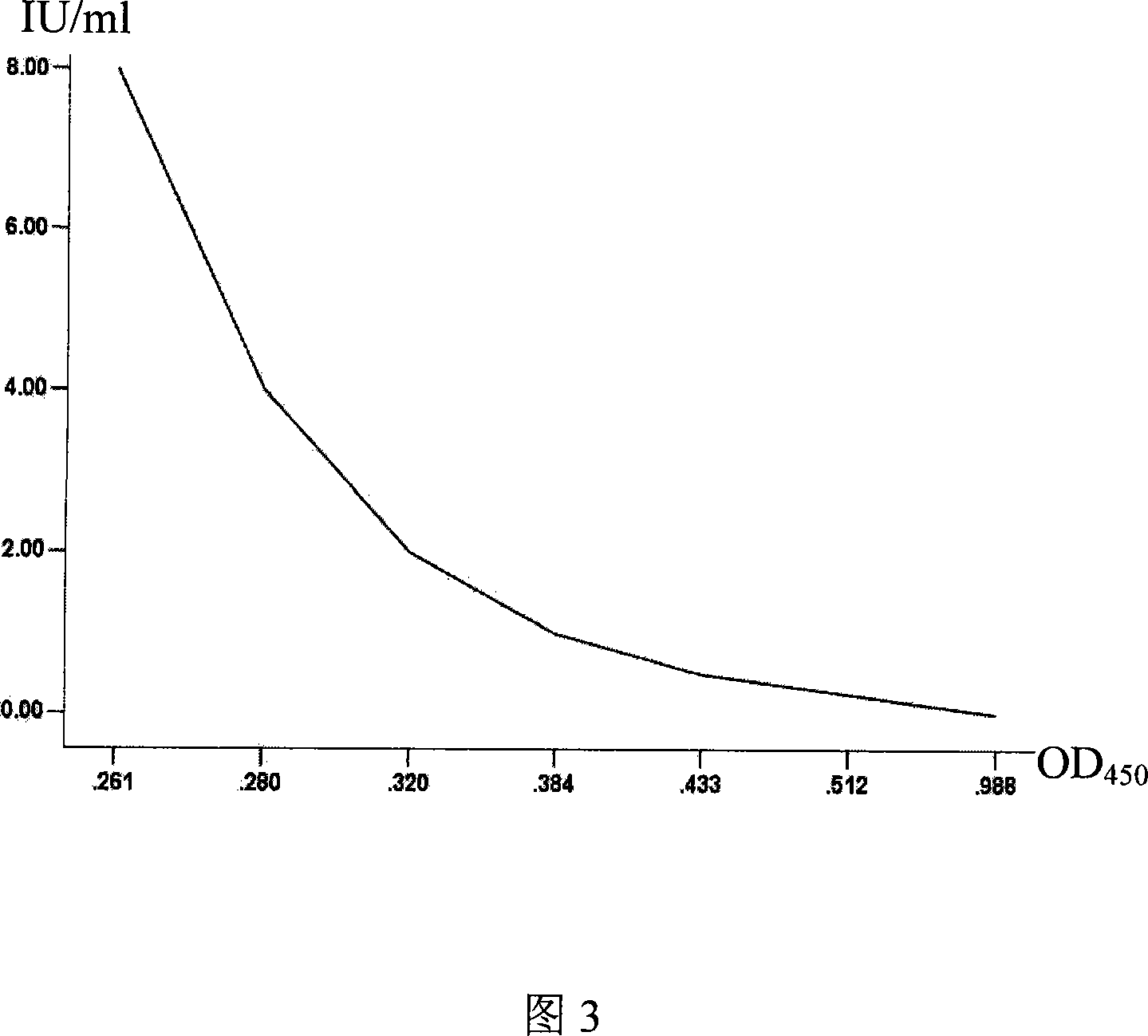
Kit for testing neutralizing antibody racing ELISA in human and animal rabies
A detection kit and kit technology, applied in the field of biological vaccine preparation, can solve problems such as long time required, poor sensitivity and specificity, large difference in antibody potency, etc., and achieve accurate quantitative determination, good consistency, and time-consuming short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation method of the kit (1)
[0038] 1. Preparation and Coating of Rabies Virus
[0039] Rabies virus was inoculated into BHK-21 monolayer cells, cultured for 3 to 4 days, frozen and thawed twice, cells were lysed, and culture medium was harvested. When coating, dilute the virus with the coating solution to a total protein concentration of 100 μg / ml, add 100 μl to the enzyme-linked plate, and coat overnight at 4°C; rinse three times with PBS-Tween 20 buffer, and wash the virus three times in each well. Add 100 μl of blocking solution (containing 5% skimmed milk powder and 0.01% thimerosal), place at 37°C for 1 hour, discard the blocking solution, seal it in a vacuum-sealed aluminum foil bag, and store at 4°C.
[0040] 2. Preparation of standard serum
[0041] 2.1 Preparation of human standard serum
[0042]Commercially available human rabies vaccine (Dalian Jingang Andy Biological Products Co., Ltd.) was purchased, and the volunteers were immunized three times ...
Embodiment 2
[0067] The preparation method of the kit (2)
[0068] 1. Preparation and Coating of Rabies Virus
[0069] Rabies virus was inoculated into BHK-21 monolayer cells, cultured for 3 to 4 days, frozen and thawed twice, cells were lysed, and culture medium was harvested. When coating, dilute the virus with the coating solution to a total protein concentration of 100 μg / ml, add 100 μl to the enzyme-linked plate, and coat overnight at 4°C; rinse three times with PBS-Tween 20 buffer, and wash the virus three times in each well. Add 100 μl of blocking solution (containing 5% skimmed milk powder and 0.01% thimerosal), place at 37°C for 1 hour, discard the blocking solution, seal it in a vacuum-sealed aluminum foil bag, and store at 4°C.
[0070] 2. Preparation of standard serum
[0071] 2.1 Preparation of human standard serum
[0072] Commercially available human rabies vaccine (Dalian Jingang Andy Biological Products Co., Ltd.) was purchased, and the volunteers were immunized three t...
Embodiment 3
[0098] The preparation method of the kit (3)
[0099] 1. Preparation and Coating of Rabies Virus
[0100] Rabies virus was inoculated into BHK-21 monolayer cells, cultured for 3 to 4 days, frozen and thawed twice, cells were lysed, and culture medium was harvested. When coating, dilute the virus with the coating solution to a total protein concentration of 100 μg / ml, add 100 μl to the enzyme-linked plate, and coat overnight at 4°C; rinse three times with PBS-Tween 20 buffer, and wash the virus three times in each well. Add 100 μl of blocking solution (containing 5% skimmed milk powder and 0.01% thimerosal), place at 37°C for 1 hour, discard the blocking solution, seal it in a vacuum-sealed aluminum foil bag, and store at 4°C.
[0101] 2. Preparation of standard serum
[0102] 2.1 Preparation of human standard serum
[0103] Commercially available human rabies vaccine (Dalian Jingang Andy Biological Products Co., Ltd.) was purchased, and the volunteers were immunized three t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com